Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application
Abstract
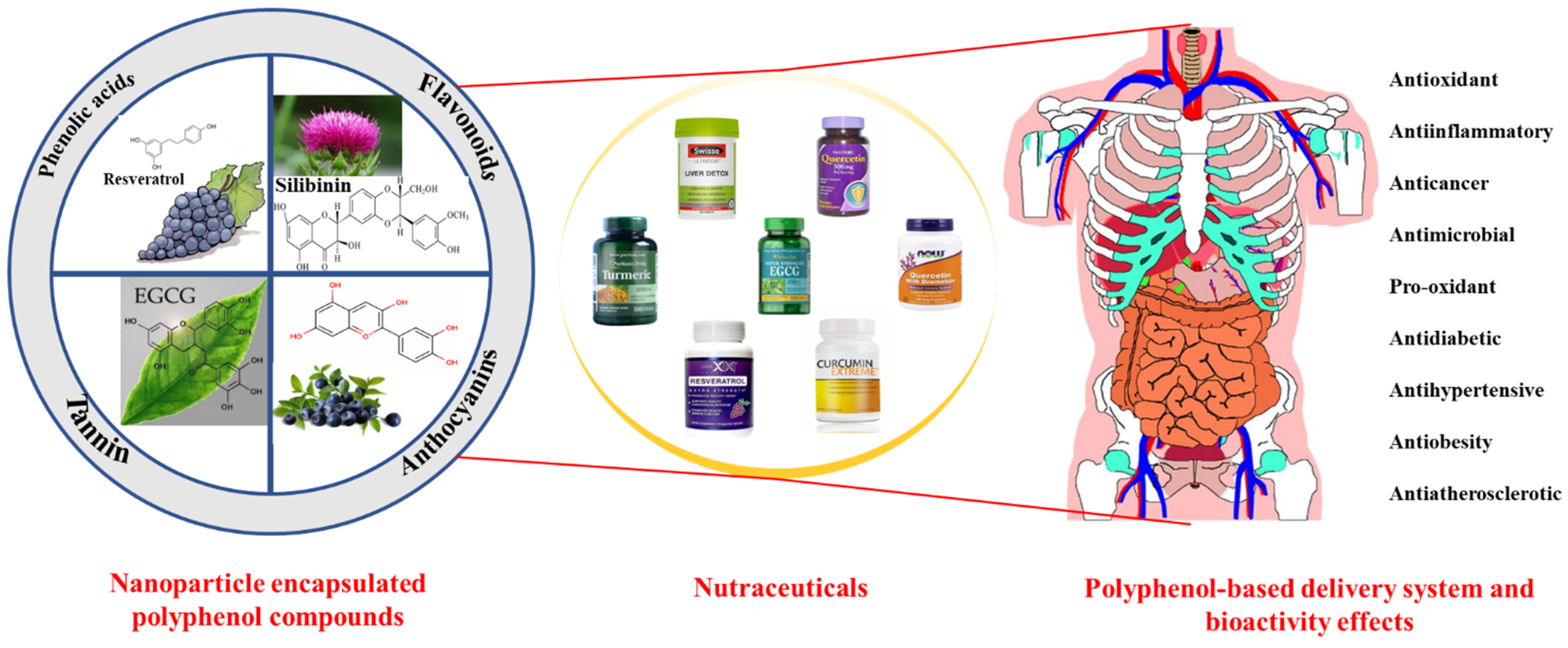
:1. Introduction
2. Classification, Properties, and Beneficial Effects of Plant Polyphenols
2.1. Classification and Properties
2.2. Beneficial Effects
2.2.1. Antioxidant Effect
2.2.2. Anti-Inflammatory Effect
2.2.3. Anticancer Effect
2.2.4. Antimicrobial Effect
2.2.5. Pro-Oxidant Effect
2.2.6. Antidiabetic Effect
2.2.7. Antihypertensive Effect
2.2.8. Antiobesity Effect
2.2.9. Antiatherosclerotic Effect
3. Common Bio-Based Polymer Nano-Delivery of Polyphenolic Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Qiu, C.; Li, X.; McClements, D.J.; Jiao, A.; Wang, J.; Jin, Z. Advances in research on interactions between polyphenols and biology-based nano-delivery systems and their applications in improving the bioavailability of polyphenols. Trends Food Sci. Technol. 2021, 116, 492–500. [Google Scholar] [CrossRef]
- Guo, Q.; Xiao, X.; Lu, L.; Ai, L.; Xu, M.; Liu, Y.; Goff, H. Polyphenol-Polysaccharide Complex: Preparation, Characterization, and Potential Utilization in Food and Health. Annu. Rev. Food Sci. Technol. 2022, 13, 59–87. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Rezaei, M.; McClements, D.J. Bioactive functional ingredients from aquatic origin: A review of recent progress in marine-derived nutraceuticals. Crit. Rev. Food Sci. 2022, 62, 1242–1269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Wang, J.; Jin, Z.; Qiu, C. A review of nanostructured delivery systems for the encapsulation, protection, and delivery of silymarin: An emerging nutraceutical. Food Res. Int. 2022, 156, 111314. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Chen, Y. Polyphenol supplementation benefits human health via gut microbiota: A systematic review via meta-analysis (Review). J. Funct. Foods 2020, 66, 103829. [Google Scholar] [CrossRef]
- Ashwin, K.; Pattanaik, A.K.; Howarth, G.S. Polyphenolic bioactives as an emerging group of nutraceuticals for promotion of gut health: A review. Food Biosci. 2021, 44, 101376. [Google Scholar] [CrossRef]
- Fan, G.; Beta, T. Discrimination of geographical origin of Napirira bean (Phaseolus vulgaris L.) based on phenolic profiles and antioxidant activity. J. Food Compos. Anal. 2017, 62, 217–222. [Google Scholar] [CrossRef]
- Mehmood, A.; Usman, M.; Patil, P.; Zhao, L.; Wang, C. A review on management of cardiovascular diseases by olive polyphenols. Food Sci. Nutr. 2020, 8, 4639–4655. [Google Scholar] [CrossRef]
- Delmas, D.; Hichami, A.; Rebe, C.; Ghiringhelli, F. Immunomodulation and Anti-inflammatory Roles of Polyphenols as Anticancer Agents. Anti-Cancer Agent Med. 2012, 12, 852–873. [Google Scholar] [CrossRef]
- Qiu, C.; Hu, Y.; Jin, Z.; McClements, D.J.; Qin, Y.; Xu, X.; Wang, J. A review of green techniques for the synthesis of size-controlled starch-based nanoparticles and their applications as nanodelivery systems (Review). Trends Food Sci. Technol. 2019, 92, 138–151. [Google Scholar] [CrossRef]
- García-Conesa, M.; Larrosa, M. Polyphenol-rich foods for human health and disease (Editorial). Nutrients 2020, 12, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, G.; Wang, X.; Wu, C.; Pan, H.; Yang, J.; Li, T.; Cao, F. Effect of heating on the content and composition of ginkgolic acids in ginkgo seeds. Qual. Assur. Saf. Crop. 2017, 9, 195–199. [Google Scholar] [CrossRef]
- Thilakarathna, W.W.; Langille, M.G.; Rupasinghe, H.V. Polyphenol-based prebiotics and synbiotics: Potential for cancer chemoprevention (Review). Curr. Opin. Food Sci. 2018, 20, 51–57. [Google Scholar] [CrossRef]
- Guan, T.; Zhang, Z.; Li, X.; Cui, S.; McClements, D.J.; Wu, X.; Chen, L.; Long, J.; Jiao, A.; Qiu, C.; et al. Preparation, Characteristics, and Advantages of Plant Protein-Based Bioactive Molecule Delivery Systems. Foods 2022, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Hundshammer, C.; Schön, C.; Kimura, M.; Furune, T.; Terao, K.; Elgeti, D.; Mohr, R. Enhanced metabolic bioavailability of tetrahydrocurcumin after oral supplementation of a γ-cyclodextrin curcumin complex. J. Funct. Foods 2021, 79, 104410. [Google Scholar] [CrossRef]
