Application of High-Intensity Ultrasound to Improve Food Processing Efficiency: A Review
Abstract
1. Introduction
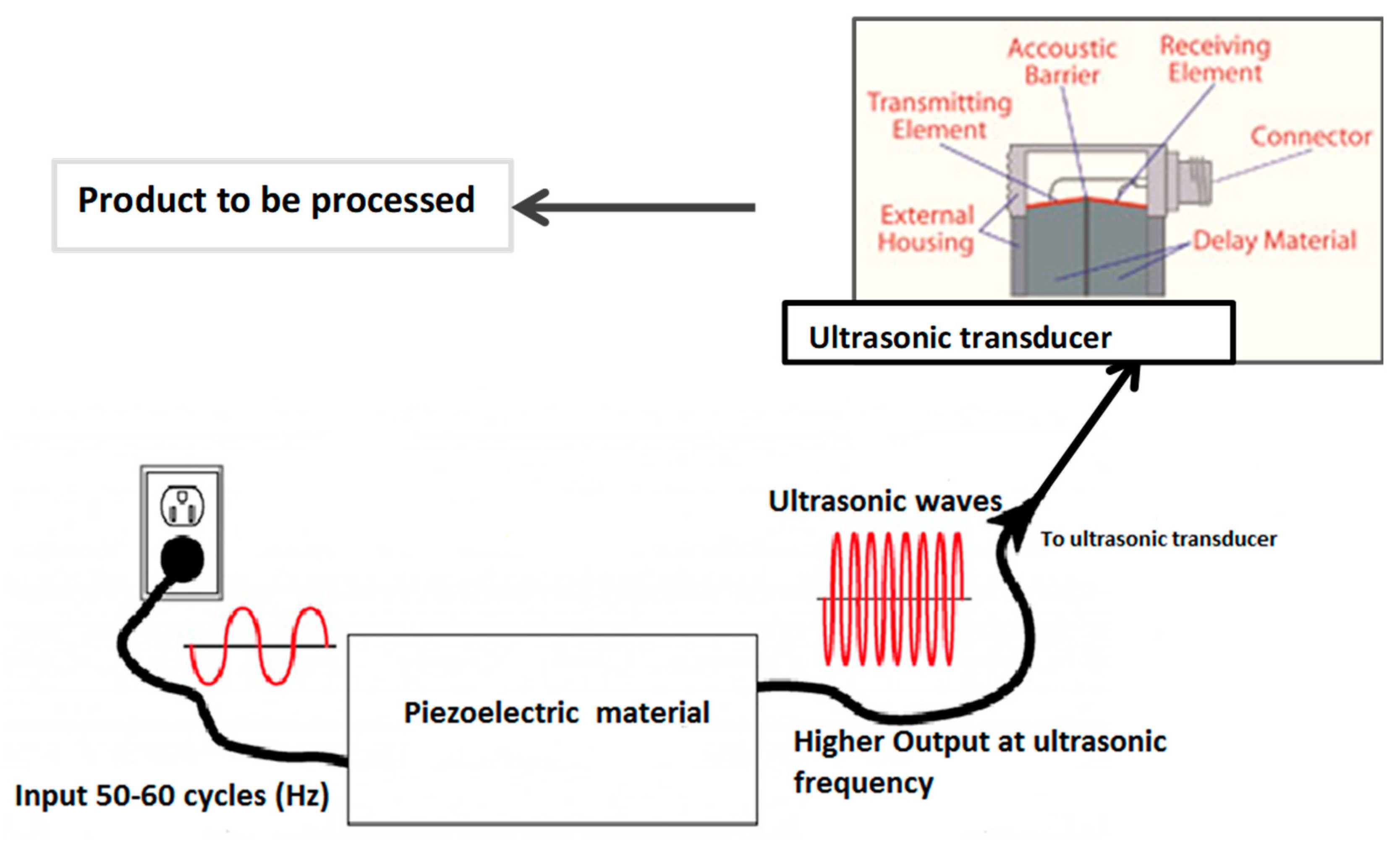
2. Generation of Ultrasound
3. Classification of Ultrasound Applications
4. Cavitation
4.1. Factors Affecting the Cavitation
4.1.1. Frequency
4.1.2. Intensity
4.1.3. State of the Material
4.1.4. Temperature, Pressure, and Viscosity
5. Applications of Ultrasonic Waves in Food Processing
5.1. Microbial Inactivation
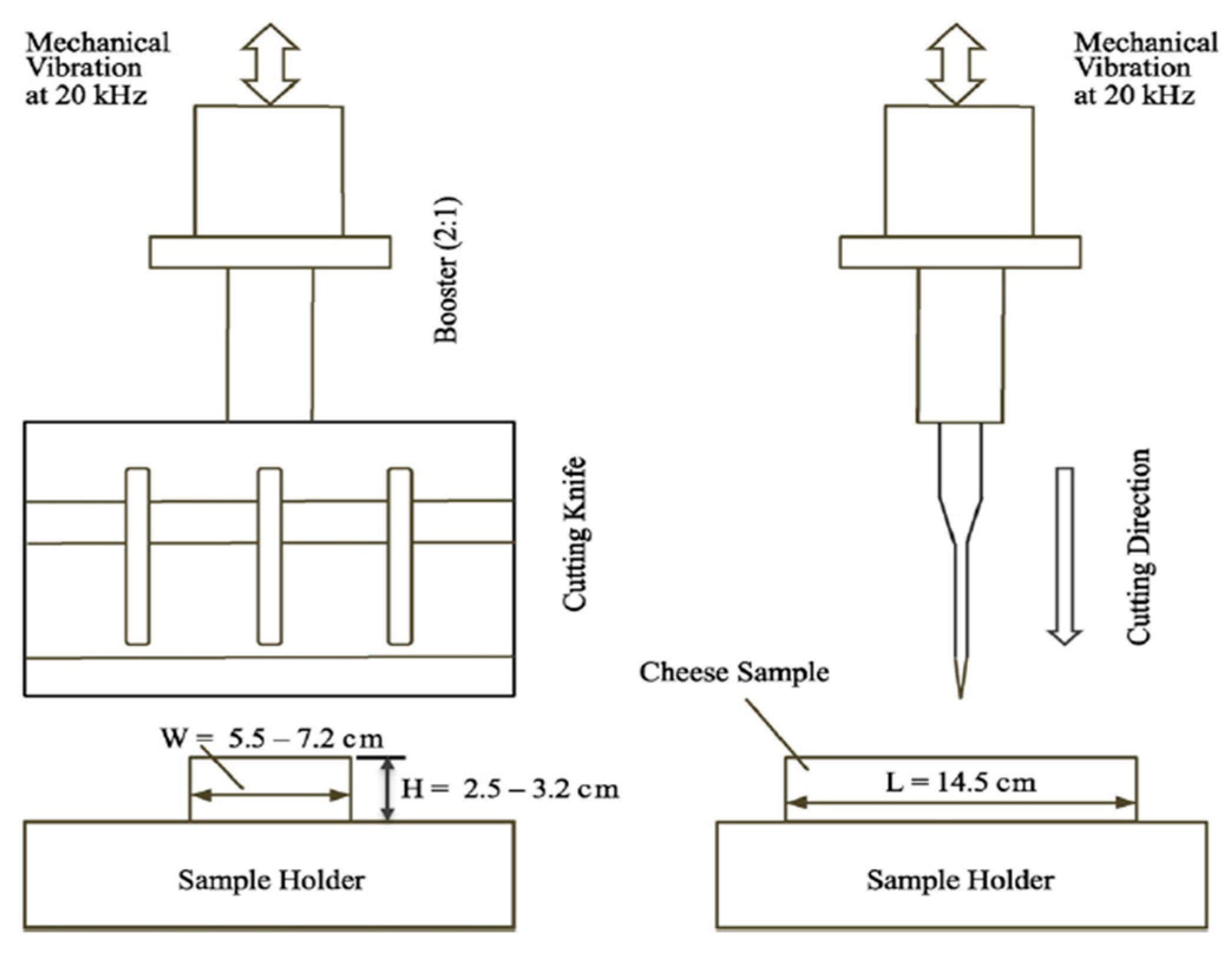
5.2. Ultrasonic Cutting
5.3. Ultrasonic Drying
5.4. Ultrasound-Assisted Freezing
5.5. Ultrasound-Assisted Extraction
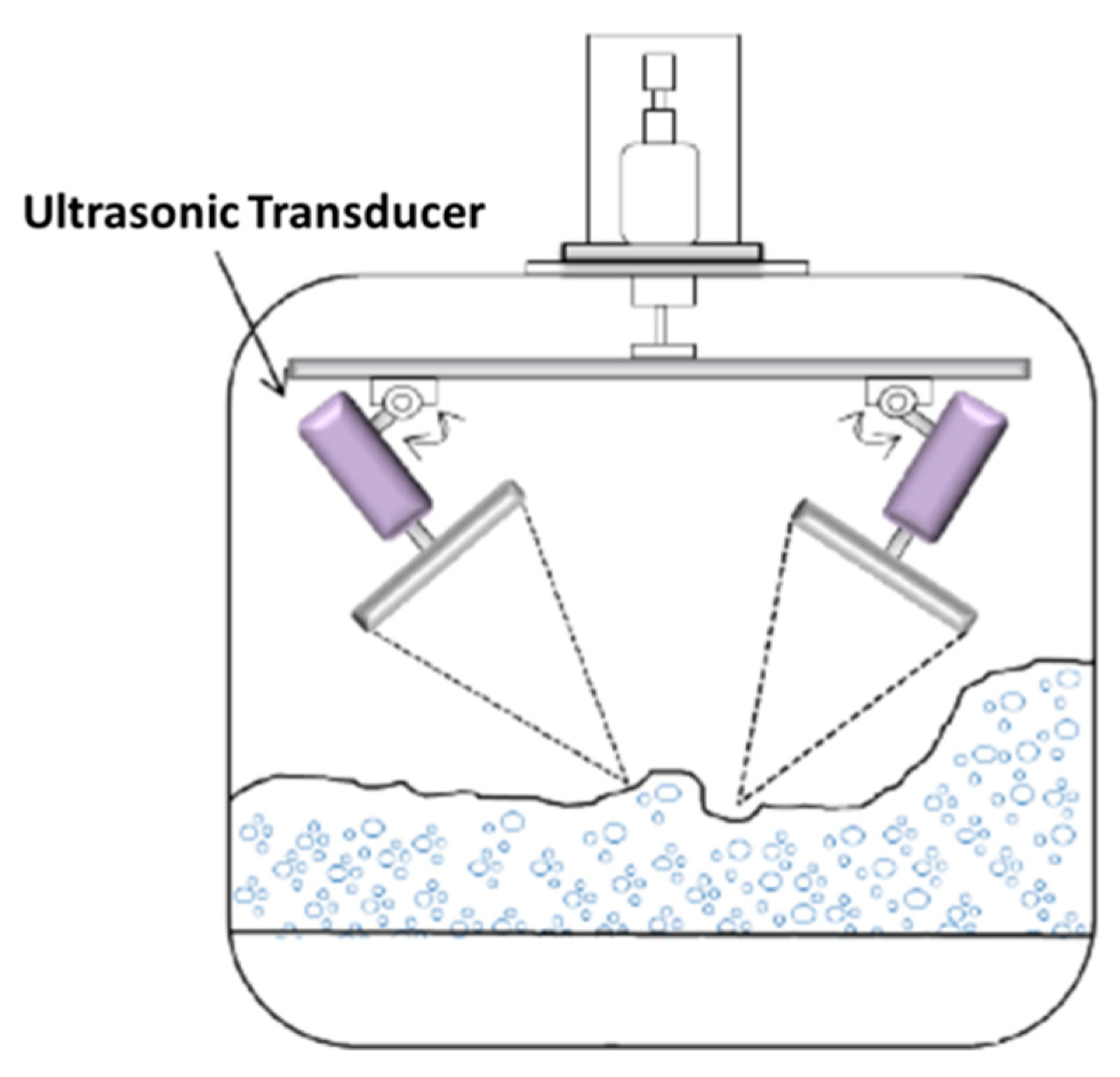
5.6. Ultrasonic Foaming and Defoaming
5.7. Functional Food Emulsions
6. Pros and Cons of Using Ultrasonication
- Mechanical effects generated by ultrasound, turbulence, shock waves, microstreaming, etc., increase mass transfer within the medium that may positively influence chemical reactions and other processes. Ultrasound waves are non-toxic, safe, and environmentally benign. Ultrasonication is a microbial inactivation approach that can be used with a variety of thermal and non-thermal treatments;
- Ultrasound has the ability to start reactions without the use of external chemicals. External reagents are not required because the collapsing bubbles generate radicals that can perform redox reactions. The products produced without external chemical reagents would be purer, and the processing atmosphere would be ‘greener’;
- Processes, such as emulsion polymerisation and microsphere creation, necessitate both physical and chemical effects, which sonication can provide. In addition to the substantially faster reaction rates and higher monomer-to-polymer conversion rates, ultrasonic polymerization has been proven to produce polymer particles with a high molecular weight;
- When compared to other juice-extraction processes, ultrasound will be more efficient in increasing juice production, cutting processing time by 55% and temperature by 16%;
- During processing, ultrasonic-treated items will lose the least amount of flavour, colour, and other nutritional ingredients.
