Modulation of Fabrication and Nutraceutical Delivery Performance of Ovalbumin-Stabilized Oleogel-Based Nanoemulsions via Complexation with Gum Arabic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
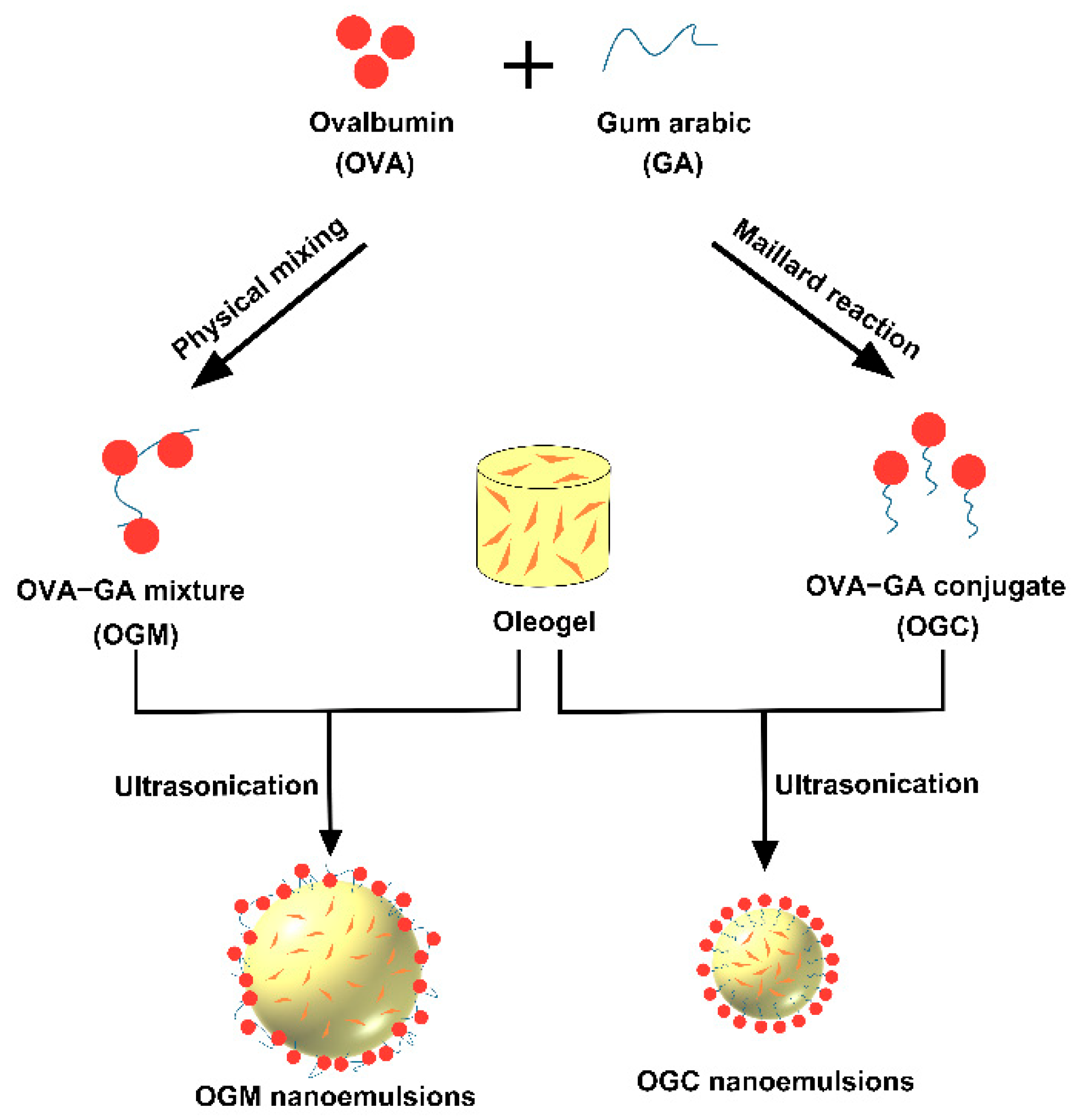
2.2. Preparation of OVA–GA Complexes Conjugate (OGC)
2.3. Preparation of Carnauba Wax-Based Oleogel
2.4. Characterization of OGC
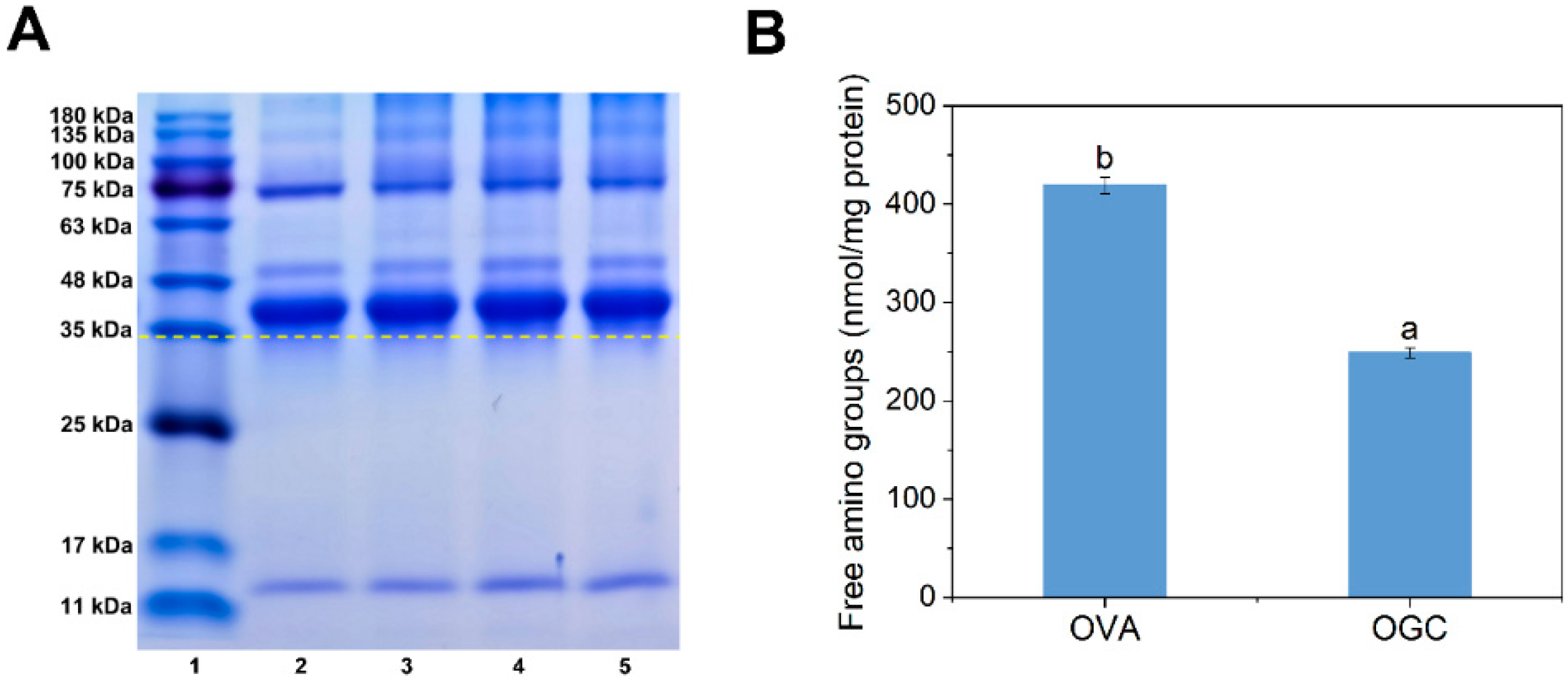
2.4.1. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
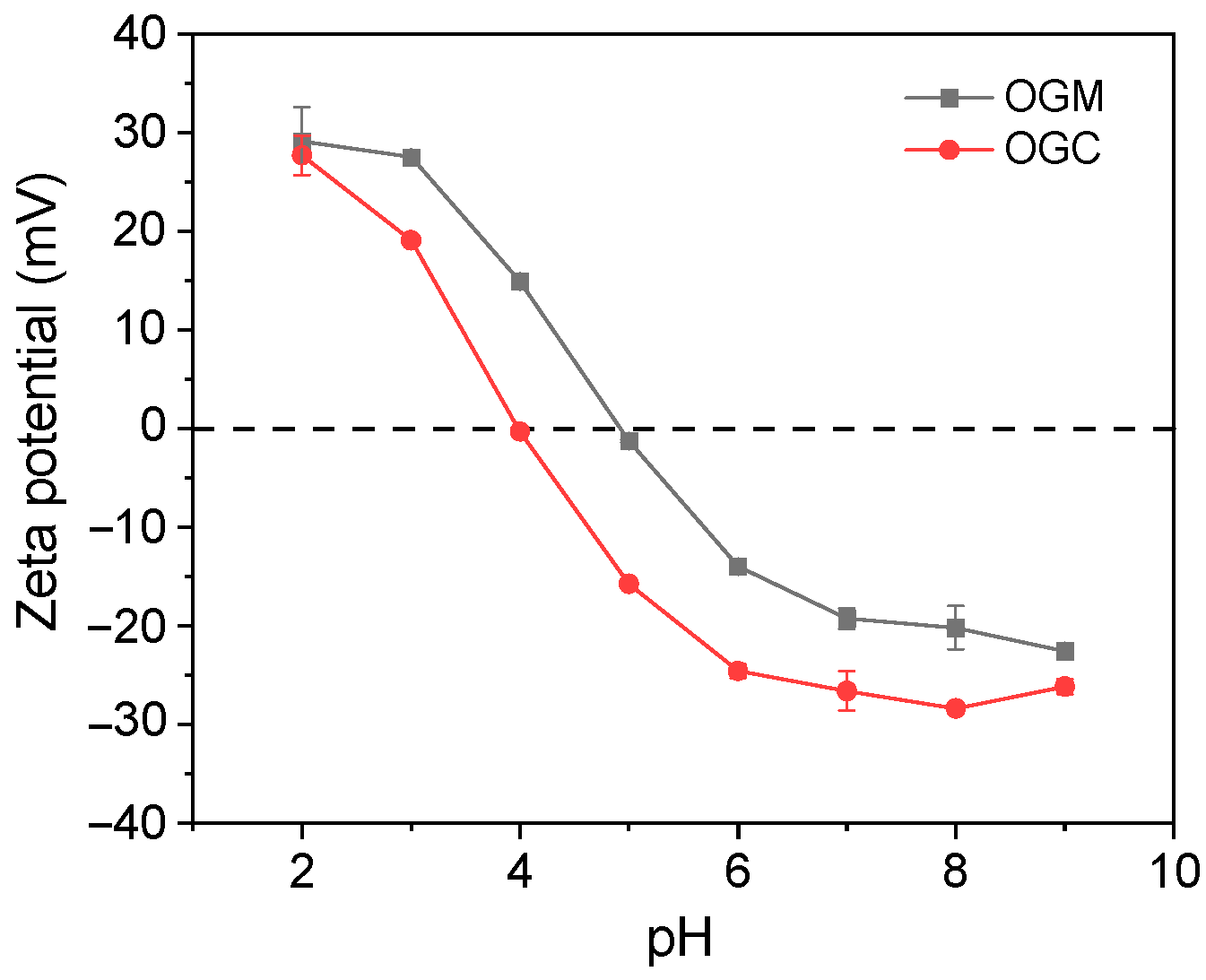
2.4.2. Zeta Potential
2.4.3. Free Amino Groups
2.5. Preparation of Oleogel-Based Nanoemulsions
2.6. Characterization of Oleogel-Based Nanoemulsions
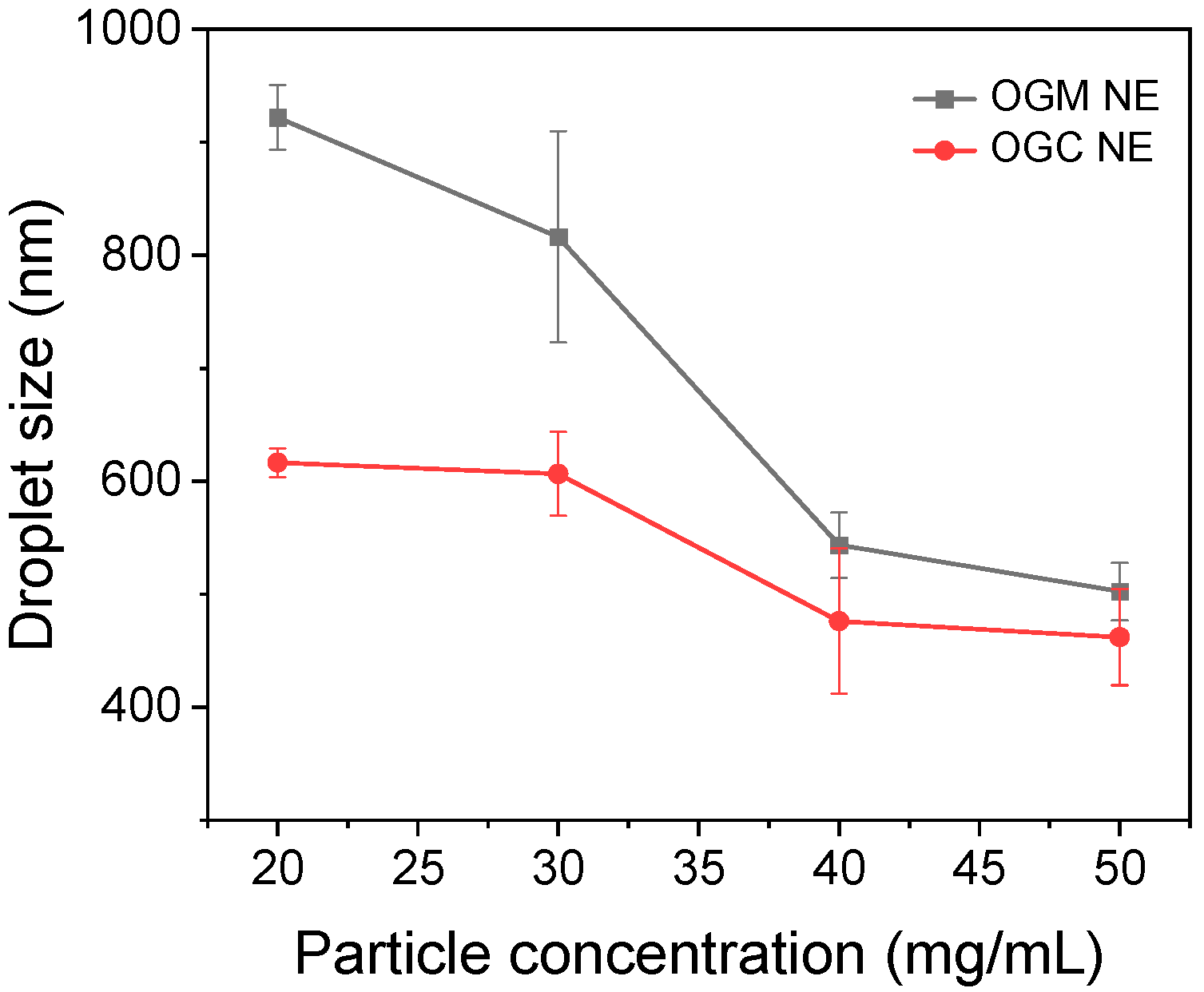
2.6.1. Droplet Size of Oleogel-Based Nanoemulsions
2.6.2. Storage Stability Analysis
2.7. Lipolysis and Bioaccessibility of Astaxanthin in Oleogel-Based Nanoemulsions
2.7.1. Digestion of Oleogel-Based Nanoemulsions
2.7.2. High-Performance Liquid Chromatography (HPLC) Analysis of Astaxanthin
2.7.3. Determination of Astaxanthin Bioaccessibility
2.8. Statistical Analysis
3. Results and Discussion
3.1. Formation and Characterization of Oleogel
3.2. Formation and Characterization of OVA–GA Conjugate (OGC)
3.2.1. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
3.2.2. Free Amino Groups
3.2.3. Zeta Potential
3.3. Formation and Characterization of Oleogel-Based Nanoemulsions
3.3.1. Droplet Size of Oleogel-Based Nanoemulsions
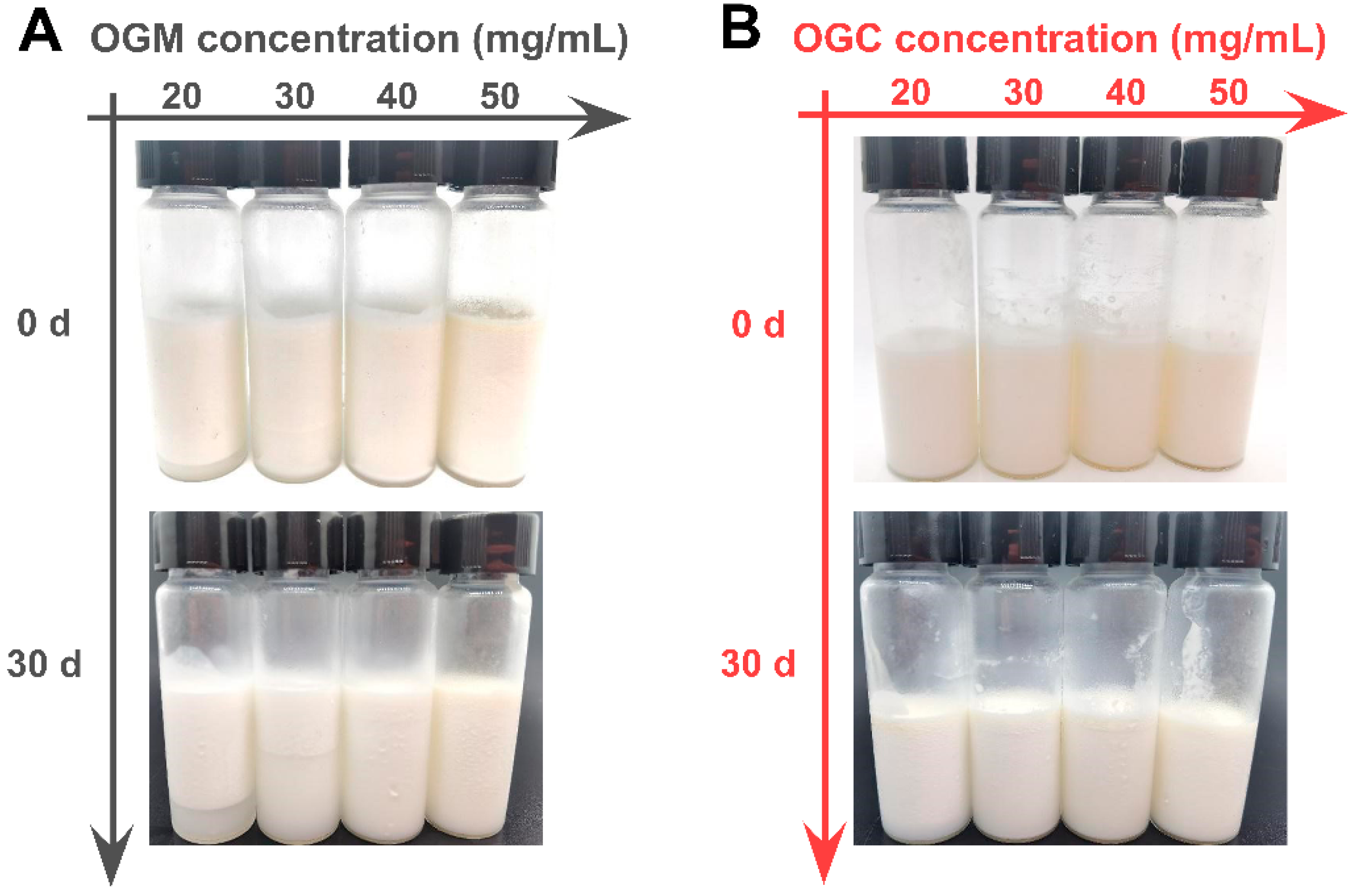
3.3.2. Storage Stability of Oleogel-Based Nanoemulsions
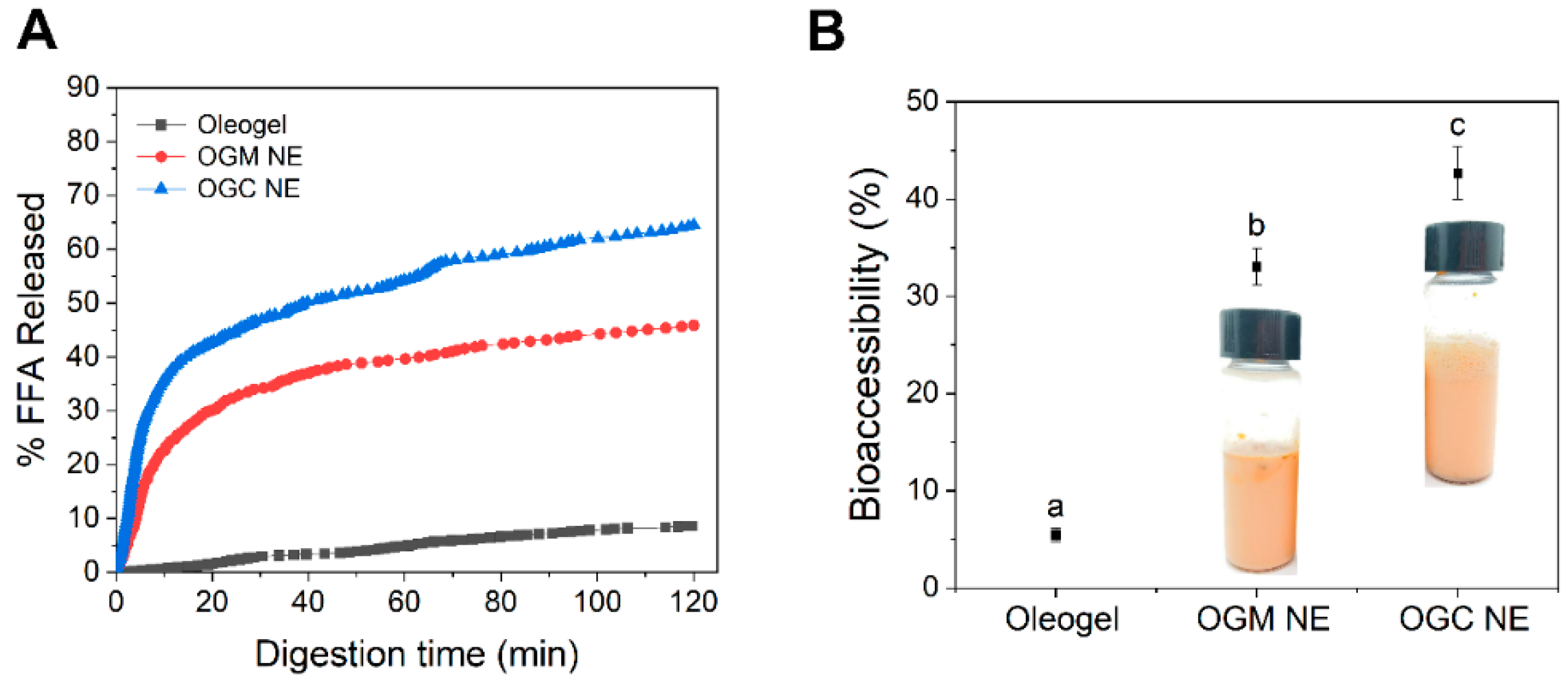