- Naksuriya, O.; Okonogi, S.; Schiffelers, R.M.; Hennink, W.E. Curcumin nanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials 2014, 35, 3365–3383. [Google Scholar] [CrossRef]
- Bennourab, N.; Mighria, H.; Eljania, H.; Zammouria, T.; Akrouta, A. Effect of solvent evaporation method on phenolic compounds and the antioxidant activity of Moringa oleifera cultivated in Southern Tunisia. S. Afr. J. Bot. 2020, 129, 181–190. [Google Scholar] [CrossRef]
- Rashmi, H.B.; Negi, P.S. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef]
- Petrillo, A.D.; Orru, G.; Fais, A.; Fantini, M.C. Quercetin and its derivates as antiviral potentials: A comprehensive review. Phytother. Res. 2022, 36, 266–278. [Google Scholar] [CrossRef]
- Semwal, R.; Joshi, S.K.; Semwal, R.B.; Semwal, D.K. Health benefits and limitations of rutin-A natural flavonoid with high nutraceutical value. Phytochem. Lett. 2021, 46, 119–128. [Google Scholar] [CrossRef]
- Ibrahim, A.; Nasr, M.; El-Sherbiny, I.M. Baicalin as an emerging magical nutraceutical molecule: Emphasis on pharmacological properties and advances in pharmaceutical delivery. J. Drug Deliv. Sci. Technol. 2022, 70, 103269. [Google Scholar] [CrossRef]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; El-Ghorab, A.; Ahmad, I.; Pezzani, R.; Arshad, M.U.; et al. Myricetin: A comprehensive review on its biological potentials. Food Sci. Nutr. 2021, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Farsad, N.A.; Mohammad, A. Antioxidant properties of the flavonoid fisetin: An updated review of in vivo and in vitro studies. Trends Food Sci. Technol. 2017, 70, 34–44. [Google Scholar] [CrossRef]
- Ramalingam, M.; Kim, H.; Lee, Y.; Lee, Y.I. Phytochemical and Pharmacological role of liquiritigenin and isoliquiritigenin from radix glycyrrhizae in human health and disease models. Front. Aging Neurosci. 2018, 10, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Wang, F.; Zhou, R.; Song, X.; Xie, M. Apigenin: A current review on its beneficial biological activities. J. Food Biochem. 2017, 41, e12376. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ahmad, N.; Ahmed, Z.; Siddique, R.; Zeng, X.; Rahaman, A.; Muhammad, A.R.; Wahab, A. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J. Food Biochem. 2019, 43, e12974. [Google Scholar] [CrossRef]
- Thakur, K.; Zhu, Y.; Feng, J.; Zhang, J.; Hu, F.; Prasad, C.; Wei, Z. Morin as an imminent functional food ingredient: An update on its enhanced efficacy in the treatment and prevention of metabolic syndromes. Food Funct. 2020, 11, 8424–8443. [Google Scholar] [CrossRef]
- Mayo, B.; Vzquez, L.; Flrez, A.B. Equol: A Bacterial Metabolite from The Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients 2019, 11, 2231. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, S.; Wang, S.; Gao, P.; Dai, L. A comprehensive review on Pueraria: Insights on its chemistry and medicinal value. Biomed. Pharmacother. 2020, 131, 110734. [Google Scholar] [CrossRef]
- Leis, K.; Kulczyńska, A.; Racinowski, M.; Kaczor, P.; Golebiewski, J.; Januszko-Giergielewicz, B. Genistein-a supplement improving efficiency of the human body: A review. Sci. Sport 2021, 36, 359–367. [Google Scholar] [CrossRef]
- Raheja, S.; Girdhar, A.; Lather, V.; Pandita, D. Biochanin A: A phytoestrogen with therapeutic potential (Review). Trends Food Sci. Technol. 2018, 79, 55–66. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, R.; Shi, W.; Li, L.; Liu, H.; Liu, Z.; Wu, L. The Multifunctional Benefits of Naturally Occurring Delphinidin and Its Glycosides. J. Agric. Food Chem. 2019, 67, 11288–11306. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Chen, L.; Qian, L.; Lin, X.; Fan, X.; Teng, H.; Cao, H. Fabrication of caseins nanoparticles to improve the stability of cyanidin 3-O-glucoside. Food Chem. 2020, 317, 126418. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Schluesener, H. Health-promoting effects of the citrus flavanone hesperidin. Crit. Rev. Food Sci. 2017, 57, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.H.; Augustin, M.A. Nano- and micro-particles for delivery of catechins: Physical and biological performance. Crit. Rev. Food Sci. 2019, 59, 1563–1579. [Google Scholar] [CrossRef]