- Their inability to obtain large-scale ultrasonic reactors is a problem. Ultrasonic equipment is now custom-made for individual uses, which raises the expense of implementing this technology in businesses;
- In some applications, direct contact between processing liquids and ultrasonic horns is a problem. This could be avoided by using a flow-through ultrasonic reactor with short residence durations and creating non-contact reactors;
- The free radicals produced through cavitation could impact the product and harm the consumer.
7. Health Effects of Ultrasound
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Li, W.; Gamlath, C.J.; Pathak, R.; Martin, G.J.O.; Ashokkumar, M. Ultrasound—The physical and chemical effects integral to food processing. In Innovative Food Processing Technologies; Knoerzer, K., Juliano, P., Smithers, G., Eds.; Elsevier Inc.: Cambridge, UK, 2021; pp. 329–358. [Google Scholar] [CrossRef]
- Ramesh, T.; Nayak, B.; Amirbahman, A.; Tripp, C.P.; Mukhopadhyay, S. Application of ultraviolet light assisted titanium dioxide photocatalysis for food safety: A review. Innov. Food Sci. Emerg. Technol. 2016, 38, 105–115. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Wang, L.-H.; Zeng, X.-A.; Han, Z.; Brennan, C.S. Non-thermal technologies and its current and future application in the food industry: A review. Int. J. Food Sci. Technol. 2019, 54, 1–13. [Google Scholar] [CrossRef]
- Firouz, M.S.; Farahmandi, A.; Hosseinpour, S. Recent advances in ultrasound application as a novel technique in analysis, processing and quality control of fruits, juices and dairy products industries: A review. Ultrason. Sonochem. 2019, 57, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Singla, M.; Sit, N. Application of ultrasound in combination with other technologies in food processing: A review. Ultrason. Sonochem. 2021, 73, 105506. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Ferrara, L.; Naviglio, D. Application of Ultrasound in Food Science and Technology: A Perspective. Foods 2018, 7, 164. [Google Scholar] [CrossRef]
- Khadhraoui, B.; Ummat, V.; Tiwari, B.; Fabiano-Tixier, A.; Chemat, F. Review of ultrasound combinations with hybrid and innovative techniques for extraction and processing of food and natural products. Ultrason. Sonochem. 2021, 76, 105625. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Belwal, T.; Cravotto, G.; Luo, Z. Sono-physical and sono-chemical effects of ultrasound: Primary applications in extraction and freezing operations and influence on food components. Ultrason. Sonochem. 2020, 60, 104726. [Google Scholar] [CrossRef]
- Wu, X.-F.; Zhang, M.; Adhikari, B.; Sun, J. Recent developments in novel freezing and thawing technologies applied to foods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3620–3631. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef]
- Ortega-Rivas, E. Ultrasound in Food Preservation. Food Eng. Ser. 2012, 5, 251–262. [Google Scholar] [CrossRef]
- Kentish, S.; Feng, H. Applications of Power Ultrasound in Food Processing. Annu. Rev. Food Sci. Technol. 2014, 5, 263–284. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Chitrakar, B.; Bhandari, B. Application of power ultrasound in freezing and thawing Processes: Effect on process efficiency and product quality. Ultrason. Sonochem. 2020, 68, 105230. [Google Scholar] [CrossRef]
- Charoux, C.M.; Inguglia, E.S.; O’Donnell, C.P.; Tiwari, B.K. Ultrasonic Waves: Inactivation of foodborne microorganisms using power ultrasound. In Innovative Food Processing Technologies; Knoerzer, K., Juliano, P., Smithers, G., Eds.; Elsevier: Cambridge, UK, 2021. [Google Scholar] [CrossRef]
- Chew, S.C.; Ali, M.A. Recent advances in ultrasound technology applications of vegetable oil refining. Trends Food Sci. Technol. 2021, 116, 468–479. [Google Scholar] [CrossRef]
- Yao, Y.; Pan, Y.; Liu, S. Power ultrasound and its applications: A state-of-the-art review. Ultrason. Sonochem. 2019, 62, 104722. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Ravikumar, M. Ultrasonication: An Advanced Technology for Food Preservation. Int. J. Pure Appl. Biosci. 2017, 5, 363–371. [Google Scholar] [CrossRef]
- Li, Z.; Dong, J.; Wang, L.; Zhang, Y.; Zhuang, T.; Wang, H.; Cui, X.; Wang, Z. A power-triggered preparation strategy of nano-structured inorganics: Sonosynthesis. Nanoscale Adv. 2021, 3, 2423–2447. [Google Scholar] [CrossRef]
- Suslick, K.S.; Eddingsaas, N.C.; Flannigan, D.J.; Hopkins, S.D.; Xu, H. The Chemical History of a Bubble. Acc. Chem. Res. 2018, 51, 2169–2178. [Google Scholar] [CrossRef]
- McKenzie, T.G.; Karimi, F.; Ashokkumar, M.; Qiao, G.G. Ultrasound and Sonochemistry for Radical Polymerization: Sound Synthesis. Chem. Eur. J. 2019, 25, 5372–5388. [Google Scholar] [CrossRef]
- Chen, Z. Microbial Inactivation in Foods by Ultrasound. J. Food Microbiol. Saf. Hyg. 2017, 2, 9–10. [Google Scholar] [CrossRef]
- Kentish, S.; Ashokkumar, M. The physical and chemical effects of ultrasound. In Ultrasound Technologies for Food and Bioprocessing; Feng, H., Barbosa-Cánovas, G.V., Weiss, J., Eds.; Springer: London, UK, 2011; pp. 1–13. [Google Scholar]
- Leong, T.; Ashokkumar, M.; Kentish, S.E. The fundamentals of power ultrasound—A review. Acoust. Aust. 2011, 39, 43–52. [Google Scholar]
