Recent Developments and Applications of Nanosystems in the Preservation of Meat and Meat Products
Abstract
:1. Introduction
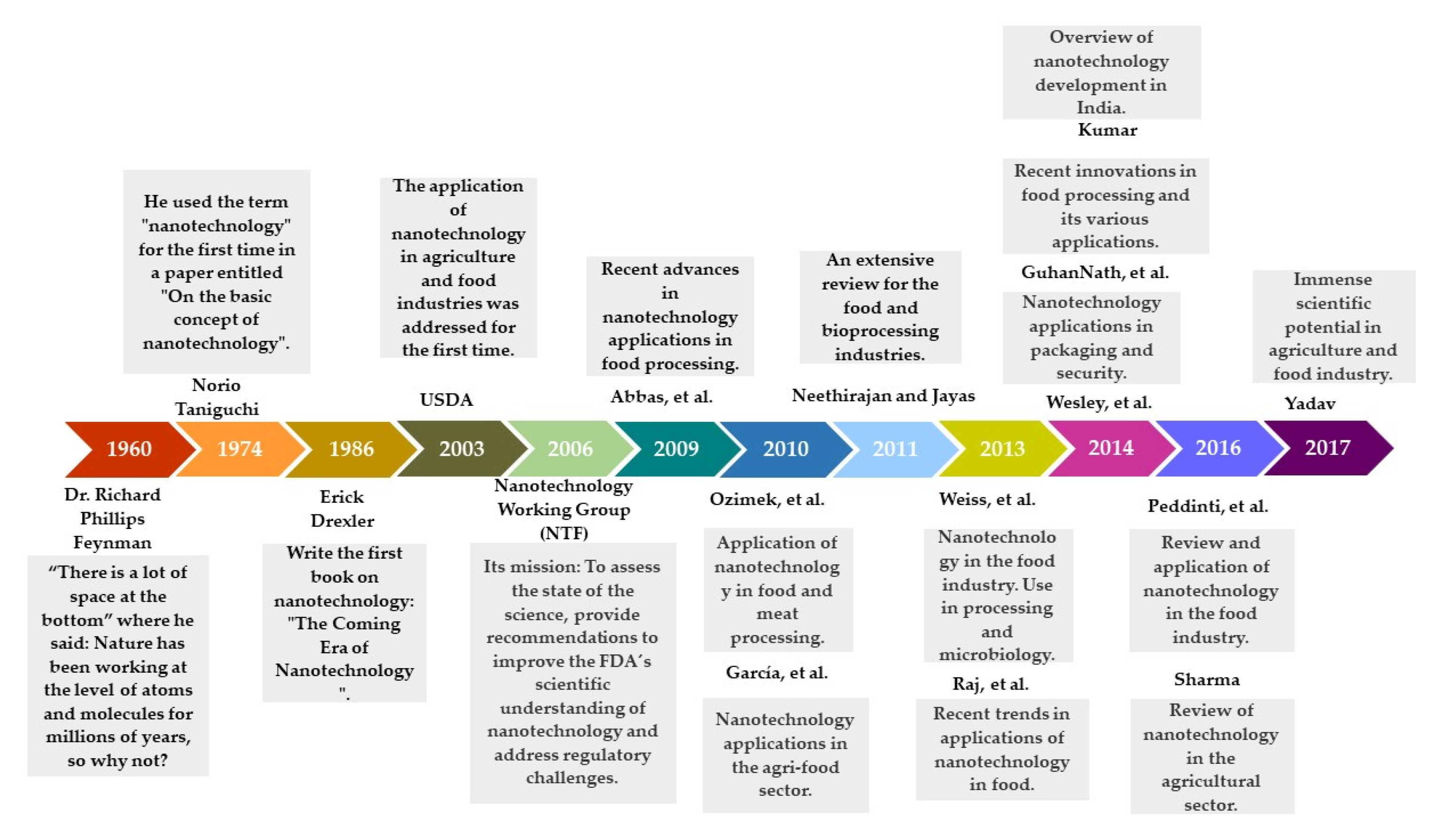
2. Evolution of Nanotechnology in Food Applications
3. Effects of Nanosystems on Sensory Properties of Food
4. Nanosystems in Food and Meat Preservation
5. Nanosystems and the Preservation of Meat and Meat Products
5.1. Effect of Nanosystems on Lipid Oxidation Control
5.2. Application of Nanosystems to Control Microbial Growth in Meat
| Nanosystem | Size (nm) | Applications and Effects | Product | Ref. |
|---|---|---|---|---|
| ZnO nanoparticle suspension containing acetic acid ZnO–AA NP | 20–25 | The meat samples were inoculated with L. monocytogenes, E. coli, and S. aureus (7 log CFU/g) and treated with various concentrations of ZnO NP suspensions (6 mM and 8 mM) plus 1% acetic acid by meat surface spraying and stored at refrigeration temperature. Higher concentrations of ZnO–AA NP significantly decreased the population of all microorganisms compared with the control. Populations of L. monocytogenes, E. coli, and S. aureus in meat decreased by 4.09 to 4.72, 0.84 to 1.24, and 2.12 to 2.75 log CFU/g, suggesting that treatments of ZnO–AA NP suspensions containing acetic acid can reduce the growth of the studied bacteria in meat. | Sheep meat | [82] |
| Chitosan–sodium tripolyphosphate nanoparticles CS–TPP NP | - | The authors reported that aerobic plate counts of chitosan nanoparticle treated shrimp were lower compared to other treatments during the entire storage time. CS–TPP NP have a greater surface area per unit volume and higher charge density than CS. Both of these factors greatly contribute to their interaction with anionic bacterial cell membrane. Fresh white shrimp meat was vacuum-tumbled with treatment solution (CS–TPP NP) and stored at 20 °C for 120 days. | Shrimp | [58] |
| Cinnamon essential oil-chitosan nanoparticles CEO–CS NP | 235.6 | According to the authors, no significant differences were observed among the treatments in the beginning of storage, but encapsulated CEO showed a distinguished inhibition pattern of S. aureus in the following days and at the end of the storage where the control samples reached 6.85 log CFU/g while meat treated with nanoparticles reached 3.99 and 4.59 CFU/g (0.05 and 0.1% of encapsulated CEO). The use of CS NP as a carrier of CEO looks promising, as lower microbial growth was obtained. The nanoparticles were added to the meat as part of the formulation, samples were stored at 4 °C for 8 days. | Beef Patties | [3] |
| Chitosan–benzoic acid nanogels loaded with Rosmarinus officinalis essential oil REO–CS-BA NG | <100 | The antimicrobial effect of REO–CS-BA NG against S. typhimurium on beef cutlet samples was evaluated in vivo during 12 days of refrigerated storage at 4 °C. 1 mL of the NC REOs (at various entrapped REOs concentrations) was spayed over the surface of the meat batches. REO–CS-BA NG caused an immediate reduction in S. typhimurium population since day 1. Moreover, after 12 days of storage, the population of the bacteria on the coated samples was significantly lower than that of the control. | Beef cuttles | [78] |
| Nanoemulsion encapsulating oregano essential oil OEO NE | 35–55 | Regarding contamination of chicken pâté by E. coli, on the last day of analysis, the OEO NE antibacterial effect was significantly higher (p < 0.05) than the other treatments. For S. aureus, the growth inhibition effects were similar for both pure and nanoencapsulated oregano EO, whereas treatment with nitrite (synthetic food preservative) was the most efficient in reducing the bacterial growth. The nanoemulsion was added to the chicken pâté as part of the formulation. | Chicken pâté | [79] |
| Clove essential oils encapsulated by chitosan myristic acid nanogel CEO–CS-MA NG | <100 | The surface treatment of beef with 1 and 2 mg CEO–CS-MA NG resulted in significant reductions in Salmonella populations by 0.58 and 0.68 log CFU/g, respectively, compared with the positive control after 3 d of storage. These results show that encapsulation of bioactive compounds may theoretically enhance the antimicrobial activity of essential oils, by increasing their dispersion in aqueous environment and stimulating passive adsorption mechanisms into cells. Beef cuttles were stored at 4 °C for 12 days. | Beef cuttles | [67] |
| Lysozyme, nisin, EDTA, ZnO NP (LNEZ) | <110 | Antimicrobial solution was directly applied on the meat surface after attachment and spread uniformly, meat was packaged and stored at 4 °C for 15 days. Treated and control samples were inoculated with 7 log CFU/cm2 of E. coli, L. monocytogenes and B. cereus. The application of LNEZ against E. coli O157:H7 showed a reduction (2.7 log CFU/cm2) in these bacteria after 15 days of storage, while control samples reached a population of 8.1 log CFU/cm2 over the course of the experiment. Same results were observed for L. monocytogenes and B. cereus, showing a final concentration of 2 and 4 log CFU/cm2 after 15 days at refrigerated conditions while control samples reached more than 8 log CFU/cm2, respectively. | Minced beef | [59] |