3.4. Lipolysis and Bioaccessibility of Astaxanthin in Oleogel-Based Nanoemulsions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, H.; Huang, Q. Improving the oral bioavailability of curcumin using novel organogel-based nanoemulsions. J. Agric. Food Chem. 2012, 60, 5373–5379. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Gao, Y.; Liu, Y.; Wei, Z.; Xue, C. Lactoferrin particles assembled via transglutaminase-induced crosslinking: Utilization in oleogel-based Pickering emulsions with improved curcumin bioaccessibility. Food Chem. 2022, 374, 131779. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wei, Z.; Xue, C. Recent advances on food-grade oleogels: Fabrication, application and research trends. Crit. Rev. Food Sci. Nutr. 2021, 1–18. [Google Scholar] [CrossRef]
- Chen, X.W.; Yin, W.J.; Yang, D.X.; Wan, Z.L.; Ma, C.G.; Yang, X.Q. One-pot ultrasonic cavitational emulsification of phytosterols oleogel-based flavor emulsions and oil powder stabilized by natural saponin. Food Res. Int. 2021, 150, 110757. [Google Scholar] [CrossRef]
- Pan, J.; Tang, L.; Dong, Q.; Li, Y.; Zhang, H. Effect of oleogelation on physical properties and oxidative stability of camellia oil-based oleogels and oleogel emulsions. Food Res. Int. 2021, 140, 110057. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, Q. Developing organogel-based Pickering emulsions with improved freeze-thaw stability and hesperidin bioaccessibility. Food Hydrocoll. 2019, 93, 68–77. [Google Scholar] [CrossRef]
- McClements, D.J. Edible nanoemulsions: Fabrication, properties, and functional performance. Soft Matter 2011, 7, 2297–2316. [Google Scholar] [CrossRef] [Green Version]
- Müller, W.A.; Sarkis, J.R.; Marczak, L.D.F.; Muniz, A.R. Molecular dynamics study of the effects of static and oscillating electric fields in ovalbumin. Innov. Food Sci. Emerg. Technol. 2022, 75, 10211. [Google Scholar] [CrossRef]