- Xiao, Y.; Lee, I. Effects of Microbial Transformation on the Biological Activities of Prenylated Chalcones from Angelica keiskei. Foods 2022, 11, 543. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Druzga, A.; Katarzyna, J.; Skonieczna-Zydecka, K. Antioxidant Potential of Curcumin-A Meta-Analysis of Randomized Clinical Trials. Antioxidants 2020, 9, e1092. [Google Scholar] [CrossRef]
- Grzesik, M.; Naparlo, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Song, H.; Wang, Q.; He, A.; Li, S.; Guan, X.; Hu, Y.; Feng, S. Antioxidant activity, storage stability and in vitro release of epigallocatechin-3-gallate (EGCG) encapsulated in hordein nanoparticles. Food Chem. 2022, 388, 132903. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Wu, S. Health Benefits of Silybum marianum: Phytochemistry, Pharmacology, and Applications. J. Agric. Food Chem. 2020, 68, 11644–11664. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, X.; Zhang, N.; Zhou, Q.; Fan, D.; Wang, M. Lipophilized apigenin derivatives produced during the frying process as novel antioxidants. Food Chem. 2022, 379, 132178. [Google Scholar] [CrossRef] [PubMed]
- Sneharani, A.H. Curcumin-sunflower protein nanoparticles—A potential antiinflammatory agent. J. Food Biochem. 2019, 43, e12909. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; McClements, D.J.; Jin, Z.; Qin, Y.; Hu, Y.; Xu, X.; Wang, J. Resveratrol-loaded core-shell nanostructured delivery systems: Cyclodextrin-based metal-organic nanocapsules prepared by ionic gelation. Food Chem. 2020, 317, 126328. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Huang, S.; Chen, Y.; Wang, Q.; Luo, S.; Li, Y.; Wang, X.; Chen, J.; Luo, X.; Zhou, L. Synergistic effect of combined treatment with baicalin and emodin on DSS-induced colitis in mouse. Phytother. Res. 2021, 35, 5708–5719. [Google Scholar] [CrossRef]
- Singhai, A.K.; Malik, J.; Sont, H. Antimicrobial and Antiinflammatory Activity of the Hydrogels Containing Rutin Delivery. Asian J. Chem. 2013, 25, 8371–8373. [Google Scholar] [CrossRef]
- Ding, H.; Huang, A.; Zhang, Y.; Li, B.; Huang, C.; Ma, T.; Meng, X.; Li, J. Design, synthesis and biological evaluation of hesperetin derivatives as potent anti-inflammatory agent. Fitoterapia 2017, 121, 212–222. [Google Scholar] [CrossRef]
- Stanca, E.; Serviddio, G.; Bellanti, F.; Vendemiale, G.; Siculella, L.; Giudetti, A.M. Down-regulation of LPCAT expression increases platelet-activating factor level in cirrhotic rat liver: Potential antiinflammatory effect of silybin. BBA-Mol. Basis Dis. 2019, 1832, 2019–2026. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Lee, Y.J. Synergistic anticancer activity of resveratrol in combination with docetaxel in prostate carcinoma cells. Nutr. Res. Pract. 2021, 15, 12–25. [Google Scholar] [CrossRef]
- Davoodvandi, A.; Shabani, V.M.; Clark, C.C.T.; Jafarnejad, S. Quercetin as an anticancer agent: Focus on esophageal cancer. J. Food Biochem. 2020, 44, e1337. [Google Scholar] [CrossRef]
- Liu, H.T.; Ho, Y.S. Anticancer effect of curcumin on breast cancer and stem cells. Food Sci. Hum. Well. 2018, 7, 134–137. [Google Scholar] [CrossRef]
- Bimonte, S.; Cascella, M.; Barbieri, A.; Arra, C.; Cuomo, A. Current shreds of evidence on the anticancer role of EGCG in triple negative breast cancer: An update of the current state of knowledge. Infect. Agents Cancer 2020, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Verebova, V.; Benes, J.; Stanicova, J. Biophysical Characterization and Anticancer Activities of Photosensitive Phytoanthraquinones Represented by Hypericin and Its Model Compounds. Molecules 2020, 25, E5666. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.; Bhattacharjee, M.; Bhattacharya, S.; Ahir, M.; Adhikary, A.; Patra, P. Silymarin-Loaded, Lactobionic Acid-Conjugated Porous PLGA Nanoparticles Induce Apoptosis in Liver Cancer Cells. ACS Appl. Bio Mater. 2020, 3, 7178–7192. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Y.; Jiang, X.; Wu, J.; Le, X. Molecular interactions, characterization and antimicrobial activity of curcumin-chitosan blend films (Article). Food Hydrocolloid. 2016, 52, 564–572. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, T. Antimicrobial Activities of Tea Polyphenol on Phytopathogens: A Review. Molecules 2019, 24, 816. [Google Scholar] [CrossRef] [Green Version]