- Astráin-Redín, G.J. Basic principles of ultrasound. In Ultrasound in Food Processing. Recent Advances; Villamiel, M., Montilla, A., García-Pérez, J.V., Cárcel, J.A., Benedito, J., Eds.; Wiley Blackwell: Chichester, UK, 2017; pp. 4–26. [Google Scholar]
- Astráin-Redín, L.; Ciudad-Hidalgo, S.; Raso, J.; Condón, S.; Cebrián, G.; Álvarez, I. Application of High-Power Ultrasound in the Food Industry. In Sonochemical Reactions; Selcan Karakuş, S., Ed.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Kentish, S. Engineering principles of ultrasound technology. In Ultrasound: Advances in Food Processing and Preservation; Bermúdez-Aguirre, D., Ed.; Academic Press: London, UK, 2017. [Google Scholar]
- Starek, A.; Kobus, Z.; Sagan, A.; Chudzik, B.; Pawłat, J.; Kwiatkowski, M.; Terebun, P.; Andrejko, D. Influence of ultrasound on selected microorganisms, chemical and structural changes in fresh tomato juice. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Evelyn; Silva, F.V. Ultrasound assisted thermal inactivation of spores in foods: Pathogenic and spoilage bacteria, molds and yeasts. Trends Food Sci. Technol. 2020, 105, 402–415. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.; Cadwallader, K.R.; Feng, H.; Martin, S.E. Sonication in combination with heat and low pressure as an alternative pasteurization treatment—Effect on Escherichia coli K12 inactivation and quality of apple cider. Ultrason. Sonochem. 2013, 20, 1131–1138. [Google Scholar] [CrossRef]
- Kapturowska, A.; Stolarzewicz, I.; Chmielewska, I.; Bialecka-Florjanczyk, E. Ultrasounds—A tool to inactivate yeast and to extract intracellular protein. Food Sci. Technol. Qual. 2011, 18, 160–171. [Google Scholar] [CrossRef]
- Sulaiman, A.; Soo, M.J.; Farid, M.; Silva, F.V. Thermosonication for polyphenoloxidase inactivation in fruits: Modeling the ultrasound and thermal kinetics in pear, apple and strawberry purees at different temperatures. J. Food Eng. 2015, 165, 133–140. [Google Scholar] [CrossRef]
- Park, S.Y.; Mizan, F.R.; Ha, S.-D. Inactivation of Cronobacter sakazakii in head lettuce by using a combination of ultrasound and sodium hypochlorite. Food Control 2016, 60, 582–587. [Google Scholar] [CrossRef]
- Muñoz, R.; Viveros, N.; Bevilacqua, A.; Pérez, M.S.; Arévalo-Villena, M. Effects of ultrasound treatments on wine microorganisms. Ultrason. Sonochem. 2021, 79, 105775. [Google Scholar] [CrossRef] [PubMed]
- Costello, K.M.; Velliou, E.; Gutierrez-Merino, J.; Smet, C.; El Kadri, H.; Van Impe, J.F.; Bussemaker, M. The effect of ultrasound treatment in combination with nisin on the inactivation of Listeria innocua and Escherichia coli. Ultrason. Sonochem. 2021, 79, 105776. [Google Scholar] [CrossRef] [PubMed]
- Wordon, B.; Mortimer, B.; McMaster, L. Comparative real-time analysis of Saccharomyces cerevisiae cell viability, injury and death induced by ultrasound (20 kHz) and heat for the application of hurdle technology. Food Res. Int. 2012, 47, 134–139. [Google Scholar] [CrossRef]
- Lee, H.; Zhou, B.; Liang, W.; Feng, H.; Martin, S.E. Inactivation of Escherichia coli cells with sonication, manosonication, thermosonication, and manothermosonication: Microbial responses and kinetics modeling. J. Food Eng. 2009, 93, 354–364. [Google Scholar] [CrossRef]
- Arroyo, C.; Cebrián, G.; Pagán, R.; Condón, S. Synergistic combination of heat and ultrasonic waves under pressure for Cronobacter sakazakii inactivation in apple juice. Food Control 2012, 25, 342–348. [Google Scholar] [CrossRef]
- Lopez-Malo, A.; Palou, E.; Jimenez-Fernandez, M.; Al-Zamora, S.M.; Guerrero, S. Multifactorial fungal inactivation combining thermosonication and antimicrobials. J. Food Eng. 2005, 67, 87–93. [Google Scholar] [CrossRef]
- Bermúdez-Aguirre, D.; Barbosa-Cánovas, G.V. Study of butter fat content in milk on the inactivation of Listeria innocua ATCC 51742 by thermo-sonication. Innov. Food Sci. Emerg. Technol. 2008, 9, 176–185. [Google Scholar] [CrossRef]
- Rossi, A.P.; Kalschne, D.L.; Byler, A.P.I.; Flores, E.L.D.M.; Leite, O.D.; dos Santos, D.; Barin, J.S.; Canan, C. Effect of ultrasound and chlorine dioxide on Salmonella Typhimurium and Escherichia coli inactivation in poultry chiller tank water. Ultrason. Sonochem. 2021, 80, 105815. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jia, W.; Xu, D.; Li, R. Applications of Ultrasonic Cutting in Food Processing. J. Food Process. Preserv. 2014, 39, 1762–1769. [Google Scholar] [CrossRef]
- Yildiz, G.; Rababah, T.M.; Feng, H. Ultrasound-assisted cutting of cheddar, mozzarella and Swiss cheeses—Effects on quality attributes during storage. Innov. Food Sci. Emerg. Technol. 2016, 37, 1–9. [Google Scholar] [CrossRef]
- Arnold, G.; Zahn, S.; Legler, A.; Rohm, H. Ultrasonic cutting of foods with inclined moving blades. J. Food Eng. 2011, 103, 394–400. [Google Scholar] [CrossRef]
- McCulloch, E. Experimental and Finite Element Modelling of Ultrasonic Cutting of Food. Doctoral Dissertation, University of Glasgow, Glasgow, UK, 2008. [Google Scholar]
- Zahn, S.; Schneider, Y.; Rohm, H. Ultrasonic cutting of foods: Effects of excitation magnitude and cutting velocity on the reduction of cutting work. Innov. Food Sci. Emerg. Technol. 2006, 7, 288–293. [Google Scholar] [CrossRef]