- Tang, C.H. Emulsifying properties of soy proteins: A critical review with emphasis on the role of conformational flexibility. Crit. Rev. Food Sci. Nutr. 2017, 57, 2636–2679. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, Q. Assembly of protein-polysaccharide complexes for delivery of bioactive ingredients: A perspective paper. J. Agric. Food Chem. 2019, 67, 1344–1352. [Google Scholar] [CrossRef]
- Zhang, F.; Cai, X.; Ding, L.; Wang, S. Effect of pH, ionic strength, chitosan deacetylation on the stability and rheological properties of O/W emulsions formulated with chitosan/casein complexes. Food Hydrocoll. 2021, 111, 106211. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, R.; Feng, W.; Chen, Z.; Wang, T. High internal phase Pickering emulsions stabilized by co-assembled rice proteins and carboxymethyl cellulose for food-grade 3D printing. Carbohydr. Polym. 2021, 273, 118586. [Google Scholar] [CrossRef] [PubMed]
- Artiga-Artigas, M.; De Abreu-Martins, H.H.; Zeeb, B.; Piccoli, R.H.; Martín-Belloso, O.; Salvia-Trujillo, L. Antimicrobial Kinetics of Nanoemulsions Stabilized with Protein:Pectin Electrostatic Complexes. Food Bioprocess Technol. 2020, 13, 1893–1907. [Google Scholar] [CrossRef]
- Zhou, Y.; Yue, W.; Luo, Y.; Luo, Q.; Liu, S.; Chen, H.; Qin, W.; Zhang, Q. Preparation and stability characterization of soybean protein isolate/sodium alginate complexes-based nanoemulsions using high-pressure homogenization. LWT-Food Sci. Technol. 2022, 154, 112607. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, Q. Modification of ovotransferrin by Maillard reaction: Consequences for structure, fibrillation and emulsifying property of fibrils. Food Hydrocoll. 2019, 97, 105186. [Google Scholar] [CrossRef]
- Khoo, K.S.; Lee, S.Y.; Ooi, C.W.; Fu, X.; Miao, X.; Ling, T.C.; Show, P.L. Recent advances in biorefinery of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2019, 288, 121606. [Google Scholar] [CrossRef]