| Bay leaf encapsulated nanoliposomes BL NL | 99 | The meat was sprayed with 1000 and 1500 ppm of nano-extract and stored at 4 °C for 12 days. For Staphylococcus aureus, the highest values were observed in control treatment on most days while in the treatments containing the nanoencapsulated extract, no bacterial S. aureus was observed in at 1500 ppm non-extract on day 4 of storage. E. coli, higher concentrations of the extract showed better results and also the results were better with the treatments containing the NC extract so that from day 8 no E. coli were observed in the NC extract at 1500 ppm. | Minced beef | [81] |
| Eugenol nanocapsules embedded with gelatin –chitosan Eug–Gel-CS NP | 229.09 nm | According to the results, the total bacteria count (TBC) of the meat samples in each treatment group increased as the storage period was extended. The Eug–Gel-CS NP group exhibited the lowest TBC growth rate. The Eug nanoparticles were verified to exert a sustained release effect on Eug, which could prolong its antiseptic and antibacterial effects. Meat was immersed in Eug–Gel-CS NCs formulation and stored for 15 days at 4 °C. | Chilled pork meat | [62] |
| ε-polylysine nanoparticles with plant extracts ε-PLN | - | Sausages formulated with ε-PLN significantly increased shelf life of frankfurter-type sausages stored at 4 °C for 45 days, since values of total viable count microorganisms did not increase significantly during storage, reaching to 4.03 Log CFU/g after 30 days. Regarding Clostridium perfringens, the percentage of decrease was significantly higher in ɛ-PLN samples than in the other groups, not being detected at the end of storage. The same behavior was observed in Staphylococcus aureus, one of the most important bacteria in meat and meat products due to production of enterotoxins. ε-PLN were efficient against E. coli compared to the other groups. After 45 days of storage ɛ-PLN sausages reached to not detected values, while control samples displayed values of 0.21 Log CFU/g. | Frankfurter-type sausages | [63] |
5.3. Application of Nanosystems in Meat Processing
6. Application Methods
6.1. Marinating
6.2. Edible Coatings
6.3. Processing Ingredients
7. Future Trends
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, X.; Ahn, D.U. Lipid Oxidation and Its Implications to Meat Quality and Human Health. Food Sci. Biotechnol. 2019, 28, 1275–1285. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, S.; Ahn, D.U. Protein Oxidation: Basic Principles and Implications for Meat Quality. Crit. Rev. Food Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.A.; Ahmadi Gavlighi, H.; Gardini, F.; Gharderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.A.; Ahmadi Gavlighi, H.; Gardini, F. Chitosan-Cinnamon Essential Oil Nano-Formulation: Application as a Novel Additive for Controlled Release and Shelf Life Extension of Beef Patties. Int. J. Biol. Macromol. 2017, 102, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-zaragoza, M.L.; González-Reza, R.; Mendoza-Muñoz, N.; Miranda-Linares, V.; Bernal-Couoh, T.F.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Nanosystems in Edible Coatings: A Novel Strategy for Food Preservation. Int. J. Mol. Sci. 2018, 19, 705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, C.D.; Nunes, I.L. Oil Nanoencapsulation: Development, Application, and Incorporation into the Food Market. Nanoscale Res. Lett. 2019, 14, 9. [Google Scholar] [CrossRef] [Green Version]
- Ramachandraiah, K.; Hong, G.P. Polymeric Nanomaterials for the Development of Sustainable Plant Food Value Chains. Food Biosci. 2021, 41, 2212–4292. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; Mendoza-Muñoz, N.; Leyva-Gómez, G. Nanoemulsions and Nanosized Ingredients for Food Formulations. In Handbook of Food Nanotechnology; Jafari, S.M., Ed.; Academic Press, Elsevier: Chennai, India, 2020; pp. 207–256. [Google Scholar]
- Ghaderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.A.; Azizi, M.H. Nanoencapsulation Approach to Improve Antimicrobial and Antioxidant Activity of Thyme Essential Oil in Beef Burgers During Refrigerated Storage. Food Bioprocess Technol. 2016, 9, 1187–1201. [Google Scholar] [CrossRef]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible Films/Coating with Tailored Properties for Active Packaging of Meat, Fish and Derived Products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Ekonomou, S.I.; Akshay Thanekar, P.; Lamprou, D.A.; Weaver, E.; Doran, O.; Stratakos, A.C. Development of Geraniol-Loaded Liposomal Nanoformulations against Salmonella Colonization in the Pig Gut. J. Agric. Food Chem. 2022, 70, 7004–7014. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef] [Green Version]
- Rai, M.; Paralikar, P.; Jogee, P.; Agarkar, G.; Ingle, A.P.; Derita, M.; Zacchino, S. Synergistic Antimicrobial Potential of Essential Oils in Combination with Nanoparticles: Emerging Trends and Future Perspectives. Int. J. Pharm. 2017, 519, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Rajakumar, G.; Gomathi, T.; Park, S.K.; Kim, S.H.; Thiruvengadam, M. Nanotechnology for Human Food: Advances and Perspective. Front. Life Sci. 2017, 10, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.; Sahar, A.; Rahman, U. Nanotechnology in Healthier Meat Processing. In Novel Approaches of Nanotechnology in Food; Grumezescu, A.M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 313–345. ISBN 9780128043080. [Google Scholar]