- Canedo-Santos, J.C.; Carrillo-Garmendia, A.; Mora-Martinez, I.; Gutierrez-Garcia, I.K.; Ramirez-Romero, M.G.; Regalado-Gonzalez, C.; Nava, G.M.; Madrigal-Perez, L.A. Resveratrol shortens the chronological lifespan of Saccharomyces cerevisiae by a pro-oxidant mechanism. Yeast 2022, 39, 193–207. [Google Scholar] [CrossRef]
- Cao, Y.; True, A.D.; Chen, J.; Xiong, Y. Dual Role (Anti- and Pro-oxidant) of Gallic Acid in Mediating Myofibrillar Protein Gelation and Gel in vitro Digestion. J. Agric. Food Chem. 2016, 64, 3054–3061. [Google Scholar] [CrossRef]
- Chen, W.; Ma, X.; Lin, Y.; Xiong, Y.; Zheng, C.; Hu, Y.; Yu, D.; Jiang, Z. Dietary supplementation with a high dose of daidzein enhances the antioxidant capacity in swine muscle but experts pro-oxidant function in liver and fat tissues. J. Anim. Sci. Biotechnol. 2016, 7, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Lucas, M.; Freitas, M.; Xavier, J.A.; Moura, F.A.; Goulart, M.O.F.; Ribeiro, D.; Fernandes, E. The scavenging effect of curcumin, piperine and their combination against physiological relevant reactive pro-oxidant species using in vitro non-cellular and cellular models. Chem. Pap. 2021, 75, 5269–5277. [Google Scholar] [CrossRef]
- Natarajan, S.B.; Chandran, S.P.; Khan, S.H.; Natarajan, P.; Rengarajan, K. Versatile Health Benefits of Catechin from Green Tea (Camellia sinensis). Curr. Res. Nutr. Food Sci. 2019, 15, 3–10. [Google Scholar] [CrossRef]
- Liu, B.; Kang, Z.; Yan, W. Synthesis, Stability, and Antidiabetic Activity Evaluation of (-)-Epigallocatechin Gallate (EGCG) Palmitate Derived from Natural Tea Polyphenols. Molecules 2021, 26, E393. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Orhan, I.E.; Bawazeer, S. Health perspectives of a bioactive compound curcumin: A review. Trends Food Sci. Technol. 2018, 74, 33–45. [Google Scholar] [CrossRef]
- Eid, H.M.; Haddad, P.S. The antidiabetic potential of quercetin: Underlying mechanisms. Curr. Med. Chem. 2017, 24, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jo, C.; Choi, H.; Lee, K. Effect of Co-Administration of Curcumin with Amlodipine in Hypertension. Nutrients 2021, 13, 2797. [Google Scholar] [CrossRef] [PubMed]
- Hayat, K.; Iqbal, H.; Malik, U.; Bilal, U.; Mushtaq, S. Tea and Its Consumption: Benefits and Risks. Crit. Rev. Food Sci. 2015, 55, 939–954. [Google Scholar] [CrossRef]
- Muguerza, B.; Quinones, M.; Suarez, M.; Aleixandre, A.; Pons, Z.; Arola, L.; Guerrero, L. Low-molecular procyanidin rich grape seed extract exerts antihypertensive effect in males spontaneously hypertensive rats. Food Res. Int. 2013, 51, 587–595. [Google Scholar] [CrossRef]
- Huang, W.Y.; Davidge, S.T.; Wu, J.P. Bioactive Natural Constituents from Food Sources-Potential Use in Hypertension Prevention and Treatment. Crit. Rev. Food Sci. 2013, 53, 615–630. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, G.; Su, X.; Yang, H.; Zhang, J. Antiobesity action of a daidzein derivative on male obese mice induced by a high-fat diet. Nutr. Res. 2009, 29, 656–663. [Google Scholar] [CrossRef]
- Ting, Y.; Chang, W.; Shiau, D.K.; Chou, P.; Wu, M.; Hsu, C.L. Antiobesity Efficacy of Quercetin-Rich Supplement on Diet-Induced Obese Rats: Effects on Body Composition, Serum Lipid Profile, and Gene Expression. J. Agric. Food Chem. 2018, 66, 70–80. [Google Scholar] [CrossRef]
- Rains, T.M.; Agarwal, S.; Maki, K.C. Antiobesity effects of green tea catechins: A mechanistic review. J. Nutr. Biochem. 2011, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, I.; Piemontese, A.; Bernini, F.; Rio, D.D. Evaluation of antiatherosclerotic effects of ellagic acid metabolites in cultured macrophages. Atherosclerosis 2014, 235, e113–e114. [Google Scholar] [CrossRef]
- Tanaka, M.; Zhao, J.; Suyama, A.; Matsui, T. Epigallocatechin Gallate Promotes the Vasorelaxation Power of the Antiatherosclerotic Dipeptide Trp-His in Contracted Rat Aorta. J. Agric. Food Chem. 2012, 60, 9048–9054. [Google Scholar] [CrossRef]