- Deng, L.-Z.; Mujumdar, A.S.; Zhang, Q.; Yang, X.-H.; Wang, J.; Zheng, Z.-A.; Gao, Z.-J.; Xiao, H.-W. Chemical and physical pretreatments of fruits and vegetables: Effects on drying characteristics and quality attributes—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1408–1432. [Google Scholar] [CrossRef]
- Liu, Y.; Pu, H.; Sun, D.-W. Hyperspectral imaging technique for evaluating food quality and safety during various processes: A review of recent applications. Trends Food Sci. Technol. 2017, 69, 25–35. [Google Scholar] [CrossRef]
- Mar, V.; Riera, E.; Pérez, G.; García-Pérez, J.V. The use of ultrasound for drying, degassing and defoaming of foods. Innov. Food Processing Technol. Compr. Rev. 2021, 415–438. [Google Scholar] [CrossRef]
- Ortuño, C.; Pérez-Munuera, I.; Puig, A.; Riera, E.; Garcia-Perez, J. Influence of power ultrasound application on mass transport and microstructure of orange peel during hot air drying. Phys. Procedia 2010, 3, 153–159. [Google Scholar] [CrossRef][Green Version]
- Kowalski, S.J.; Pawłowski, A. Intensification of apple drying due to ultrasound enhancement. J. Food Eng. 2015, 156, 1–9. [Google Scholar] [CrossRef]
- Kowalski, S.J.; Pawłowski, A.; Szadzińska, J.; Łechtańska, J.; Stasiak, M. High power airborne ultrasound assist in combined drying of raspberries. Innov. Food Sci. Emerg. Technol. 2016, 34, 225–233. [Google Scholar] [CrossRef]
- Romero, J.C.; Yépez, V.B. Ultrasound as pretreatment to convective drying of Andean blackberry (Rubus glaucus Benth). Ultrason. Sonochem. 2015, 22, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.L.; Alvim, I.D.; Augusto, P.E.D. Incorporation of microencapsulated hydrophilic and lipophilic nutrients into foods by using ultrasound as a pre-treatment for drying: A prospective study. Ultrason. Sonochem. 2019, 54, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Mou, X.; Chen, Z. Study on the ultrasound-assisted drying process of deformable porous materials. J. Food Eng. 2021, 306, 110612. [Google Scholar] [CrossRef]
- Wu, B.; Guo, X.; Guo, Y.; Ma, H.; Zhou, C. Enhancing jackfruit infrared drying by combining ultrasound treatments: Effect on drying characteristics, quality properties and microstructure. Food Chem. 2021, 358, 129845. [Google Scholar] [CrossRef]
- Kek, S.; Chin, N.; Yusof, Y. Direct and indirect power ultrasound assisted pre-osmotic treatments in convective drying of guava slices. Food Bioprod. Process. 2013, 91, 495–506. [Google Scholar] [CrossRef]
- Gamboa-Santos, J.; Montilla, A.; Cárcel, J.A.; Villamiel, M.; Garcia-Perez, J.V. Air-borne ultrasound application in the convective drying of strawberry. J. Food Eng. 2014, 128, 132–139. [Google Scholar] [CrossRef]
- Kowalski, S.; Szadzińska, J. Convective-intermittent drying of cherries preceded by ultrasonic assisted osmotic dehydration. Chem. Eng. Process. Process. Intensif. 2014, 82, 65–70. [Google Scholar] [CrossRef]
- do Nascimento, E.M.G.C.; Mulet, A.; Ascheri, J.L.R.; de Carvalho, C.W.P.; Cárcel, J.A. Effects of high-intensity ultrasound on drying kinetics and antioxidant properties of passion fruit peel. J. Food Eng. 2016, 170, 108–118. [Google Scholar] [CrossRef]
- Wang, L.; Xu, B.; Wei, B.; Zeng, R. Low frequency ultrasound pretreatment of carrot slices: Effect on the moisture migration and quality attributes by intermediate-wave infrared radiation drying. Ultrason. Sonochem. 2018, 40, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-G.; Guo, X.-Y.; Wu, T. A novel dehydration technique for carrot slices implementing ultrasound and vacuum drying methods. Ultrason. Sonochem. 2016, 30, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-Y.; Xia, Q.; He, J.; Sun, Y.-Y.; Dang, Y.-L.; Ou, C.-R.; Pan, D.-D.; Cao, J.-X.; Zhou, G.-H. Improvement of ultrasound-assisted thermal treatment on organoleptic quality, rheological behavior and flavor of defective dry-cured ham. Food Biosci. 2021, 43, 101310. [Google Scholar] [CrossRef]
- Abbaspour-Gilandeh, Y.; Kaveh, M.; Aziz, M. Ultrasonic-Microwave and Infrared Assisted Convective Drying of Carrot: Drying Kinetic, Quality and Energy Consumption. Appl. Sci. 2020, 10, 6309. [Google Scholar] [CrossRef]
- Beaupere, N.; Soupremanien, U.; Zalewski, L. Nucleation triggering methods in supercooled phase change materials (PCM), a review. Thermochim. Acta 2018, 670, 184–201. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, M.; Adhikari, B. The effects of ultrasound-assisted freezing on the freezing time and quality of broccoli (Brassica oleracea L. var. botrytis L.) during immersion freezing. Int. J. Refrig. 2014, 41, 82–91. [Google Scholar] [CrossRef]
- Tan, M.; Mei, J.; Xie, J. The Formation and Control of Ice Crystal and Its Impact on the Quality of Frozen Aquatic Products: A Review. Crystals 2021, 11, 68. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, Z.; Sun, D.-W. Using power ultrasound to accelerate food freezing processes: Effects on freezing efficiency and food microstructure. Crit. Rev. Food Sci. Nutr. 2017, 58, 2842–2853. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, X.; Zhang, C.; Xia, X.; Sun, F.; Kong, B. Ultrasound-assisted immersion freezing accelerates the freezing process and improves the quality of common carp (Cyprinus carpio) at different power levels. LWT 2019, 108, 106–112. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, M.; Xu, B.; Adhikari, B.; Sun, J. The principles of ultrasound and its application in freezing related processes of food materials: A review. Ultrason. Sonochem. 2015, 27, 576–585. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, Q.; Xia, X.; Kong, B.; Diao, X. Effects of ultrasound-assisted freezing at different power levels on the structure and thermal stability of common carp (Cyprinus carpio) proteins. Ultrason. Sonochem. 2019, 54, 311–320. [Google Scholar] [CrossRef]