- García-Pinilla, S.; Villalobos-Espinosa, J.C.; Cornejo-Mazón, M.; Gutiérrez-López, G.F. Nanotechnology in Food Processing. Adv. Process. Technol. Bio-Based Nanosyst. Food 2019, 259–276. [Google Scholar] [CrossRef]
- Liu, J.; Ellies-Oury, M.-P.; Stoyanchev, T.; Hocquette, J.-F. Consumer Perception of Beef Quality and How to Control, Improve and Predict It? Focus on Eating Quality. Foods 2022, 11, 1732. [Google Scholar] [CrossRef]
- Amiri, E.; Aminzare, M.; Azar, H.H.; Mehrasbi, M.R. Combined Antioxidant and Sensory Effects of Corn Starch Films with Nanoemulsion of Zataria Multiflora Essential Oil Fortified with Cinnamaldehyde on Fresh Ground Beef Patties. Meat Sci. 2019, 153, 66–74. [Google Scholar] [CrossRef]
- González-Reza, R.M.; Zambrano-Zaragoza, M.L.; Hernández-Sánchez, H. Polymeric Nanoparticles in Foods. In Nanotechnology in the Life Sciences; Springer Science and Business Media B.V.: Berlin/Heidelberg, Germany, 2019; pp. 217–233. [Google Scholar]
- Perinelli, D.R.; Palmieri, G.F.; Cespi, M.; Bonacucina, G.; Montesano, D.; Petrelli, R. Molecules Encapsulation of Flavours and Fragrances into Polymeric Capsules and Cyclodextrins Inclusion Complexes: An Update. Molecules 2020, 25, 5878. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Turcuş, V.; Predoi, G.; Iordache, F. Nanoencapsulation Techniques for Compounds and Products with Antioxidant and Antimicrobial Activity—A Critical View. Eur. J. Med. Chem. 2018, 157, 1326–1345. [Google Scholar] [CrossRef]
- Taylor, T.M.; Davidson, P.M.; Bruce, B.D.; Weiss, J. Liposomal Nanocapsules in Food Science and Agriculture. Crit. Rev. Food Sci. Nutr. 2007, 45, 587–605. [Google Scholar] [CrossRef]
- Gupta, M.; Aggarwal, R.; Raina, N.; Khan, A. Vitamin-Loaded Nanocarriers as Nutraceuticals in Healthcare Applications. In Nanomedicine for Bioactives: Healthcare Applications; Springer: Singapore, 2020; pp. 451–470. ISBN 9789811516641. [Google Scholar]
- Sáiz-Abajo, M.J.; González-Ferrero, C.; Moreno-Ruiz, A.; Romo-Hualde, A.; González-Navarro, C.J. Thermal Protection of β-Carotene in Re-Assembled Casein Micelles during Different Processing Technologies Applied in Food Industry. Food Chem. 2013, 138, 1581–1587. [Google Scholar] [CrossRef]
- Cerqueira, M.Â.; Pinheiro, A.C.; Ramos, O.L.; Silva, H.; Bourbon, A.I.; Vicente, A.A. Advances in Food Nanotechnology. In Emerging Nanotechnologies in Food Science; Busquets, R., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 11–38. ISBN 9780323429993. [Google Scholar]
- Acevedo-Fani, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Nanostructured Emulsions and Nanolaminates for Delivery of Active Ingredients: Improving Food Safety and Functionality. Trends Food Sci. Technol. 2017, 60, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Muñoz, N.; Urbán-Morlán, Z.; Leyva-Gómez, G.; De La, M.; Zambrano-Zaragoza, L.; Piñón-Segundo, E.; Quintanar-Guerrero, D. Solid Lipid Nanoparticles: An Approach to Improve Oral Drug Delivery. J. Pharm. Pharm. Sci. 2021, 24, 509–532. [Google Scholar] [CrossRef] [PubMed]
- Katopodi, A.; Detsi, A. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers of Natural Products as Promising Systems for Their Bioactivity Enhancement: The Case of Essential Oils and Flavonoids. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127529. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Rostamabadi, H.; Babazadeh, A.; Tarhan, Ö.; Rashidinejad, A.; Boostani, S.; Khoshnoudi-Nia, S.; Akbari-Alavijeh, S.; Shaddel, R.; Jafari, S.M. Lycopene Nanodelivery Systems; Recent Advances. Trends Food Sci. Technol. 2022, 119, 378–399. [Google Scholar] [CrossRef]
- Coelho, S.C.; Estevinho, B.N.; Rocha, F. Encapsulation in Food Industry with Emerging Electrohydrodynamic Techniques: Electrospinning and Electrospraying—A Review. Food Chem. 2020, 339, 127850. [Google Scholar] [CrossRef] [PubMed]
- Horuz, T.İ.; Belibağlı, K.B. Nanoencapsulation of Carotenoids Extracted from Tomato Peels into Zein Fibers by Electrospinning. J. Sci. Food Agric. 2019, 99, 759–766. [Google Scholar] [CrossRef]
- Hosseini, R.S.; Rajaei, A. Potential Pickering Emulsion Stabilized with Chitosan-Stearic Acid Nanogels Incorporating Clove Essential Oil to Produce Fish-Oil-Enriched Mayonnaise. Carbohydr. Polym. 2020, 241, 116340. [Google Scholar] [CrossRef]
- Raghunath, A.; Perumal, E. Metal Oxide Nanoparticles as Antimicrobial Agents: A Promise for the Future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef]
- Duncan, T.V. Applications of Nanotechnology in Food Packaging and Food Safety: Barrier Materials, Antimicrobials and Sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef]
- Pereda, M.; Marcovich, N.E.; Ansorena, M.R. Nanotechnology in Food Packaging Applications: Barrier Materials, Antimicrobial Agents, Sensors, and Safety Assessment. In Handbook of Ecomaterials; Springer: Cham, Switzerland, 2018; pp. 2035–2056. ISBN 783319682556. [Google Scholar]
- Carbone, M.; Donia, D.T.; Sabbatella, G.; Antiochia, R. Silver Nanoparticles in Polymeric Matrices for Fresh Food Packaging. J. King Saud Univ.-Sci. 2016, 28, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.; Viswanathan, K.; Kasi, G.; Sadeghi, K.; Seo, J. ZnO Nanostructures in Active Antibacterial Food Packaging: Preparation Methods, Antimicrobial Mechanisms, Safety Issues, Future Prospects, and Challenges ZnO Nanostructures in Active Antibacterial Food Packaging: Preparation Methods, Antimicrobial. Food Rev. Int. 2020, 38, 537–565. [Google Scholar] [CrossRef] [Green Version]