- Zheng, S.; Feng, Q.; Cheng, J.; Zheng, J. Maternal resveratrol consumption and its programming effects on metabolic health in offspring mechanisms and potential implications. Biosci. Rep. 2018, 38, BSR20171741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunes-Bayir, A.; Toprak, A.; Kiziltan, H.S.; Kocyigit, A.; Karatas, E.; Guler, E.M. Effects of natural phenolic compound carvacrol on the human gastric adenocarcinoma (AGS) cells in vitro. Anti-Cancer Drug 2017, 28, 522–530. [Google Scholar] [CrossRef]
- Domínguez, A.J.A.; Rodrigo, G.J.; González, A.G.A.; Rosa, L.A. The Antidiabetic Mechanisms of Polyphenols Related to Increased Glucagon-Like Peptide-1 (GLP1) and Insulin Signaling (Review). Molecules 2017, 22, 903. [Google Scholar] [CrossRef] [Green Version]
- Vayalil, P.K. Date Fruits (Phoenix dactylifera Linn): An Emerging Medicinal Food. Crit. Rev. Food Sci. 2012, 52, 249. [Google Scholar] [CrossRef]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef]
- Sung, H.; Hong, C.; Suh, Y.S. Role of (-)-epigallocatechin-3-gallate in cell viability, lipogenesis, and retinol-binding protein 4 expression in adipocytes. N-S Arch. Pharmacol. 2010, 382, 303–310. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Ebrahimi, F.; Farzaei, M.H.; Baratpourmoghaddam, A.; Ahmadi, P.; Rostamiasrabadi, P.; Rasouli, A.; Amir, H.; Rahimi, R. Dietary polyphenols for atherosclerosis: A comprehensive review and future perspectives. Crit. Rev. Food Sci. 2019, 59, 114–132. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, J.; Zhang, H.; Qin, Y.; Xu, X.; Jin, Z. Novel Approach with Controlled Nucleation and Growth for Green Synthesis of Size-Controlled Cyclodextrin-Based Metal-Organic Frameworks Based on Short-Chain Starch Nanoparticles. J. Agric. Food Chem. 2018, 66, 9785–9793. [Google Scholar] [CrossRef] [PubMed]
- Kasaai, M.R. Zein and zein -based nano-materials for food and nutrition applications: A review (Review). Trends Food Sci. Technol. 2018, 79, 184–197. [Google Scholar] [CrossRef]
- Tang, C.H. Nanostructured soy proteins: Fabrication and applications as delivery systems for bioactives (a review). Food Hydrocolloid. 2019, 91, 92–116. [Google Scholar] [CrossRef]
- Kelemen, V.; Pichler, A.; Ivić, I.; Buljeta, I.; Šimunović, J.; Kopjar, M. Brown rice proteins as delivery system of phenolic and volatile compounds of raspberry juice. Int. J. Food Sci. Technol. 2022, 57, 1866–1874. [Google Scholar] [CrossRef]
- Zang, J.C.; Chen, H.; Zhao, G.H.; Wang, F.D.; Ren, F.Z. Ferritin cage for encapsulation and delivery of bioactive nutrients: From structure, property to applications. Crit. Rev. Food Sci. 2017, 57, 3673–3683. [Google Scholar] [CrossRef] [PubMed]
- Visentini, F.F.; Perez, A.A.; Santiago, L.G. Bioactive compounds: Application of albumin nanocarriers as delivery systems. Crit. Rev. Food Sci. 2022, 63, 1–31. [Google Scholar] [CrossRef]
- Silvia, V.; Massimo, F.; Donato, C. Gliadins as versatile biomaterials for drug delivery applications. J. Control. Release 2021, 329, 385–400. [Google Scholar] [CrossRef]
- Sadiq, U.; Gill, H.; Chandrapala, J. Casein Micelles as an Emerging Delivery System for Bioactive Food Components. Foods 2021, 10, 1965. [Google Scholar] [CrossRef]
- Ha, H.K.; Rankin, S.A.; Lee, M.R.; Lee, W.J.; Luo, Y.C. Development and Characterization of Whey Protein-Based Nano-Delivery Systems: A Review. Molecules 2019, 24, 3254. [Google Scholar] [CrossRef] [Green Version]
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Starch-based nanocarriers as cutting-edge natural cargos for nutraceutical delivery (Review). Trends Food Sci. Technol. 2019, 88, 397–415. [Google Scholar] [CrossRef]
- Mu, R.J.; Hong, X.; Ni, Y.S.; Li, Y.Z.; Pang, J.; Wang, Q.; Xiao, J.B.; Zheng, Y.F. Recent trends and applications of cellulose nanocrystals in food industry. Trends Food Sci. Technol. 2019, 93, 136–144. [Google Scholar] [CrossRef]
- Christian, J.W.; Suryadi, I.; Setiyo, G. A Review of Lignocellulosic-Derived Nanoparticles for Drug Delivery Applications: Lignin Nanoparticles, Xylan Nanoparticles, and Cellulose Nanocrystals. Molecules 2021, 26, 676. [Google Scholar] [CrossRef]