- Fratter, A.; Biagi, D.; Cicero, A.F.G. Sublingual delivery of astaxanthin through a novel ascorbyl palmitate-based nanoemulsion: Preliminary data. Mar. Drugs 2019, 17, 508. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, R.; McClements, D.J.; Li, F.; Liu, H.; Cao, Y.; Xiao, H. Nanoemulsion-based delivery systems for nutraceuticals: Influence of long-chain triglyceride (LCT) type on in vitro digestion and astaxanthin bioaccessibility. Food Biophys. 2018, 13, 412–421. [Google Scholar] [CrossRef]
- Chen, Z.; Shu, G.; Taarji, N.; Barrow, C.J.; Nakajima, M.; Khalid, N.; Neves, M.A. Gypenosides as natural emulsifiers for oil-in-water nanoemulsions loaded with astaxanthin: Insights of formulation, stability and release properties. Food Chem. 2018, 261, 322–328. [Google Scholar] [CrossRef]
- Fraterrigo Garofalo, S.; Tommasi, T.; Fino, D. A short review of green extraction technologies for rice bran oil. Biomass Convers. Biorefin. 2021, 11, 569–587. [Google Scholar] [CrossRef]
- Punia, S.; Kumar, M.; Siroha, A.K.; Purewal, S.S. Rice bran oil: Emerging trends in extraction, health benefit, and its industrial application. Rice Sci. 2021, 28, 217–232. [Google Scholar] [CrossRef]
- Charoen, R.; Jangchud, A.; Jangchud, K.; Harnsilawat, T.; Naivikul, O.; McClements, D.J. Influence of biopolymer emulsifier type on formation and stability of rice bran oil-in-water emulsions: Whey protein, gum arabic, and modified starch. J. Food Sci. 2011, 76, E165–E172. [Google Scholar] [CrossRef] [PubMed]
- Piriyaprasarth, S.; Juttulapa, M.; Sriamornsak, P. Stability of rice bran oil-in-water emulsions stabilized by pectin–zein complexes: Effect of composition and order of mixing. Food Hydrocoll. 2016, 61, 589–598. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, H.; Jafari, S.M.; Rajabzadeh, G.; Sarfarazi, M.; Sedaghati, S. Chitosan-gum Arabic complex nanocarriers for encapsulation of saffron bioactive components. Colloids Surf. A 2019, 578, 123644. [Google Scholar] [CrossRef]