- Panea, B.; Ripoll, G.; González, J.; Fernández-Cuello, Á.; Albertí, P. Effect of Nanocomposite Packaging Containing Different Proportions of ZnO and Ag on Chicken Breast Meat Quality. J. Food Eng. 2014, 123, 104–112. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Vasile, B. Ștefan; Andronescu, E. Inorganic Nanoparticles and Composite Films for Antimicrobial Therapies. Int. J. Mol. Sci. 2021, 22, 4595. [Google Scholar] [CrossRef] [PubMed]
- Ramachandraiah, K.; Han, S.G.; Chin, K.B. Nanotechnology in Meat Processing and Packaging: Potential Applications—A Review. Asian-Australas. J. Anim. Sci. 2015, 28, 290–302. [Google Scholar] [CrossRef] [Green Version]
- El-Seedi, H.R.; El-Shabasy, R.M.; Khalifa, S.A.M.; Saeed, A.; Shah, A.; Shah, R.; Iftikhar, F.J.; Abdel-Daim, M.M.; Omri, A.; Hajrahand, N.H.; et al. Metal Nanoparticles Fabricated by Green Chemistry Using Natural Extracts: Biosynthesis, Mechanisms, and Applications. RSC Adv. 2019, 9, 24539–24559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, D.; El Kurdi, R. El Curcumin as a Novel Reducing and Stabilizing Agent for the Green Synthesis of Metallic Nanoparticles. Green Chem. Lett. Rev. 2021, 14, 474–487. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbühler, K. What Is a Green Solvent? A Comprehensive Framework for the Environmental Assessment of Solvents. Green Chem. 2007, 9, 927–993. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and Techniques for Solvent Selection: Green Solvent Selection Guides. Sustain. Chem. Process. 2016, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Gómez, B.; Barba, F.J.; Domínguez, R.; Putnik, P.; Bursać Kovačević, D.; Pateiro, M.; Toldrá, F.; Lorenzo, J.M. Microencapsulation of Antioxidant Compounds through Innovative Technologies and Its Specific Application in Meat Processing. Trends Food Sci. Technol. 2018, 82, 135–147. [Google Scholar] [CrossRef]
- Barreras-Urbina, C.G.; Ramírez-Wong, B.; López-Ahumada, G.A.; Burruel-Ibarra, S.E.; Martínez-Cruz, O.; Tapia-Hernández, J.A.; Rodríguez Félix, F. Nano- and Micro-Particles by Nanoprecipitation: Possible Application in the Food and Agricultural Industries. Int. J. Food Prop. 2016, 19, 1912–1923. [Google Scholar] [CrossRef]
- Wei, Y.; Sun, C.; Dai, L.; Zhan, X.; Gao, Y. Structure, Physicochemical Stability and in Vitro Simulated Gastrointestinal Digestion Properties of β-Carotene Loaded Zein-Propylene Glycol Alginate Composite Nanoparticles Fabricated by Emulsification-Evaporation Method. Food Hydrocoll. 2018, 81, 149–158. [Google Scholar] [CrossRef]
- Smaoui, S.; Ben Hlima, H.; Braïek, O.B.; Ennouri, K.; Mellouli, L.; Khaneghah, A.M. Recent Advancements in Encapsulation of Bioactive Compounds as a Promising Technique for Meat Preservation. Meat Sci. 2021, 181, 108585. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.E.; Lamprecht, A. Spray Freeze Drying for Dry Powder Inhalation of Nanoparticles. Eur. J. Pharm. Biopharm. 2014, 87, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Prieto, C.; Calvo, L. Supercritical Fluid Extraction of Emulsions to Nanoencapsulate Vitamin E in Polycaprolactone. J. Supercrit. Fluids 2017, 119, 274–282. [Google Scholar] [CrossRef]
- Lévai, G.; Albarelli, J.Q.; Santos, D.T.; Meireles, M.A.A.; Martín, Á.; Rodríguez-Rojo, S.; Cocero, M.J. Quercetin Loaded Particles Production by Means of Supercritical Fluid Extraction of Emulsions: Process Scale-Upstudy and Thermo-Economic Evaluation. Food Bioprod. Process. 2017, 103, 27–38. [Google Scholar] [CrossRef]
- Guimarães, M.; Correia, S.; Briuglia, M.L.; Niosi, F.; Lamprou, D.A. Microfluidic Manufacturing of Phospholipid Nanoparticles: Stability, Encapsulation Efficacy, and Drug Release. Int. J. Pharm. 2017, 512, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Munekata, P.E.S.; Sant’ana, A.S.; Domínguez, R.; Rodríguez-Lázaro, D.; Lorenzo, J.M. Application of Essential Oils as Antimicrobial Agents against Spoilage and Pathogenic Microorganisms in Meat Products. Int. J. Food Microbiol. 2021, 337, 108966. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Jayasena, D.D.; Jo, C. Potential Application of Essential Oils as Natural Antioxidants in Meat and Meat Products: A Review. Food Rev. Int. 2014, 30, 71–90. [Google Scholar] [CrossRef]
- Estévez, M. Critical Overview of the Use of Plant Antioxidants in the Meat Industry: Opportunities, Innovative Applications and Future Perspectives. Meat Sci. 2021, 181, 108610. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, W.; Pang, C.; Deng, W.; Xu, C.; Wang, X. Multifunctional Chitosan-Based Coating with Liposomes Containing Laurel Essential Oils and Nanosilver for Pork Preservation. Food Chem. 2019, 295, 16–25. [Google Scholar] [CrossRef]
- Elbarbary, A.M.; El-Sawy, N.M.; Hegazy, E.S.A. Antioxidative Properties of Irradiated Chitosan/Vitamin C Complex and Their Use as Food Additive for Lipid Storage. J. Appl. Polym. Sci. 2015, 132, 42105. [Google Scholar] [CrossRef]
- Chouljenko, A.; Chotiko, A.; Bonilla, F.; Moncada, M.; Reyes, V.; Sathivel, S. Effects of Vacuum Tumbling with Chitosan Nanoparticles on the Quality Characteristics of Cryogenically Frozen Shrimp. LWT-Food Sci. Technol. 2017, 75, 114–123. [Google Scholar] [CrossRef]