- Ying, Y.; Xiang, Y.; Liu, J.; Chen, X.; Hu, L.; Li, Y.; Hu, Y. Optimization of ultrasonic-assisted freezing of Penaeus chinensis by response surface methodology. Food Qual. Saf. 2021, 5, 1–9. [Google Scholar] [CrossRef]
- Cheng, X.-F.; Zhang, M.; Adhikari, B.; Islam, N.; Xu, B.-G. Effect of ultrasound irradiation on some freezing parameters of ultrasound-assisted immersion freezing of strawberries. Int. J. Refrig. 2014, 44, 49–55. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, M.; Bhandari, B.; Cheng, X. Influence of power ultrasound on ice nucleation of radish cylinders during ultrasound-assisted immersion freezing. Int. J. Refrig. 2014, 46, 1–8. [Google Scholar] [CrossRef]
- Perussello, C.; Banjac, V.; Poji, M.; Tiwari, B. Ultrasonics Sonochemistry Kinetic modelling of ultrasound-assisted extraction of phenolics from cereal brans. Ultrason. Sonochem. 2021, 79, 105761. [Google Scholar] [CrossRef]
- Jambrak, A.R. Application of High Power Ultrasound and Microwave in Food Processing: Extraction. J. Food Process. Technol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, N.; Wang, C.; Chang, T.; Jiang, H. Ultrasound-homogenization-assisted extraction of polyphenols from coconut mesocarp: Optimization study. Ultrason. Sonochem. 2021, 78, 105739. [Google Scholar] [CrossRef]
- Baite, T.N.; Mandal, B.; Purkait, M.K. Ultrasound assisted extraction of gallic acid from Ficus auriculata leaves using green solvent. Food Bioprod. Process. 2021, 128, 1–11. [Google Scholar] [CrossRef]
- Milani, G.; Vian, M.; Cavalluzzi, M.M.; Franchini, C.; Corbo, F.; Lentini, G.; Chemat, F. Ultrasound and deep eutectic solvents: An efficient combination to tune the mechanism of steviol glycosides extraction. Ultrason. Sonochem. 2020, 69, 105255. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, J.; Diao, W.; Wang, C. Ultrasound-assisted enzymatic extraction and antioxidant activity of polysaccharides from pumpkin (Cucurbita moschata). Carbohydr. Polym. 2014, 113, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Xie, C.; Fan, G.; Gu, Z.; Han, Y. Optimization of ultrasound-assisted extraction of melanin from Auricularia auricula fruit bodies. Innov. Food Sci. Emerg. Technol. 2010, 11, 611–615. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B. Ultrasound-assisted extraction of polysaccharide from Nephelium lappaceum L. fruit peel. Int. J. Biol. Macromol. 2014, 70, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gong, G.; Zhang, J.; Jia, S.; Li, F.; Wang, Y.; Wu, S. Response surface optimization of ultrasound-assisted enzymatic extraction polysaccharides from Lycium barbarum. Carbohydr. Polym. 2014, 110, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic-Malinovska, R.; Kuzmanova, S.; Winkelhausen, E. Application of ultrasound for enhanced extraction of prebiotic oligosaccharides from selected fruits and vegetables. Ultrason. Sonochem. 2015, 22, 446–453. [Google Scholar] [CrossRef]
- Grassino, A.N.; Brnčić, M.; Vikić-Topić, D.; Roca, S.; Dent, M.; Brnčić, S.R. Ultrasound assisted extraction and characterization of pectin from tomato waste. Food Chem. 2016, 198, 93–100. [Google Scholar] [CrossRef]
- Mawson, R.; Tongaonkar, J.; Bhagwat, S.S.; Pandit, A.B. Airborne ultrasound for enhanced defoaming applications. In Innovative Food Processing: Technologies-Extraction, Separation, Component Modification, and Process Intensification; Knoerzer, K., Juliano, P., Smithers, G., Eds.; Woodhead Publishing: Southston, UK, 2016; pp. 347–359. [Google Scholar]
- Leong, T.S.; Martin, G.J.; Ashokkumar, M. Ultrasonic encapsulation—A review. Ultrason. Sonochem. 2017, 35, 605–614. [Google Scholar] [CrossRef]
- Zuniga, R.N.; Kulozik, U.; Aguilera, J.M. Ultrasonic generation of aerated gelatin gels stabilized by whey protein beta-lactoglobulin. Food Hydrocolloid 2011, 25, 958–967. [Google Scholar] [CrossRef]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Dedhia, A.C.; Ambulgekar, P.V.; Pandit, A.B. Static foam destruction: Role of ultrasound. Ultrason. Sonochem. 2004, 11, 67–75. [Google Scholar] [CrossRef]
- Riera, E.; Gallego-Juárez, J.A.; Mason, T.J. Airborne ultrasound for the precipitation of smokes and powders and the destruction of foams. Ultrason. Sonochem. 2006, 13, 107–116. [Google Scholar] [CrossRef]
- Rodríguez, G.; Riera, E.; Gallego-Juárez, J.A.; Acosta, V.M.; Pinto, A.; Martínez, I.; Blanco, A. Experimental study of defoaming by air-borne power ultrasonic technology. Phys. Procedia 2010, 3, 135–139. [Google Scholar] [CrossRef]
- Charoux, C.M.; Ojha, K.S.; O’Donnell, C.P.; Cardoni, A.; Tiwari, B.K. Applications of airborne ultrasonic technology in the food industry. J. Food Eng. 2017, 208, 28–36. [Google Scholar] [CrossRef]