- Wei, Z.; Cheng, Y.; Zhu, J.; Huang, Q. Genipin-crosslinked ovotransferrin particle-stabilized Pickering emulsions as delivery vehicles for hesperidin. Food Hydrocoll. 2019, 94, 561–573. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Garavazo, B.R.; Rodrigues, C.E.C. Liquid–liquid equilibria for systems composed of rice bran oil and alcohol-rich solvents: Application to extraction and deacidification of oil. J. Food Eng. 2012, 110, 418–427. [Google Scholar] [CrossRef]
- Zheng, Y.; Chang, Y.; Luo, B.; Teng, H.; Chen, L. Molecular structure modification of ovalbumin through controlled glycosylation with dextran for its emulsibility improvement. Int. J. Biol. Macromol. 2022, 194, 1–8. [Google Scholar] [CrossRef]
- Ledesma-Osuna, A.I.; Ramos-Clamont, G.; Guzman-Partida, A.M.; Vazquez-Moreno, L. Conjugates of bovine serum albumin with chitin oligosaccharides prepared through the Maillard reaction. J. Agric. Food Chem. 2010, 58, 12000–12005. [Google Scholar] [CrossRef]
- Alahdad, Z.; Ramezani, R.; Aminlari, M.; Majzoobi, M. Preparation and properties of dextran sulfate-lysozyme conjugate. J. Agric. Food Chem. 2009, 57, 6449–6454. [Google Scholar] [CrossRef]
- Liu, G.; Liu, J.; Tu, Z.; Sha, X.; Wang, H.; Wang, Z. Investigation of conformation change of glycated ovalbumin obtained by Co-60 gamma-ray irradiation under drying treatment. Innov. Food Sci. Emerg. Technol. 2018, 47, 286–291. [Google Scholar] [CrossRef]
- Seidi, P.; Nasirpour, A.; Keramat, J.; Saeidy, S. Functional and structural properties of gum arabic complexes with casein and hydrolyzed casein achieved by Maillard reaction. J. Dispers. Sci. Technol. 2021, 1–12. [Google Scholar] [CrossRef]