- Morsy, M.K.; Elsabagh, R.; Trinetta, V. Evaluation of Novel Synergistic Antimicrobial Activity of Nisin, Lysozyme, EDTA Nanoparticles, and/or ZnO Nanoparticles to Control Foodborne Pathogens on Minced Beef. Food Control 2018, 92, 249–254. [Google Scholar] [CrossRef]
- Hajji, S.; Hamdi, M.; Boufi, S.; Li, S.; Nasri, M. Suitability of Chitosan Nanoparticles as Cryoprotectant on Shelf Life of Restructured Fish Surimi during Chilled Storage. Cellulose 2019, 26, 6825–6847. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Zeppenfeld, C.C.; Velho, M.C.; Klein, B.; Abbad, L.B.; Ourique, A.F.; Wagner, R.; Da Silva, A.S.; Baldisserotto, B. Dietary Supplementation with Nerolidol Nanospheres Improves Growth, Antioxidant Status and Fillet Fatty Acid Profiles in Nile Tilapia: Benefits of Nanotechnology for Fish Health and Meat Quality. Aquaculture 2020, 516, 734635. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Ding, W. Eugenol Nanocapsules Embedded with Gelatin-Chitosan for Chilled Pork Preservation. Int. J. Biol. Macromol. 2020, 158, 837–844. [Google Scholar] [CrossRef]
- Alirezalu, K.; Pirouzi, S.; Yaghoubi, M.; Karimi-Dehkordi, M.; Jafarzadeh, S.; Khaneghah, A.M. Packaging of Beef Fillet with Active Chitosan Film Incorporated with ε-Polylysine: An Assessment of Quality Indices and Shelf Life. Meat Sci. 2021, 176, 108475. [Google Scholar] [CrossRef]
- Eskandani, M.; Janmohammadi, H.; Mirghelenj, S.A.; Ebrahimi, M.; Kalanaky, S. Effects of Zinc Nanoparticles on Growth Performance, Carcass Characteristics, Immunity, and Meat Quality of Broiler Chickens. Iran. J. Appl. Anim. Sci. 2021, 11, 135–146. [Google Scholar]
- Ibrahim, D.; Kishawy, A.T.Y.; Khater, S.I.; Khalifa, E.; Ismail, A.; Mohammed, H.A.; Elnahriry, S.S.; Tolba, H.A.; Sherief, W.R.I.A.; Farag, M.F.M.; et al. Interactive Effects of Dietary Quercetin Nanoparticles on Growth, Flesh Antioxidant Capacity and Transcription of Cytokines and Aeromonas Hydrophila Quorum Sensing Orchestrating Genes in Nile Tilapia (Oreochromis Niloticus). Fish Shellfish Immunol. 2021, 119, 1050–4648. [Google Scholar] [CrossRef]
- Estévez, M.; Lorenzo, J.M. Impact of Antioxidants on Oxidized Proteins and Lipids in Processed Meat. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 600–608. ISBN 9780128140451. [Google Scholar]
- Rajaei, A.; Hadian, M.; Mohsenifar, A.; Rahmani-Cherati, T.; Tabatabaei, M. A Coating Based on Clove Essential Oils Encapsulated by Chitosan-Myristic Acid Nanogel Efficiently Enhanced the Shelf-Life of Beef Cutlets. Food Packag. Shelf Life 2017, 14, 137–145. [Google Scholar] [CrossRef]
- Ahmad, S.R.; Gokulakrishnan, P.; Giriprasad, R.; Yatoo, M.A. Fruit-Based Natural Antioxidants in Meat and Meat Products: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Yadav, D.N.; Ahmad, T.; Narsaiah, K. Recent Trends in the Use of Natural Antioxidants for Meat and Meat Products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 796–812. [Google Scholar] [CrossRef] [Green Version]
- Oswell, N.J.; Thippareddi, H.; Pegg, R.B. Practical Use of Natural Antioxidants in Meat Products in the U.S.: A Review. Meat Sci. 2018, 145, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Alirezalu, K.; Movlan, H.S.; Yaghoubi, M.; Pateiro, M.; Lorenzo, J.M. Ɛ-Polylysine Coating with Stinging Nettle Extract for Fresh Beef Preservation. Meat Sci. 2021, 176, 309–1740. [Google Scholar] [CrossRef] [PubMed]
- Karre, L.; Lopez, K.; Getty, K.J.K. Natural Antioxidants in Meat and Poultry Products. Meat Sci. 2013, 94, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Lamri, M.; Bhattacharya, T.; Boukid, F.; Chentir, I.; Dib, A.L.; Das, D.; Djenane, D.; Gagaoua, M. Nanotechnology as a Processing and Packaging Tool to Improve Meat Quality and Safety. Foods 2021, 10, 2633. [Google Scholar] [CrossRef] [PubMed]
- Sahraee, S.; Milani, J.M.; Regenstein, J.M.; Kafil, H.S. Protection of Foods against Oxidative Deterioration Using Edible Films and Coatings: A Review. Food Biosci. 2019, 32, 100451. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of Essential Oils and Homogenization Conditions on Properties of Chitosan-Based Films. Food Hydrocoll. 2012, 26, 9–16. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Punbusayakul, N.; Lee, D.S. Antimicrobial Packaging for Meat Products. In Antimicrobial Food Packaging; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 229–241. ISBN 9780128007235. [Google Scholar]
- Ali, S.S.; Moawad, M.S.; Hussein, M.A.; Azab, M.; Abdelkarim, E.A.; Badr, A.; Sun, J.; Khalil, M. Efficacy of Metal Oxide Nanoparticles as Novel Antimicrobial Agents against Multi-Drug and Multi-Virulent Staphylococcus Aureus Isolates from Retail Raw Chicken Meat and Giblets. Int. J. Food Microbiol. 2021, 344, 109116. [Google Scholar] [CrossRef]
- Hadian, M.; Rajaei, A.; Mohsenifar, A.; Tabatabaei, M. Encapsulation of Rosmarinus Officinalis Essential Oils in Chitosan-Benzoic Acid Nanogel with Enhanced Antibacterial Activity in Beef Cutlet against Salmonella Typhimurium during Refrigerated Storage. LWT 2017, 84, 394–401. [Google Scholar] [CrossRef]
- Moraes-Lovison, M.; Marostegan, L.F.P.; Peres, M.S.; Menezes, I.F.; Ghiraldi, M.; Rodrigues, R.A.F.; Fernandes, A.M.; Pinho, S.C. Nanoemulsions Encapsulating Oregano Essential Oil: Production, Stability, Antibacterial Activity and Incorporation in Chicken Pâté. LWT-Food Sci. Technol. 2017, 77, 233–240. [Google Scholar] [CrossRef]