- Tie, S.; Tan, M. Current Advances in Multifunctional Nanocarriers Based on Marine Polysaccharides for Colon Delivery of Food Polyphenols. J. Agric. Food Chem. 2022, 70, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, V.; Vadivelu, J.; Saravanan, S.; Al-Maleki, A.R.; Ramesh, M. A review of natural polysaccharides for drug delivery applications: Special focus on cellulose, starch and glycogen. Biomed. Pharmacother. 2018, 107, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Lu, T.; Qin, Y.; He, Y.; Cui, N.; Du, A.; Sun, J. In Vivo Effect of Resveratrol-Loaded Solid Lipid Nanoparticles to Relieve Physical Fatigue for Sports Nutrition Supplements. Molecules 2020, 25, E5302. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, Z.; Liu, H.; Hu, L. Nanoemulsion-based delivery approaches for nutraceuticals: Fabrication, application, characterization, biological fate, potential toxicity and future trends. Food Funct. 2021, 12, 1933–1953. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Azadmard-Damirchi, S.; Peighambardoust, S.H.; Valizadeh, H.; Hesari, J. Liposomes as carrier vehicles for functional compounds in food sector (Article). J. Exp. Nanosci. 2016, 11, 737–759. [Google Scholar] [CrossRef]
- Rambaran, T.F. A patent review of polyphenol nano-formulations and their commercialization. Trends Food Sci. Technol. 2022, 120, 111–122. [Google Scholar] [CrossRef]
- Ba, C.; Fu, Y.; Niu, F.; Wang, M.; Jin, B.; Li, Z.; Chen, G.; Zhang, H.; Li, X. Effects of environmental stresses on physiochemical stability of β-carotene in zein-carboxymethyl chitosan-tea polyphenols ternary delivery system. Food Chem. 2020, 311, 125878. [Google Scholar] [CrossRef]
- Lozano-Pérez, A.A.; Rivero, H.C.; Pérez, H.; María, C.; Pagán, A.; Montalbán, M.G.; Víllora, G.; Cénis, J.L. Silk fibroin nanoparticles: Efficient vehicles for the natural antioxidant quercetin. Int. J. Pharmaceut. 2017, 518, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Solghi, S.; Emam-Djomeh, Z.; Fathi, M.; Farahani, F. The encapsulation of curcumin by whey protein: Assessment of the stability and bioactivity. J. Food Process. Eng. 2020, 43, 1–10. [Google Scholar] [CrossRef]
- Marziyeh, S.; Hamed, N.; Elham, J.; Faezeh, A.; Hamidreza, K.M.; Soodabeh, D.; Hossein, D. Production of biological nanoparticles from bovine serum albumin as controlled release carrier for curcumin delivery. Int. J. Biol. Macromol. 2018, 115, 83–89. [Google Scholar] [CrossRef]
- Barclay, T.G.; Day, C.M.; Petrovsky, N.; Garg, S. Review of polysaccharide particle-based functional drug delivery. Carbohyd. Polym. 2019, 221, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xue, L.; Hu, Y.; Qiu, C.; Jin, Z.; Xu, X.; Wang, J. Green fabrication and characterization of debranched starch nanoparticles via ultrasonication combined with recrystallization. Ultrason. Sonochem. 2020, 66, 105074. [Google Scholar] [CrossRef] [PubMed]
- Geetha, K.A.; Alummoottil, N.J. Cassava starch-poly (vinyl alcohol) nanocomposites for the controlled delivery of curcumin in cancer prevention and treatment. Starch-Starke 2015, 67, 549–558. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Puligundla, P.; Gao, X.; Zhou, Y.; Wan, X. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: A review. Food Hydrocoll. 2017, 69, 286–292. [Google Scholar] [CrossRef]
- Talarico, L.; Consumi, M.; Leone, G.; Tamasi, G.; Magnani, A. Solid Lipid Nanoparticles Produced via a Coacervation Method as Promising Carriers for Controlled Release of Quercetin. Molecules 2021, 26, 2694. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, D.; Li, F.; Li, D.; Huang, Q. Cinnamon essential oil Pickering emulsion stabilized by zein-pectin composite nanoparticles: Characterization, antimicrobial effect and advantages in storage application. Int. J. Biol. Macromol. 2020, 148, 1280–1289. [Google Scholar] [CrossRef]
- Chen, S.; Li, Q.; McClements, D.J.; Han, Y.; Dai, L.; Mao, L.; Gao, Y. Co-delivery of curcumin and piperine in zein-carrageenan core-shell nanoparticles: Formation, structure, stability and in vitro gastrointestinal digestion. Food Hydrocoll. 2020, 99, 105334. [Google Scholar] [CrossRef]