- Indurthi, V.S.; Leclerc, E.; Vetter, S.W. Interaction between glycated serum albumin and AGE-receptors depends on structural changes and the glycation reagent. Arch. Biochem. Biophys. 2012, 528, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, Y.; Yang, M.; Liu, X.; Lyu, S.; Liu, B.; Liu, J.; Zhang, T. Effect of glycation degree on the structure and digestion properties of ovalbumin: A study of amino acids and peptides release after in vitro gastrointestinal simulated digestion. Food Chem. 2022, 373, 131331. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, G.; Wang, H. Investigation of the mechanism of conformational alteration in ovalbumin as induced by glycation with different monoses through conventional spectrometry and liquid chromatography high-resolution mass spectrometry. J. Agric. Food Chem. 2019, 67, 3096–3105. [Google Scholar] [CrossRef]
- Sheng, L.; Tang, G.; Wang, Q.; Zou, J.; Ma, M.; Huang, X. Molecular characteristics and foaming properties of ovalbumin-pullulan conjugates through the Maillard reaction. Food Hydrocoll. 2020, 100, 105384. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Q.; Tang, C.; Luo, J.; Wu, X.; Lu, L.; Cai, Z. Study on structural, rheological and foaming properties of ovalbumin by ultrasound-assisted glycation with xylose. Ultrason. Sonochem. 2019, 58, 104644. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, L.; Tian, S.; Wen, H.; Gou, X.; Ngai, T. Gelatin particle-stabilized high-internal phase emulsions for use in oral delivery systems: Protection effect and in vitro digestion study. J. Agric. Food Chem. 2017, 65, 900–907. [Google Scholar] [CrossRef]