- Aziz, M.; Karboune, S. Natural Antimicrobial/Antioxidant Agents in Meat and Poultry Products as Well as Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 486–511. [Google Scholar] [CrossRef] [PubMed]
- Tometri, S.S.; Ahmady, M.; Ariaii, P.; Soltani, M.S. Extraction and Encapsulation of Laurus Nobilis Leaf Extract with Nano-Liposome and Its Effect on Oxidative, Microbial, Bacterial and Sensory Properties of Minced Beef. J. Food Meas. Charact. 2020, 14, 3333–3344. [Google Scholar] [CrossRef]
- Mirhosseini, M.; Arjmand, V. Reducing Pathogens by Using Zinc Oxide Nanoparticles and Acetic Acid in Sheep Meat. J. Food Prot. 2014, 77, 1599–1604. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Cai, L.; Wu, Y.; Ji, M.; Jiang, H. Dissection of the Antibacterial Mechanism of Zinc Oxide Nanoparticles with Manipulable Nanoscale Morphologies. J. Hazard. Mater. 2022, 430, 128436. [Google Scholar] [CrossRef]
- Lallo da Silva, B.; Caetano, B.L.; Chiari-Andréo, B.G.; Pietro, R.C.L.R.; Chiavacci, L.A. Increased Antibacterial Activity of ZnO Nanoparticles: Influence of Size and Surface Modification. Colloids Surf. B Biointerfaces 2019, 177, 440–447. [Google Scholar] [CrossRef]
- Liu, Y.; He, L.; Mustapha, A.; Li, H.; Hu, Z.Q.; Lin, M. Antibacterial Activities of Zinc Oxide Nanoparticles against Escherichia Coli O157:H7. J. Appl. Microbiol. 2009, 107, 1193–1201. [Google Scholar] [CrossRef]
- Singh, P.K.; Jairath, G.; Ahlawat, S.S. Nanotechnology: A Future Tool to Improve Quality and Safety in Meat Industry. J. Food Sci. Technol. 2016, 53, 1739–1749. [Google Scholar] [CrossRef] [Green Version]
- Purslow, P.P.; Warner, R.D.; Clarke, F.M.; Hughes, J.M. Variations in Meat Colour Due to Factors Other than Myoglobin Chemistry; a Synthesis of Recent Findings (Invited Review). Meat Sci. 2020, 159, 107941. [Google Scholar] [CrossRef]
- Dong, J.; Zhou, Y.; Lu, Y.; Lv, Y.; Chi, Y.; He, Q. Effect of Tea Polyphenols on the Oxidation and Color Stability of Porcine Hemoglobin. J. Food Sci. 2019, 84, 2086–2090. [Google Scholar] [CrossRef]
- Suman, S.P.; Joseph, P. Myoglobin Chemistry and Meat Color. Annu. Rev. Food Sci. Technol. 2013, 4, 79–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Xiong, Y.L. Natural Antioxidants as Food and Feed Additives to Promote Health Benefits and Quality of Meat Products: A Review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozimek, L.; Pospiech, E.; Narine, S. Nanotechnologies in Food and Meat Processing. Acta Sci. Pol. Technol. Aliment. 2010, 9, 401–412. [Google Scholar]
- Chaudhary, S.; Kumar, V.; Sharma, V.; Sharma, R.; Kumar, S. Chitosan Nanoemulsion: Gleam into the Futuristic Approach for Preserving the Quality of Muscle Foods. Int. J. Biol. Macromol. 2022, 199, 121–137. [Google Scholar] [CrossRef]
- Rosario, D.K.A.; Rodrigues, B.L.; Bernardes, P.C.; Conte-Junior, C.A. Principles and Applications of Non-Thermal Technologies and Alternative Chemical Compounds in Meat and Fish. Crit. Rev. Food Sci. Nutr. 2021, 61, 1163–1183. [Google Scholar] [CrossRef]
- Hill, E.K.; Li, J. Current and Future Prospects for Nanotechnology in Animal Production. J. Anim. Sci. Biotechnol. 2017, 8, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yusop, S.M.; O’Sullivan, M.G.; Preuß, M.; Weber, H.; Kerry, J.F.; Kerry, J.P. Assessment of Nanoparticle Paprika Oleoresin on Marinating Performance and Sensory Acceptance of Poultry Meat. LWT-Food Sci. Technol. 2012, 46, 349–355. [Google Scholar] [CrossRef]
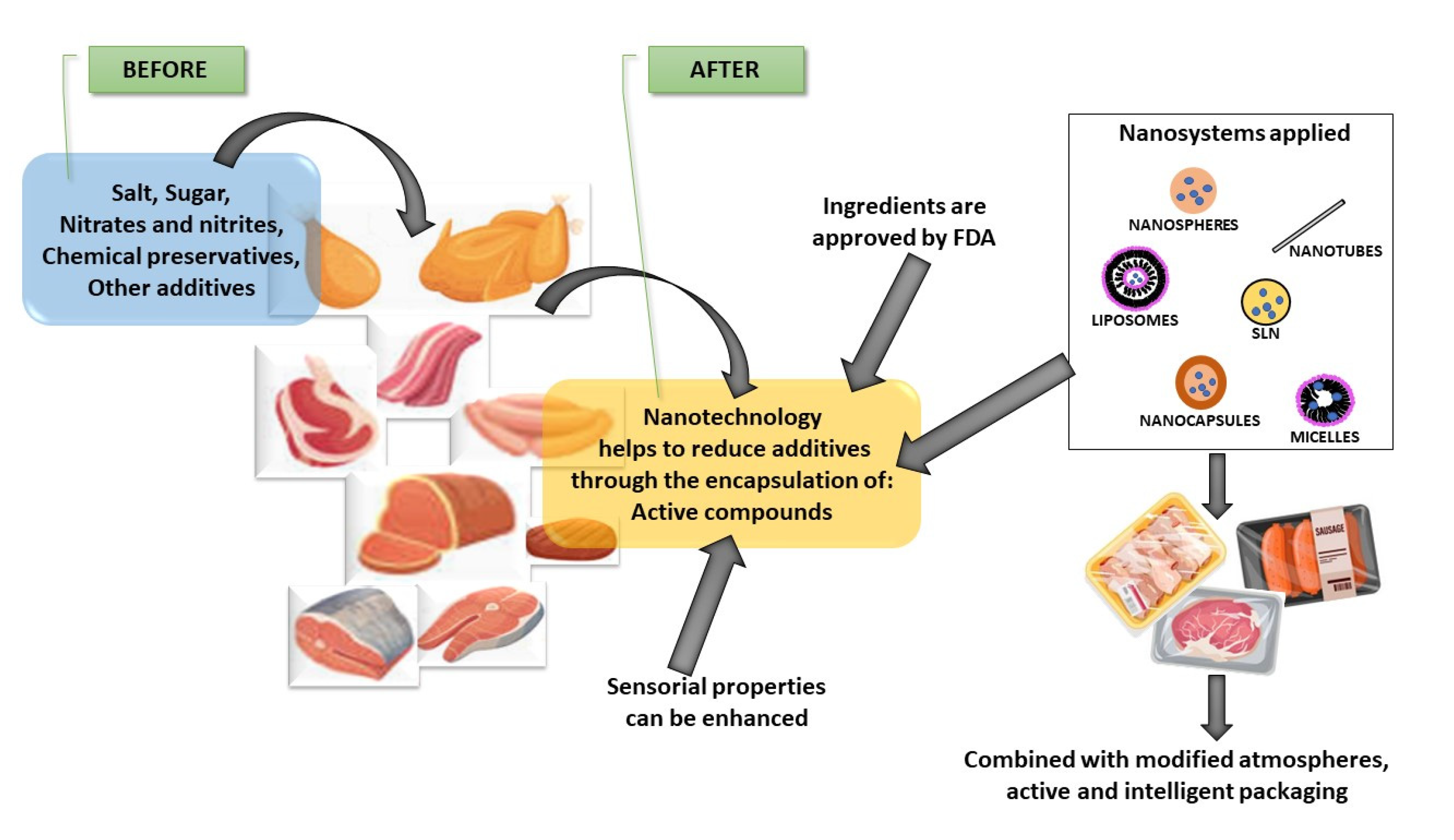
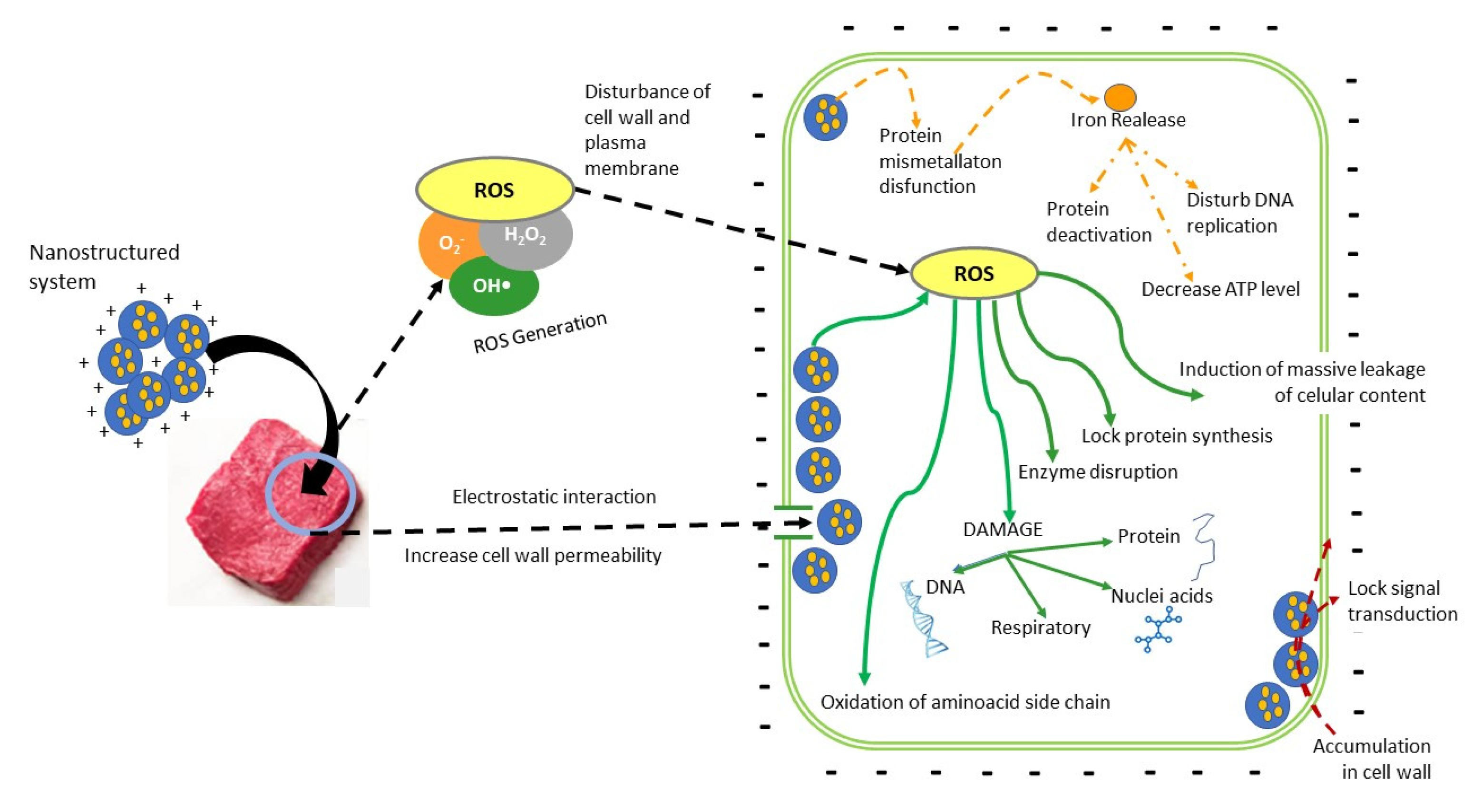
| Nanosystems | Types and Definitions | Application in Food | Ref. |
|---|---|---|---|
| Polymeric nanoparticles Nanosystems with a wall membrane or structure composed of natural or synthetic biodegradable polymers. All materials must be approved for use in food [18]. | Nanocapsules are composed of a biopolymeric wall surrounding a core that contains an active compound [19]. | Fresh cut apple, orange juice, tuna fish, lean beef, fresh cut melon | [12] |
| Nanospheres are matrix systems that incorporate a bioactive compound entrapped and chemically-bound or absorbed into a polymer matrix. The bioactive compound disperses inside the nanosphere polymers [6]. | |||
| Surfactant-based systems | Nanoliposomes are lipid vesicles and consist of concentric bilayers, each with two layers of surfactant molecules whose non-polar tail groups face each other [20]. | Dairy products | [21] |
| Micelles are thermodynamically-stable structures typically made from surfactants with groups of polar heads and non-polar tails [22]. | Bakery products | [23] | |
| Emulsion-based systems | Nanoemulsions: a lipid phase is dispersed in an aqueous continuous phase or vice versa. They are thermodynamically-unstable and improved by incorporating stabilizers, emulsifiers, weighting agents, ripening inhibitors, and texture modifiers [24]. | Cabbage, cucumber, grape berry, green beans, Orange juice | [4,25] |
| Solid lipid nanoparticles (SLN) are nanoemulsions that contain active substances prepared with lipid solids at ambient temperature (high melting points). At cold temperatures, they solidify for use as delivery systems [26]. Nanostructured lipid carriers (NLC) are the next generation of lipid nanoparticles. They are prepared from a mixture of solid and liquid lipids to reduce the melting point [27]. | Guava (Psidium guajava L.) Fortified Beverages | [4,28] | |