- Pauluk, D.; Padilha, A.K.; Khalil, N.M.; Mainardes, R.M. Chitosan-coated zein nanoparticles for oral delivery of resveratrol: Formation, characterization, stability, mucoadhesive properties and antioxidant activity (Article). Food Hydrocoll. 2019, 94, 411–417. [Google Scholar] [CrossRef]
- Dai, L.; Zhou, H.; Wei, Y.; Gao, Y.; McClements, D.J. Curcumin encapsulation in zein-rhamnolipid composite nanoparticles using a pH-driven method. Food Hydrocoll. 2019, 93, 342–350. [Google Scholar] [CrossRef]


| Flavonoids | Chemical Structure | Biological and Pharmacological Activity | Refs |
|---|---|---|---|
| Quercetin |  | Promote antioxidant, anti-inflammatory, immunoprotective effects, anticarcinogenic, antidiabetic activities | [19] |
| Rutin |  | Anti-inflammation, antioxidation, antiallergy, antivirus | [20] |
| Baicalin |  | Antibacterial, diuretic, anti-inflammatory, cholesterol-lowering, antithrombotic, asthma relief, fire relief and detoxification, hemostasis | [21] |
| Myricetin |  | Anti-inflammatory, antitumor, antimutagenic, caries prevention, antioxidant properties, elimination of free radicals in the body | [22] |
| Fisetin |  | Anti-inflammatory, antioxidant, anticoagulant, antithrombotic, antispasmodic, treatment of diabetic kidney injury | [23] |
| Liquiritigenin |  | Antispasmodic, anti-ulcer, antibacterial, hepatocyte monoamine oxidase inhibitor | [24] |
| Apigenin |  | Anticancer, antiviral drug, anti-inflammatory, antioxidant, sedative, tranquilizer, antihypertensive | [25] |
| Luteolin |  | Anti-inflammatory, antitumor, antiallergy, treatment of demyelinating disease, anti-inflammatory chemistry, uric acid lowering | [26] |
| Morin |  | Anti-inflammatory, immunomodulatory effect, antitumor effect, antioxidant effect | [27] |
| Daidzein |  | Antioxidant, estrogenic effects | [28] |
| Puerarin |  | Anti-inflammatory, anticancer, cardiovascular disease prevention | [29] |
| Genistein |  | Antioxidant, estrogenic, and antihormonal properties, anticancer activity | [30] |
| Biochanin A |  | Estrogen-like effect, can inhibit the rise of cholesterol, also has anti-fungal and antitumor effects | [31] |
| Delphinidin |  | Antioxidants, which can protect the body from damage caused by harmful substances including free radicals | [32] |
| Cyanidin-3-O-glucoside |  | Antioxidant, antitumor, neuroprotective, restores transient vision loss | [33] |
| Tangeretin |  | Antifungal effect, antitumor, inhibition of smooth muscle contraction | [34] |
| Hesperidin |  | Lower blood pressure, anti-allergy, lower bone density, cholesterol, antibacterial, anti-inflammatory, antihepatitis, antitumor | [35] |
| Catechin |  | Antioxidant, age-delaying, obesity control, antibacterial | [36] |
| Isobavachalcone |  | Antibacterial, anticancer | [37] |
| Beneficial Effect | Polyphenols Examples | References |
|---|---|---|
| Antioxidant | Curcumin | [38] |
| Catechin | [39] | |
| Epigallocatechin gallate (EGCG) | [40] | |
| Silymarin | [41] | |
| Apigenin | [42] | |
| Anti-inflammatory | Curcumin | [43] |
| Resveratrol | [44] | |
| Baicalin | [45] | |
| Rutin | [46] | |
| Hesperetin | [47] | |
| Silybin | [48] | |
| Anticancer | Resveratrol | [49] |
| Quercetin | [50] | |
| Curcumin | [51] | |
| EGCG | [52] | |
| Hypericin | [53] | |
| Silymarin | [54] | |
| Antimicrobial | Curcumin | [55] |
| Silymarin | [41] | |
| Tea polyphenol | [56] | |
| Rutin | [46] | |
| Pro-oxidant | Resveratrol | [57] |
| Gallic acid | [58] | |
| Daidzein | [59] | |
| Curcumin | [60] | |
| Antidiabetic | Catechin | [61] |
| EGCG | [62] | |
| Curcumin | [63] | |
| Quercetin | [64] | |
| Antihypertensive | Curcumin | [65] |
| Tea polyphenol | [66] | |
| Procyanidin | [67] | |
| Resveratrol | [68] | |
| Antiobesity | Daidzein | [69] |
| Curcumin | [63] | |
| Quercetin | [70] | |
| Catechin | [71] | |
| Antiatherosclerotic | Curcumin | [63] |
| Ellagic acid | [72] | |
| EGCG | [73] | |
| Resveratrol | [74] |
| Common Bio-Based Nano-Delivery Systems | Types of Carriers | Materials | Major Outcomes | Refs |
|---|---|---|---|---|