- Yukuyama, M.N.; Ghisleni, D.D.; Pinto, T.J.; Bou-Chacra, N.A. Nanoemulsion: Process selection and application in cosmetics--a review. Int. J. Cosmet. Sci. 2016, 38, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Mehmood, T.; Ahmed, A.; Ahmad, A.; Ahmad, M.S.; Sandhu, M.A. Optimization of mixed surfactants-based beta-carotene nanoemulsions using response surface methodology: An ultrasonic homogenization approach. Food Chem. 2018, 253, 179–184. [Google Scholar] [CrossRef]
- Feng, J.; Berton-Carabin, C.C.; Fogliano, V.; Schroën, K. Maillard reaction products as functional components in oil-in-water emulsions: A review highlighting interfacial and antioxidant properties. Trends Food Sci. Technol. 2022, 121, 129–141. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.; Liu, J.; Cui, Q.; Wang, X.; Chen, S.; Jiang, L. Relationship between molecular flexibility and emulsifying properties of soy protein isolate-glucose conjugates. J. Agric. Food Chem. 2019, 67, 4089–4097. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fan, L.; Yang, Y.; Jiang, Q.; Xu, Y.; Xia, W. Characterization of surimi particles stabilized novel pickering emulsions: Effect of particles concentration, pH and NaCl levels. Food Hydrocoll. 2021, 117, 106731. [Google Scholar] [CrossRef]
- Zhu, X.; Li, L.; Li, S.; Ning, C.; Zhou, C. l–Arginine/l–lysine improves emulsion stability of chicken sausage by increasing electrostatic repulsion of emulsion droplet and decreasing the interfacial tension of soybean oil-water. Food Hydrocoll. 2019, 89, 492–502. [Google Scholar] [CrossRef]
- Xu, J.; Mukherjee, D.; Chang, S.K.C. Physicochemical properties and storage stability of soybean protein nanoemulsions prepared by ultra-high pressure homogenization. Food Chem. 2018, 240, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Liu, B.; Wang, R.; Huang, Y.; Luo, J.; Li, Y. The enhanced fatty acids flavor release for low-fat cheeses by carrier immobilized lipases on O/W Pickering emulsions. Food Hydrocoll. 2021, 116, 106651. [Google Scholar] [CrossRef]