- Abismaıl, B.; Canselier, J.; Wilhelm, A.; Delmas, H.; Gourdon, C. Emulsification by ultrasound: Drop size distribution and stability. Ultrason. Sonochem. 1999, 6, 75–83. [Google Scholar] [CrossRef]
- Ashokkumar, M. The characterization of acoustic cavitation bubbles—An overview. Ultrason. Sonochem. 2011, 18, 864–872. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Cavalieri, F.; Chemat, F.; Okitsu, K.; Sambandam, A.; Yasui, K. Handbook of Ultrasonics and Sonochemistry; Springer: Singapore, 2016. [Google Scholar] [CrossRef]
- Taha, A.; Ahmed, E.; Ismaiel, A.; Ashokkumar, M.; Xu, X.; Pan, S.; Hu, H. Ultrasonic emulsification: An overview on the preparation of different emulsifiers-stabilized emulsions. Trends Food Sci. Technol. 2020, 105, 363–377. [Google Scholar] [CrossRef]
- Li, W.; Leong, T.S.H.; Ashokkumar, M.; Martin, G.J.O. A study of the effectiveness and energy efficiency of ultrasonic emulsification. Phys. Chem. Chem. Phys. 2018, 20, 86–96. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Amemiya, F.; Fuchigami, T.; Machida, K.; Takeda, S.; Tamamitsu, K.; Atobe, M. Highly clear and transparent nanoemulsion preparation under surfactant-free conditions using tandem acoustic emulsification. Chem. Commun. 2011, 47, 5765–5767. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, A.; Ashokkumar, M. Functional properties of ultrasonically generated flaxseed oil-dairy emulsions. Ultrason. Sonochem. 2014, 21, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Mawson, R.; Rout, M.; Ripoll, G.; Swiergon, P.; Singh, T.; Knoerzer, K.; Juliano, P. Production of particulates from transducer erosion: Implications on food safety. Ultrason. Sonochem. 2014, 21, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.Q.; Hansen, C.H.; Zander, A.C. A Review of Current Ultrasound Exposure Limits; School of Mechanical Engineering, University of Adelaide: Adelaide, Australia, 2004. [Google Scholar]

| Temperature (°C) | Organism | D Value (min) | Reference | |||
|---|---|---|---|---|---|---|
| Heat | Ultrasound | Thermosonication | Monosonication/Manothermosonication (MTS) | |||
| 60 | Saccharomyces cerevisiae | 3.53 | 3.1(20 kHz, 124 μm) | 1.9 (1 min US, 55 °C) 0.73 (1 min US, 60 °C) | - | [36] |
| 61 | Escherichia coli K12 | 0.79 | 1.01 | 0.44 (100 kPa) | 0.40 (300 kPa)/0.27 (MTS 300 kPa 61 °C) | [37] |
| 56 | Cronabacter sakazakii | 0.86 | - | - | 0.28 (MTS-20 kHz, 117 μm, 200 kPa, 56 °C) | [38] |
| 55 | Aspergillus flavus | 17.40 | - | 5.06 (120 μm) 4.94 (120 μm, 500 ppm vanillin (Vi)) 1.09 (120 μm, 500 ppm potassium sorbate (KS)) | [39] | |
| 60 | 2.60 | - | 1.20 (120 μm) < 0.5 (120 μm, 500 ppm Vi) < 0.5 (120 μm, 500 ppm KS) | |||
| 50 | Penicillium digitatum | 25.42 | - | 9.59 (120 μm) 8.57 (120 μm, 500 ppm Vi) 7.15 (120 μm, 500 ppm KS) | [39] | |
| 52.5 | 13.30 | - | 5.33 (120 μm) 6.47 (120 μm, 500 ppm Vi) 4.19 (120 μm, 500 ppm KS) | |||
| 63 | Listeria innocua | 30 | - | 10 (400 W, 24 kHz, 120 μm) | [40] | |
| Ambient | Mesophilic aerobic, Lactic acid bacteria, Coliform bacteria, yeast | 750 W, 20 kHz, 6.8–126 μm | [28] | |||
| 25 | Salmonella Typhimurium, Escherichia coli | 80 and 37 kHz, (330 W), pulsed modes, frequency amplitude (40% and 100%) | [41] | |||
| Drying Technique | Ultrasound Processing Parameters | Sample | Inference | Reference |
|---|---|---|---|---|
| Ultrasound-assisted osmotic drying | Indirect sonication: 25 kHz, 1.75 kW Osmotic solution: 70° Brix Immersion time: 60 min Air drying: 70 °C | Guava slices | Initial moisture content: 91.3 ± 0.6% wet basis (w.b.) Final moisture content: 19.5 ± 3 (w.b.) Total dehydration time: 300 min. Drying time reduced by 33%. | [57] |
| Ultrasound-assisted convective drying | Ultrasound: 21.8 kHz, 60 W Air drying: 70 °C | Strawberry | Initial moisture content: 90.5 ± 0.27 (w.b.) Final moisture content: 23.07% (w.b.). Total drying time: 2.2 h. Drying time reduced by 44%. | [58] |
| Ultrasound-assisted osmotic dehydration | Ultrasound: 25 kHz, 700 W | Cherries | It was proved that intermittent drying of cherries preceded by ultrasonic-assisted osmotic dehydration contributes to shorter drying time, better colour preservation, and smaller water activity. | [59] |
| Ultrasound-assisted convective drying | Ultrasound: 21.8 kHz, 30.8 W Air drying: 70 °C | Passion fruit peel | Initial moisture content: 87.5± 1.9 (w.b.) Final moisture content: 32% (w.b.) Total drying time: 3.9 ± 0.7 h. | [60] |
| Ultrasound-assisted radiation drying | Ultrasound: 1200 W, 20 kHz Sonication time: 5 s Drying: 62 °C | Carrot slices | Final moisture content: 10 ± 0.5% (d.b.) Drying time increased with increasing ultrasonic power levels. | [61] |
| Ultrasound-assisted vacuum drying | Sonication time: 10 s Drying: 65–75 °C | Carrot slices | Final moisture content: 12–13% dry basis (d.b) Drying time was decreased by 53%. | [62] |
| Ultrasound-assisted heating | 1000 W and 50 ℃ | Ham slices | Decrease of 0.65-fold in adhesiveness values. Population of free water increased from 2.71% to 11.35%. Decreased the content of rancid and sour compounds. Accelerated the formation of esters. | [63] |
| Ultrasound-assisted microwave dryer | 28 kHz, 70 W, 30 min | Carrot slices | Reduction in drying time by 63%. Least-specific energy consumption: 23.75 ±2.22 MJ/kg Lowest shrinkage: 31.8 ±1.1% | [64] |