| Nanotubes | Nanotubes are ultrathin tubes of nanometer-size diameter whose constituent elements are carbon, boron, and sand silicon that impart excellent thermal and electrical conductivity [14]. | Carrot, apples | [4] |
| Electrospinning | Nanofibers: electrospinning is used to obtain nanofibers, which are produced using high voltage that generates a field on a jet of a polymer solution. The nanosystems obtained have a large area:volume ratio, high inter-intra pores, high encapsulation efficiency, and better control of release and protection of active compounds [29]. | Food processing | [30] |
| Nanolaminates | Nanolaminates are considered to consist of two or more layers of material of nanometric dimensions that are bonded physically or chemically [20]. | Nutraceutical food, pork preservation, fresh cut and whole pears and mangoes | [25] |
| Nanohydrogels | Networks of three-dimensional hydrophilic or amphiphilic polymers that can swell in water (to around 30 times their size) and contain a relatively large amount of aqueous solvent. They maintain their structure thanks to the presence of covalent or non-covalent interactions [24]. | Enrich mayonnaise with fish oil | [31] |
| Method | Description |
|---|---|
| IONIC GELATION The aqueous phase contains a charged polymer and counterions such as calcium carbonate or calcium chloride. Phase A is compounded by a polymer added by dripping into Phase B, which contains the active agent, under constant stirring. Gelation is instantaneous when the two phases come into contact, forming a three-dimensional network. An example is using alginate–calcium chloride, which produces a structure called “egg box”, where the “eggs” are the calcium ions [8]. This technique does not require solvents or severe temperature and pH conditions. | 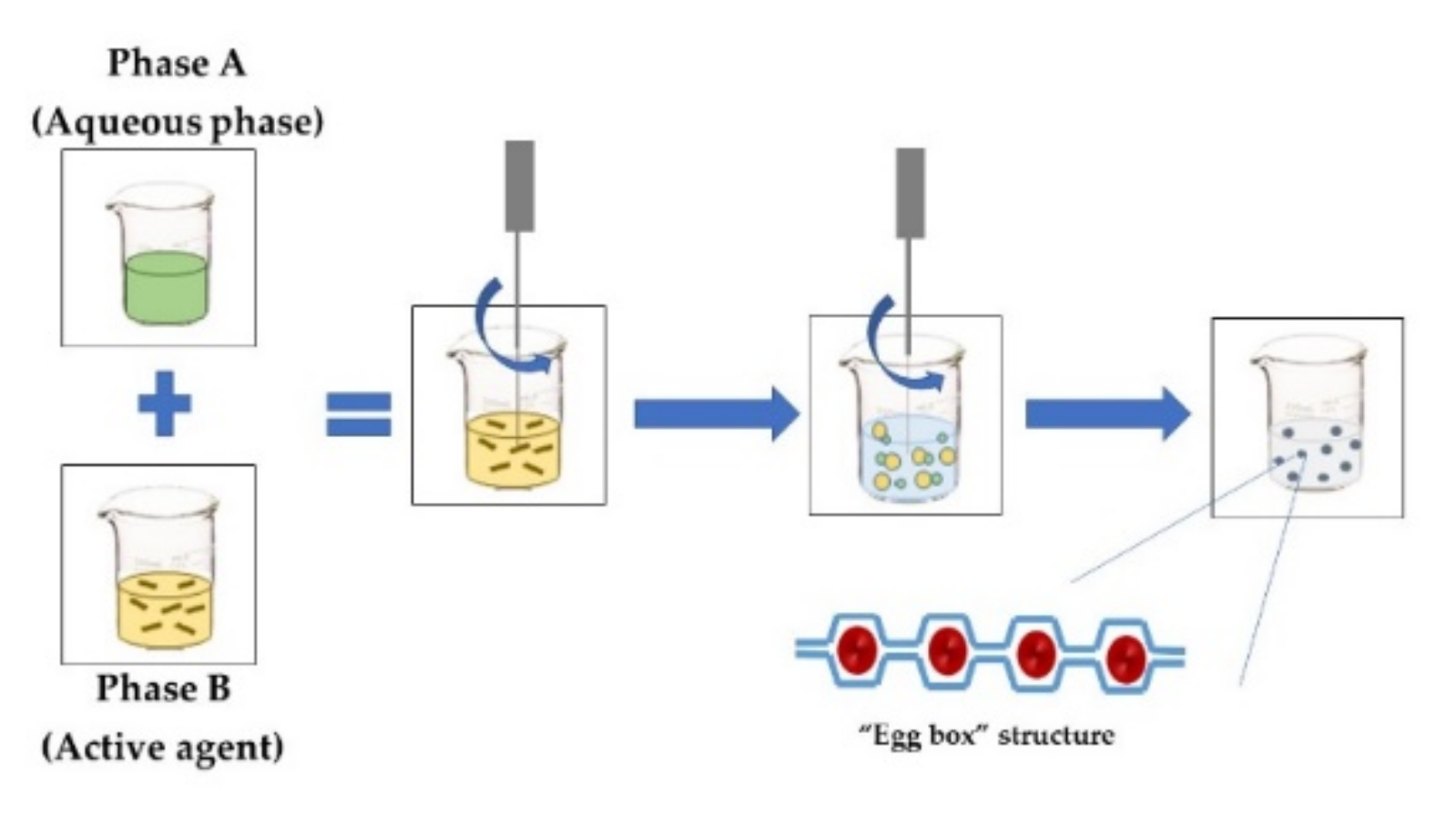 |
| MOLECULAR INCLUSION This involves interactions between two compounds, a host molecule that contains the active compound in a cavity-bearing material, such as cyclodextrin [19], which has a hydroxyl group outside a doughnut-shaped molecule and a relatively non-polar hole in the center, where the active compound of appropriate size and polarity; the second element is placed to form “host–guest” inclusion complexes in an aqueous solution [44]. |  |
| NANOPRECIPITATION OR SOLVENT DISPLACEMENT The organic phase contains an active agent and polymer that are dissolved in an organic miscible solvent (acetone, ethanol), and a protein or polymer that can be dissolved. The aqueous phase contains the stabilizer. The polymer precipitates, trapping the bioactive compound at the precise instant when the solvent containing the polymer diffuses into the dispersing medium [20,45]. | 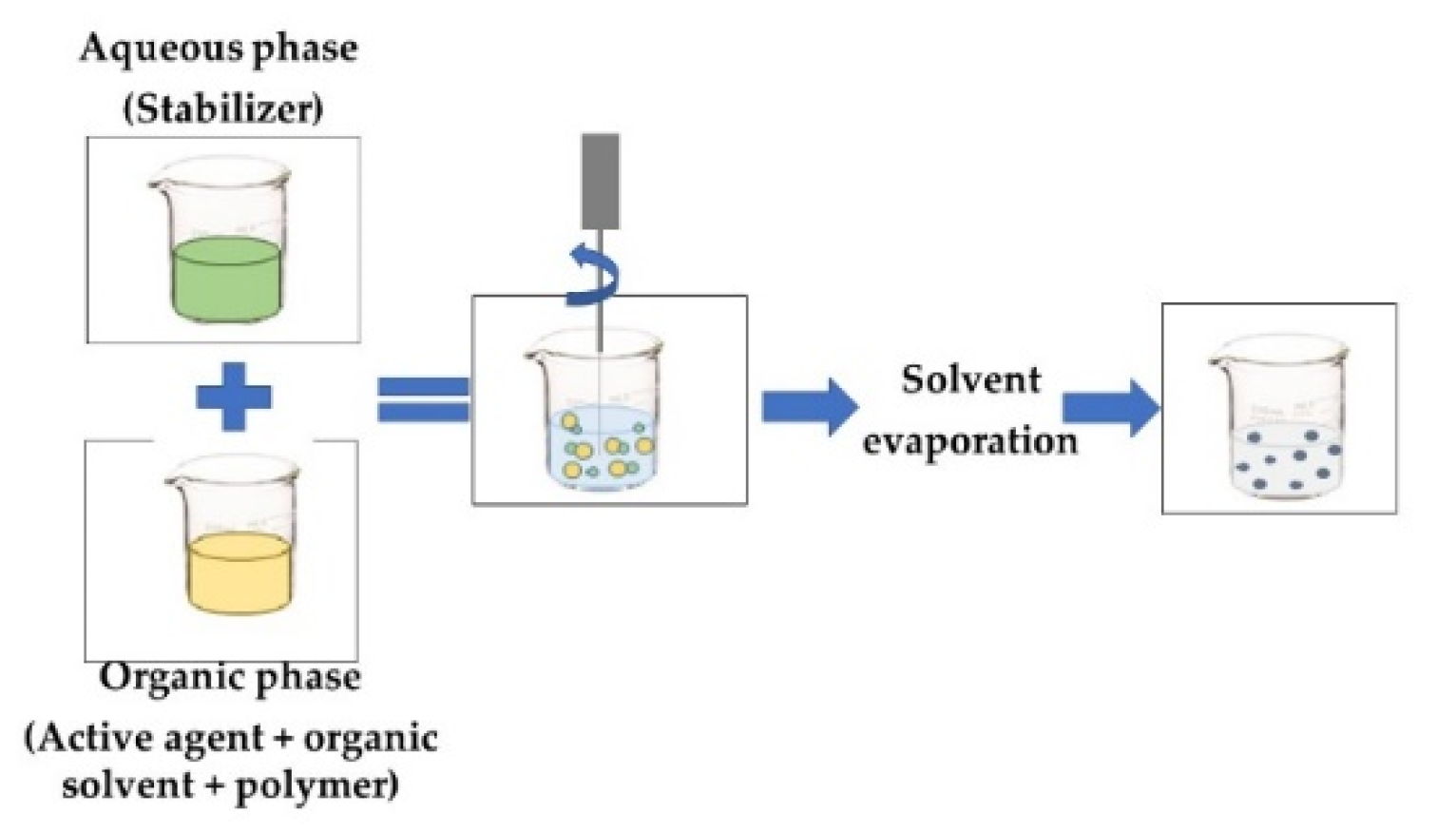 |
| EMULSIFICATION-EVAPORATION The organic phase contains the active compound and a hydrophobic polymer. The aqueous phase contains a surfactant and polymers that form the emulsion. The o/w emulsion is homogenized by a high-shear device, such as a rotor-stator, pressure homogenizer, or ultrasound. The solvent is then removed to allow the active compound and polymer to precipitate [46]. | 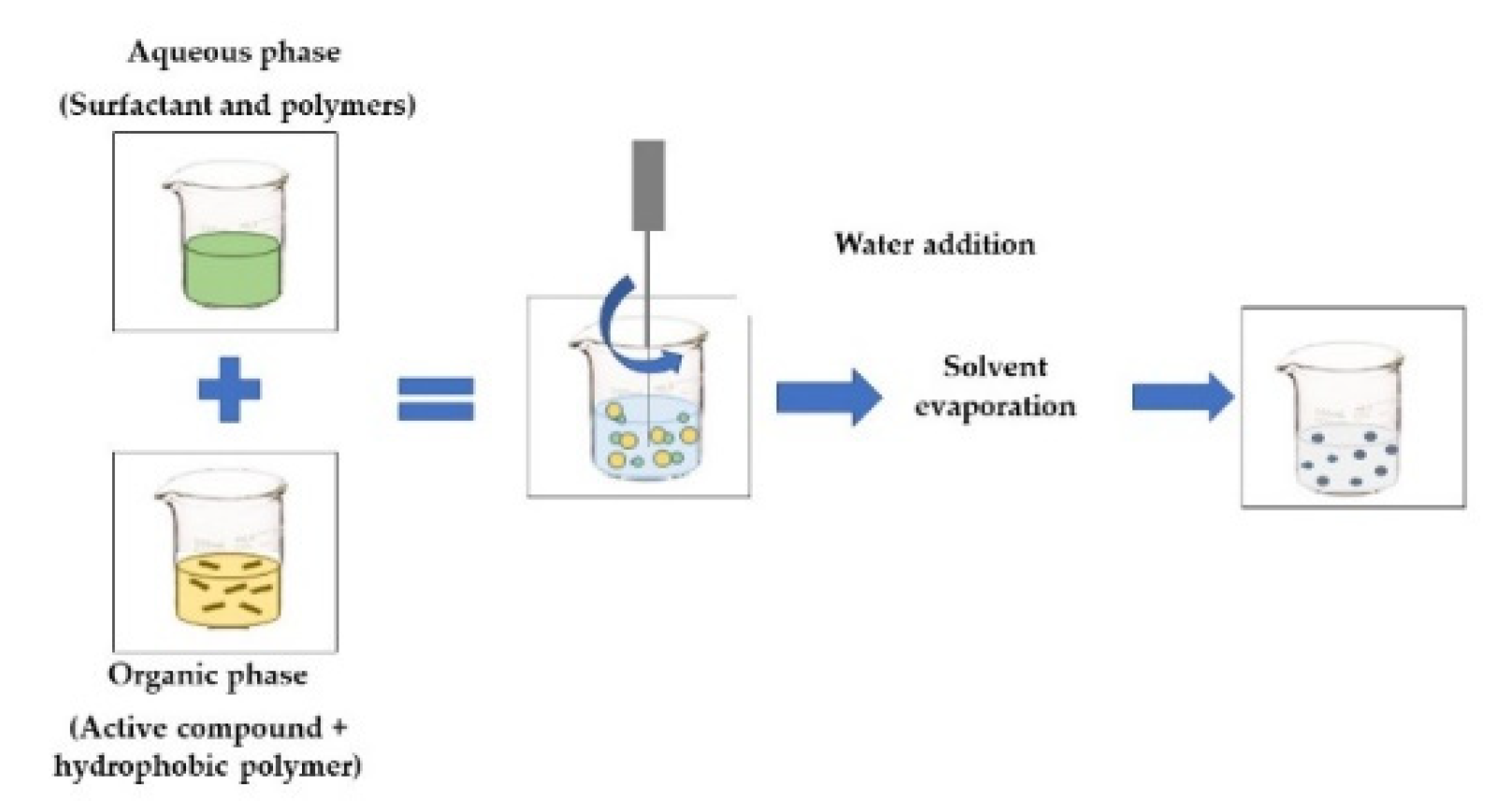 |
| SPRAY-DRYING The sample is passed through a nozzle that forms a mist of fine droplets that atomize in a drying chamber and come into contact with hot air (temperatures of 100–200 °C) that causes spontaneous evaporation of the water. Once dried, the particles are passed through a cyclone or filter, where they are collected in powder form [47]. | 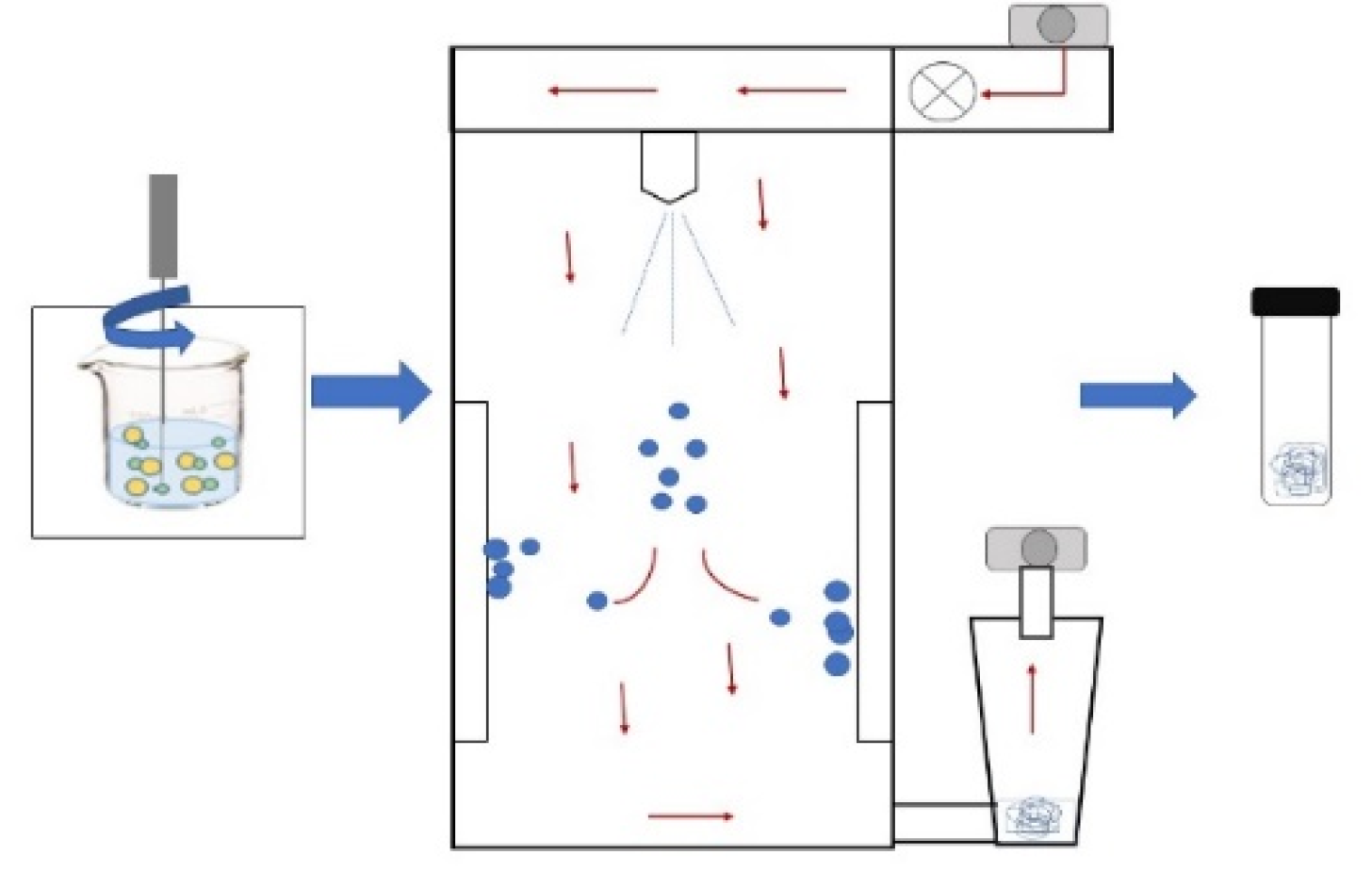 |
| LYOPHILIZATION Nanoparticle dispersions are atomized in a stainless-steel spray tower lined with a liquid nitrogen cooling jacket so that the particles freeze as they fly in the cold air, avoiding contact with the liquid nitrogen. This is a 3-stage process: droplet formation, freezing, and freeze-drying [48]. |  |
| SUPERCRITICAL FLUID EXTRACTION OF EMULSIONS A supercritical fluid is used to rapidly extract the organic phase of a nanoemulsion, in which a bioactive compound and its coating polymer were previously dissolved. Upon removing the solvent, both compounds precipitate, generating a suspension of particles in water [49]. The supercritical fluid chosen should have high affinity for the organic solvent, but negligible affinity for the active compound. Due to the rapid supersaturation of the dissolution medium by the active compound, the latter precipitates on a nanometric scale, encapsulated in the surfactant material [50]. |  |
| MICROFLUIDIC This method helps to reduce problems to obtain liposomes such as poly-disperse in size and lamellarity. It could be solved by applying different flow rate ratios (FRR) and the total flow rate (TFR) using a volume of fluids around 10−9–10−8 l within channels with dimensions of 10–100 mm. An aqueous buffer and solvent with lipid solution is necessary [51] | 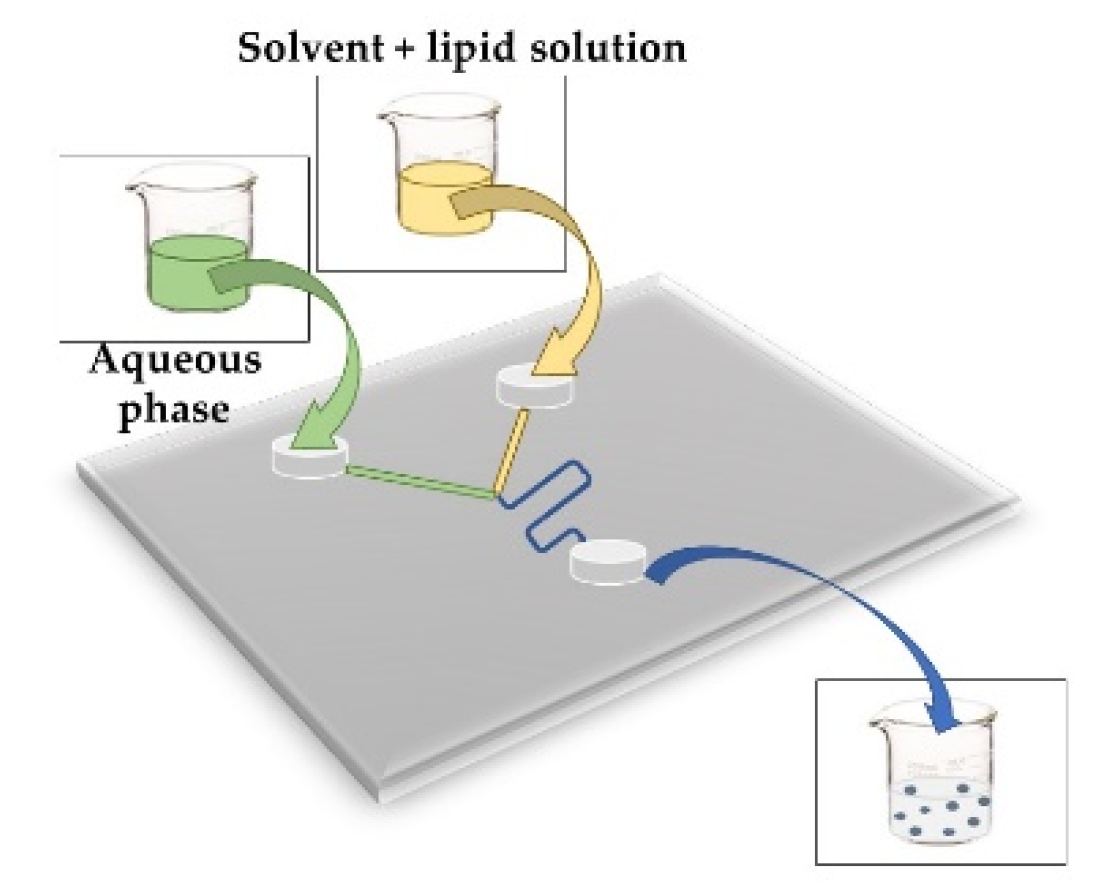 |
| Nanosystem | Applications and Effects | Product | Ref. |
|---|---|---|---|