- Qazi, H.J.; Ye, A.; Acevedo-Fani, A.; Singh, H. In vitro digestion of curcumin-nanoemulsion-enriched dairy protein matrices: Impact of the type of gel structure on the bioaccessibility of curcumin. Food Hydrocoll. 2021, 117, 106692. [Google Scholar] [CrossRef]
- Sun, R.; Xia, Q. In vitro digestion behavior of (W1/O/W2) double emulsions incorporated in alginate hydrogel beads: Microstructure, lipolysis, and release. Food Hydrocoll. 2020, 107, 105950. [Google Scholar] [CrossRef]
- Yang, J.; Hua, S.; Huang, Z.; Gu, Z.; Cheng, L.; Hong, Y. Comparison of bioaccessibility of astaxanthin encapsulated in starch-based double emulsion with different structures. Carbohydr. Polym. 2021, 272, 118475. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Wang, Z.; Xue, C.; Wei, Z. Modulation of Fabrication and Nutraceutical Delivery Performance of Ovalbumin-Stabilized Oleogel-Based Nanoemulsions via Complexation with Gum Arabic. Foods 2022, 11, 1859. https://doi.org/10.3390/foods11131859
Gao Y, Wang Z, Xue C, Wei Z. Modulation of Fabrication and Nutraceutical Delivery Performance of Ovalbumin-Stabilized Oleogel-Based Nanoemulsions via Complexation with Gum Arabic. Foods. 2022; 11(13):1859. https://doi.org/10.3390/foods11131859
Chicago/Turabian StyleGao, Yuxing, Zihua Wang, Changhu Xue, and Zihao Wei. 2022. "Modulation of Fabrication and Nutraceutical Delivery Performance of Ovalbumin-Stabilized Oleogel-Based Nanoemulsions via Complexation with Gum Arabic" Foods 11, no. 13: 1859. https://doi.org/10.3390/foods11131859
APA StyleGao, Y., Wang, Z., Xue, C., & Wei, Z. (2022). Modulation of Fabrication and Nutraceutical Delivery Performance of Ovalbumin-Stabilized Oleogel-Based Nanoemulsions via Complexation with Gum Arabic. Foods, 11(13), 1859. https://doi.org/10.3390/foods11131859