| Processing Parameter | Nucleation Temperature Range | Sample | Inference | Reference |
|---|---|---|---|---|
| Ultrasound: −0.1 to −2.1 °C Ultrasound Intensity: 0.09 to 0.51 W/cm2 Sonication: 30 s | −3.43 to −2.36 °C −3.43 to −2.38 °C | Strawberry | Nucleation temperature increased with increase of US temperature. No linear relationship between USI and the degree of super cooling. Shortest CFT observed at 0.51 W/cm2. | [73] |
| Ultrasound: −0.5 to −2.0 °C Ultrasound Intensity: 0.09–0.37 W/cm2 Sonication: 3–15 s | −1.6 to −2.75 °C | Radish | Nucleation temperature increased with increase of US temperature. Increasing USI, NT increases first and then decreases with increasing US duration. US 0.226 W/cm2 at −0.5 °C for 7 s is enough for commencement of nucleation. | [74] |
| Ultrasound: 125–190 W; 20–30 kHz Sonication: 60 s | - | Broccoli | Higher freezing rate can be achieved at relatively low-power ultrasound. Shorter freezing times were observed at relatively lower ultrasound frequency. At relatively low power levels (125 W), the freezing time was found to be significantly shorter at 30 kHz than at 20 kHz, whereas at relatively high power levels (175 W), the freezing time at 30 kHz was significantly longer than at 20 kHz. | [66] |
| Ultrasound: −30 °C; 90–630 W; 45 kHz | −2 to −4 °C | Penaeus chinensis | Optimized ultrasonic parameters were power 191.97 W, 4.92 s on/3 s off. Optimized ultrasonic parameters provide an economic and effective way for obtaining high-quality frozen Penaeus chinensis. | [72] |
| Ultrasound Process Parameter | Extract | Solvent | Sample | Inference | Reference |
|---|---|---|---|---|---|
| Ultrasound: 100–250 W Temperature: 45–60 °C Liquid-to-Solid Ratio (LSR): 30–50 mL/g Time: 20–40 min. | Melanin | NaOH | Auricularia Auricula | Increasing US power, temperature, LSR, and duration may enhance the melanin yield. Optimum extraction conditions: (120.05 mg/g)−63 °C, 43 mL/g, 36 min, two extractions. | [82] |
| Ultrasound: 80–120 W LSR: 20:1–40:1 mL/g Temperature: 40–60 °C Time: 30–50 min. | Polysaccharide | Distilled water | Nephelium Lappaceum L. | Polysaccharide yield increases with increasing ultrasound power (USP 80–100 W, temperature: 40–50 °C, duration up to 40 min, LSR 20:1–30:1 mL/g), respectively, and then decreases accordingly. Optimum extraction conditions: (8.32%)–53 °C, 110 W, 41 min, 32:1 mL/g. | [83] |
| Ultrasound: 40–120 W; 40 kHz Temperature: 40–80 °C Enzyme concentration: 0.5 to 2.5% Time: 10–80 min. | Polysaccharides | Cellulose | Lycium barbarum | Polysaccharide yield increases with increasing (USP 40–80 W, temperature: 40–60 °C, duration up to 20 min, Cellulose concentration: 0.5% to 2.0%), respectively, and then decreases accordingly. Optimum extraction conditions: (6.32%)–55.79 °C, 78.6 W, Cellulose concentration: 2.15%, 20.29 min. | [84] |
| Fructo-oligosaccharides (1-kestone), Nystone, 1F-β-fructofuranosylnystose; Raffinose family oligosaccharides (Raffinose and Stachyose) | Ethanol, Methanol and Acetone | Fruits (Blueberry, Nectarine, Raspberry, Watermelon) Vegetables (Garlic, Jerusalem Artichoke, Leek, Scallion, Spring Garlic, White Onion) | USAE was an efficient method for the extraction of oligosaccharides at extraction time of 10 min, temperature: 40 °C, and ethanol concentration 63% v/v. Aqueous acetone was slightly better than aqueous methanol and aqueous ethanol in USAE of oligosaccharides under the same extraction conditions (50% v/v, 50 °C, and extraction: 10 min). Extraction of the oligosaccharides increased with increase of the ethanol concentration up to 60%. The concentration of the extracted oligosaccharide increased when the extraction temperature increased from 20 °C to 50 °C. The highest increase of total oligosaccharide was spotted in the Jerusalem artichoke (1.96% conventional extractions) to (7.17% USAE). | [85] | |
| Ultrasound: 37 kHz;Temperature: 60–80 °C Sonication time: 15 min | Pectin | Ammonium oxalate/oxalic acid | Tomato waste | At 60 °C, the pectin yield obtained was higher for the conventional extraction, which obtained a higher yield than ultrasound-assisted extraction. At 80 °C, the pectin yield obtained was comparable for both the methods, but better quality of pectin was obtained with ultrasound-assisted extraction. | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavan, P.; Sharma, P.; Sharma, S.R.; Mittal, T.C.; Jaiswal, A.K. Application of High-Intensity Ultrasound to Improve Food Processing Efficiency: A Review. Foods 2022, 11, 122. https://doi.org/10.3390/foods11010122
Chavan P, Sharma P, Sharma SR, Mittal TC, Jaiswal AK. Application of High-Intensity Ultrasound to Improve Food Processing Efficiency: A Review. Foods. 2022; 11(1):122. https://doi.org/10.3390/foods11010122
Chicago/Turabian StyleChavan, Prasad, Pallavi Sharma, Sajeev Rattan Sharma, Tarsem Chand Mittal, and Amit K. Jaiswal. 2022. "Application of High-Intensity Ultrasound to Improve Food Processing Efficiency: A Review" Foods 11, no. 1: 122. https://doi.org/10.3390/foods11010122
APA StyleChavan, P., Sharma, P., Sharma, S. R., Mittal, T. C., & Jaiswal, A. K. (2022). Application of High-Intensity Ultrasound to Improve Food Processing Efficiency: A Review. Foods, 11(1), 122. https://doi.org/10.3390/foods11010122