| Protein-based | Nanoparticles Nanogels Nanofilms Nanofibers Nanoemulsion | Zein | Renewable resources with performance and efficiency advantages | [82] |
| Soy protein | Various food active ingredients with high nutritional value, functional activity and health effects | [83] | ||
| Rice protein | High value-added protein complexes | [84] | ||
| Ferritin | Natural iron storage protein with a hollow shell for encapsulation and delivery of bioactive nutrients | [85] | ||
| Albumin | Safe and well-tolerated in humans | [86] | ||
| Gliadin | Natural and sustainable resources; environmentally friendly and safe manufacturing process; good biocompatibility | [87] | ||
| Casein | With both hydrophilic and hydrophobic properties | [88] | ||
| Whey Protein | Binding ability to hydrophobic active substances, gelation and emulsification properties | [89] | ||
| Polysaccharide-based | Nanoparticles Nanogels Nanofilms Nanofibers Nanoemulsion | Starch | Wide range of raw material sources; non-toxic, biocompatible, ideal material choice for nano-delivery carriers | [90] |
| Cellulose | Natural fiber extraction, high crystallinity and high Young’s modulus | [91] | ||
| Lignin | Amphiphilic nanoparticles with multiple interactions with hydrophobic and hydrophilic polyphenols | [92] | ||
| Marine Polysaccharides | Versatility and good biocompatibility as a wall material for colon-targeted delivery of polyphenols for disease intervention | [93] | ||
| Glycogen | Molecular weight is above the renal threshold and is restricted to renal clearance in the blood stream without biodegradation | [94] | ||
| Lipid-based | Solid Lipid Nanoparticles Nanoemulsion Liposomes | Lipid compounds | Enhances the resistance of active molecules to environmental, enzymatic, and chemical static stress; improves intestinal solubility; provides a larger surface-to-mass ratio; increases intestinal absorption | [95,96,97] |
| Nano-Formulation Trade Name | Form | Main Functional Component | Country Manufactured |
|---|---|---|---|
| CurcuminRich Theracurmin® | Capsule | Optimized curcumin | Canada |
| CAVACURCUMIN@ | Capsule | Curcumin | Germany |
| Theracurcumin® | Capsule | Curcumin | USA |
| Turmeric ultra-Nano Curcumin with Piperine | Capsule | Nano curcumin | Jordan |
| Nano Curcumin | Capsule | Nano curcumin | India |
| Nano Curcumin Plus | Capsule | Nano curcumin | USA |
| Nanocurcumin Double Plus | Capsule | Nano curcumin extract | Vietnam |
| Nano-curcumin | Liquid (Drink) | Curcuminoids | Sweden |
| Nanocumin Super Food | Liquid (Drink) | Turmeric powder extract | South Korea |
| Healing Cell Gold Nano CurcuminSerum | Liquid (Serum) | Curcumin extract | Singapore |
| Nano Resveratrol Facial Serum | Oil | Nano resveratrol | Brazil |
| CumarGOLD Gel-Nano Curcumin Skincare | Gel | Nano curcumin | Vietnam |
| Nano Food | Liquid (Oral supplement) | Polyphenol-rich extract | Indonesia |
| Nano Resveratrol (Nano Red Wine) | Liquid (wine) | Nano resveratrol, grape seed extract | Japan |
| Nanoemulsified Milk Thistle | Liquid (Oral supplement) | Milk thistle seed extract | USA |
| Nano Food Kids | Liquid (Oral supplement) | Polyphenol-rich extract | Indonesia |
| Nanoceuticals™ Slim Shake Chocolate | Liquid (drink) | Polyphenol-rich extract | USA |
| Alqunus Nano Curcumin | Liquid (Oral supplement) | Turmeric | India |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Jin, Z.; Qiu, C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods 2022, 11, 2189. https://doi.org/10.3390/foods11152189
Zhang Z, Li X, Sang S, McClements DJ, Chen L, Long J, Jiao A, Jin Z, Qiu C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods. 2022; 11(15):2189. https://doi.org/10.3390/foods11152189
Chicago/Turabian StyleZhang, Zhiheng, Xiaojing Li, Shangyuan Sang, David Julian McClements, Long Chen, Jie Long, Aiquan Jiao, Zhengyu Jin, and Chao Qiu. 2022. "Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application" Foods 11, no. 15: 2189. https://doi.org/10.3390/foods11152189
APA StyleZhang, Z., Li, X., Sang, S., McClements, D. J., Chen, L., Long, J., Jiao, A., Jin, Z., & Qiu, C. (2022). Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods, 11(15), 2189. https://doi.org/10.3390/foods11152189