| Chitosan–vitamin C nanoparticle complexes CS–VC NP (23.2–82 nm) | Compared to controls and groups of meat treated only with CS or VC, the samples treated with CS–VC NP showed a good effect on delaying peroxidation by extending the induction period thanks to their antioxidant properties. CS–VC NP were incorporated onto the meat by dipping, then stored at 4 °C for 21 days. | Fresh ground meat | [57] |
| Chitosan–sodium tripolyphosphate nanoparticles CS–TPP NP | A CS–TPP NP solution was developed and applied to shrimp by vacuum tumbling. Quality characteristics were evaluated for 120 days during frozen storage (−20 °C). The CS-TPP NP treatments generated the highest reduction in lipid oxidation compared to other treatments under the same conditions since it showed the lowest TBARS value. | Shrimp | [58] |
| Cinnamon essential oil–chitosan nanoparticles CEO–CS NP (235.6 nm) | The efficacy of encapsulated CEO as a natural antioxidant that inhibits primary and secondary lipid oxidation in beef patties was assessed by measuring PV and TBARS values. The authors reported that CEO encapsulation can effectively retard lipid oxidation of beef patties even at low concentrations (0.05 and 0.1%) during storage at 4 °C for 8 days. At the end of storage, the 0.1 CEO–CS NP treatment had lower PV and TBARS values than the other treatments and the greatest resistance to oxidation. | Beef patties | [8] |
| Lyophilized pomegranate peel nanoparticles LPP NP (80 nm) | PV and TBARS values were lower in the LPP NP-treated samples than in the control for 15 days. LPP NP were added to the samples as part of the formulation of the meat-balls. The meat was stored at 4 °C for 15 days. | Meatballs | [59] |
| Chitosan–tripolyphosphate nanoparticles CS–TPP NP (12.6–321.5 nm) | CS-TPP NP added to the formulation of surimi exhibited an inhibitory role against lipid oxidation by reducing TBARS and the formation of conjugated dienes during 9 days of storage at 4 °C. | Surimi | [60] |
| Nerolidol nanospheres Ne NS (133 nm) | Nile tilapia fish were fed dietary supplements that contained 0.5 and 1 mL of nanoencapsulated nerolidol/Kg. Significant reductions in meat ROS and LPO levels were seen in the fish fed the 1.0-mL Ne NS/kg, suggesting a lower free radical content and reduced lipid damage, respectively. | Nile tilapia fish (Oreochromis niloticus) | [61] |
| Eugenol nanocapsules embedded with gelatin–chitosan (229.09 nm) Eug–Gel-CS NC | Eug-Gel-CS NC were applied to chilled pork meat by immersion. This treatment showed a lower rate of increase in TBARS than control samples. A fat-soluble free radical scavenger, Eug mediated hydroperoxide-protected muscles from oxidation and acted as an effective natural antioxidant by inhibiting lipid oxidation in the muscle. Storage temperature was 4 °C for 15 days. | Chilled pork meat | [62] |
| ε-polylysine nanoparticles with plant extracts ε-PLN | ɛ-PLN combined with a mixed extract (green tea, stinging nettle, olive leaf) can be used as a potential nitrite replacer in frankfurter-type sausages due to its effective antioxidant activity during refrigerated storage at 4 °C for 45 days. The authors reported that the sausages containing ε-PLN and the extract mixture had significantly lower TBARS values after 45 days. At the end of storage, samples of the sausage with ε-PLN had a TBARS value of 2.39, which is within acceptable limits (2–2.5 mg MDA/kg). Neither the meat itself nor the meat products tested showed rancidity. | Frankfurter-type sausages | [63] |
| Zinc nanoparticles Zn NP | Results indicated that feeding chicken broilers Zn NPs reduced the MDA content of breast meat, suggesting lower oxidative damage and lipid peroxidation due to improvement in the broilers’ antioxidant status. | Breast and thigh chicken fillets | [64] |
| Quercetin nanoparticles QT NPz | Five dietary treatments were formulated with increasing percentages of QT NPs (0, 100, 200, 300, and 400 mg/kg of diet). ROS production and malondialdehyde (MDA) content in muscle were reduced markedly at higher levels of dietary QT NP supplementation, suggesting a decrease in free radical contents and less lipid damage. Administering QT NP-enriched diets decreased ROS production by increasing cellular resistance to oxidative stress, causing a subsequent decrease in lipid peroxidation and higher numbers of healthy cells. | Nile tilapia fish (Oreochromis niloticus) | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulloa-Saavedra, A.; García-Betanzos, C.; Zambrano-Zaragoza, M.; Quintanar-Guerrero, D.; Mendoza-Elvira, S.; Velasco-Bejarano, B. Recent Developments and Applications of Nanosystems in the Preservation of Meat and Meat Products. Foods 2022, 11, 2150. https://doi.org/10.3390/foods11142150
Ulloa-Saavedra A, García-Betanzos C, Zambrano-Zaragoza M, Quintanar-Guerrero D, Mendoza-Elvira S, Velasco-Bejarano B. Recent Developments and Applications of Nanosystems in the Preservation of Meat and Meat Products. Foods. 2022; 11(14):2150. https://doi.org/10.3390/foods11142150
Chicago/Turabian StyleUlloa-Saavedra, Araceli, Claudia García-Betanzos, María Zambrano-Zaragoza, David Quintanar-Guerrero, Susana Mendoza-Elvira, and Benjamín Velasco-Bejarano. 2022. "Recent Developments and Applications of Nanosystems in the Preservation of Meat and Meat Products" Foods 11, no. 14: 2150. https://doi.org/10.3390/foods11142150







