Antibacterial Activity and Mechanism of Action of Whey Protein-ε-Polylysine Complexes against Staphylococcus aureus and Bacillus subtilis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Determination of Minimum Inhibitory Concentration
2.3. Determination of Minimum Bactericidal Concentration
2.4. Analysis of Inhibition Zone
2.5. Scanning Electron Microscopy (SEM) Analysis
2.6. Molecular Dynamics Simulation
2.7. Antibacterial Effect of Whey Protein-ε-PL Complexes on Total Mesophilic Counts and Native Staphylococcus aureus in Fresh Meat
2.8. Data Analysis
3. Results and Discussion
3.1. Minimum Inhibitory Concentration of Whey Protein-ε-PL Complexes
3.2. Minimum Bactericidal Concentration of Whey Protein-ε-PL Complexes
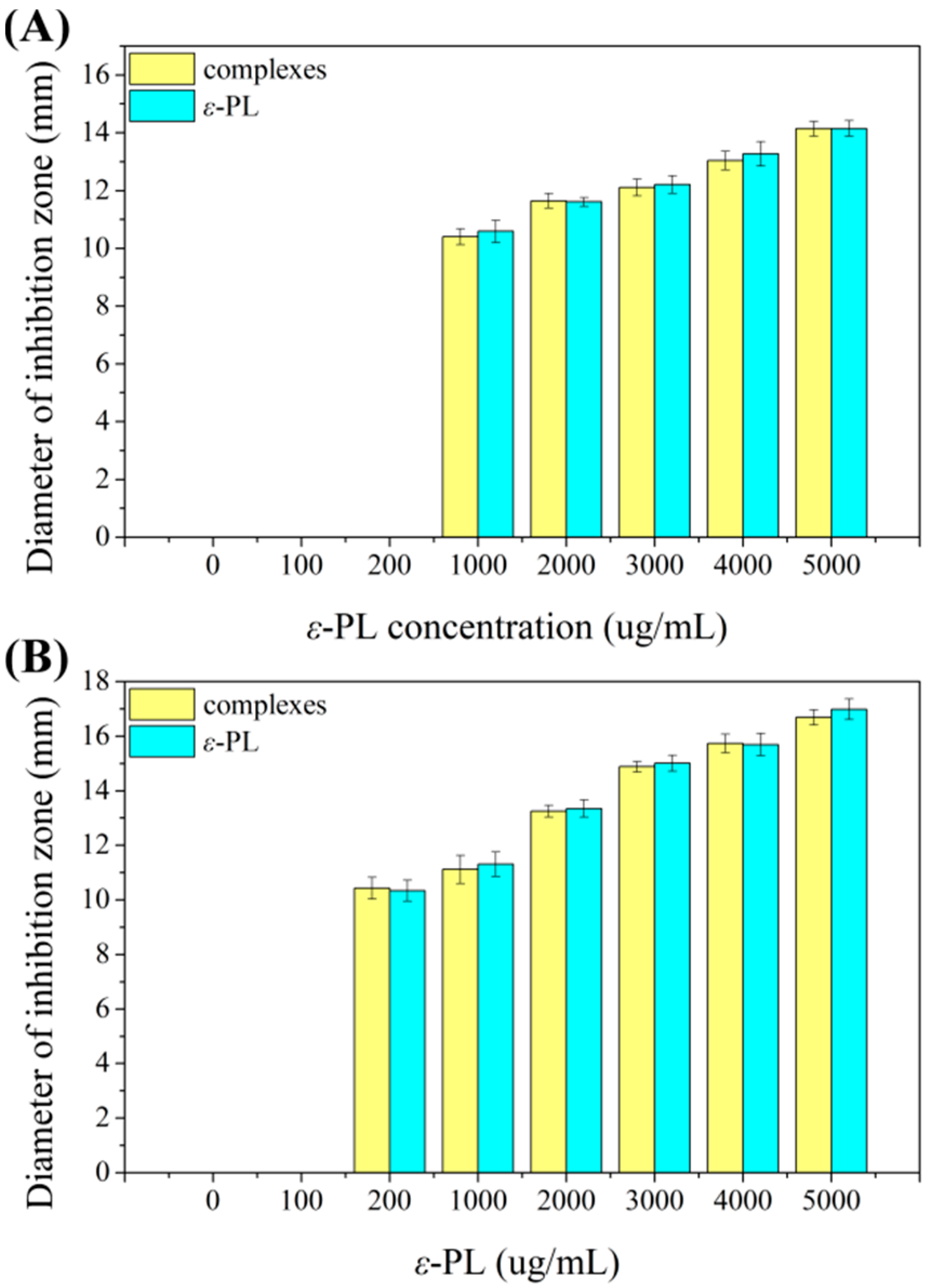
3.3. Inhibition Zone of Whey Protein-ε-PL Complexes
3.4. Morphological Changes of Staphylococcus aureus and Bacillus subtilis
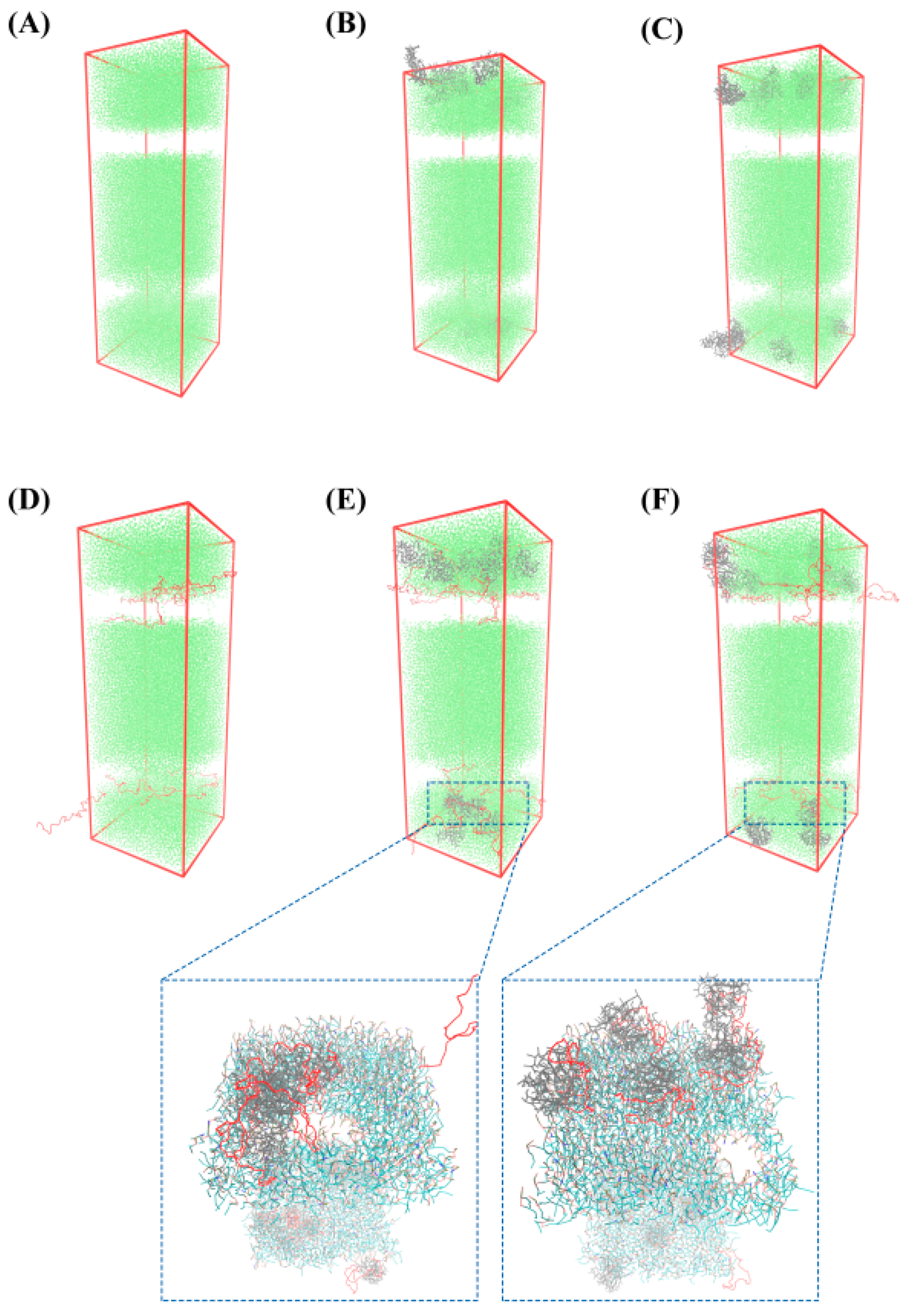
3.5. MD Simulation
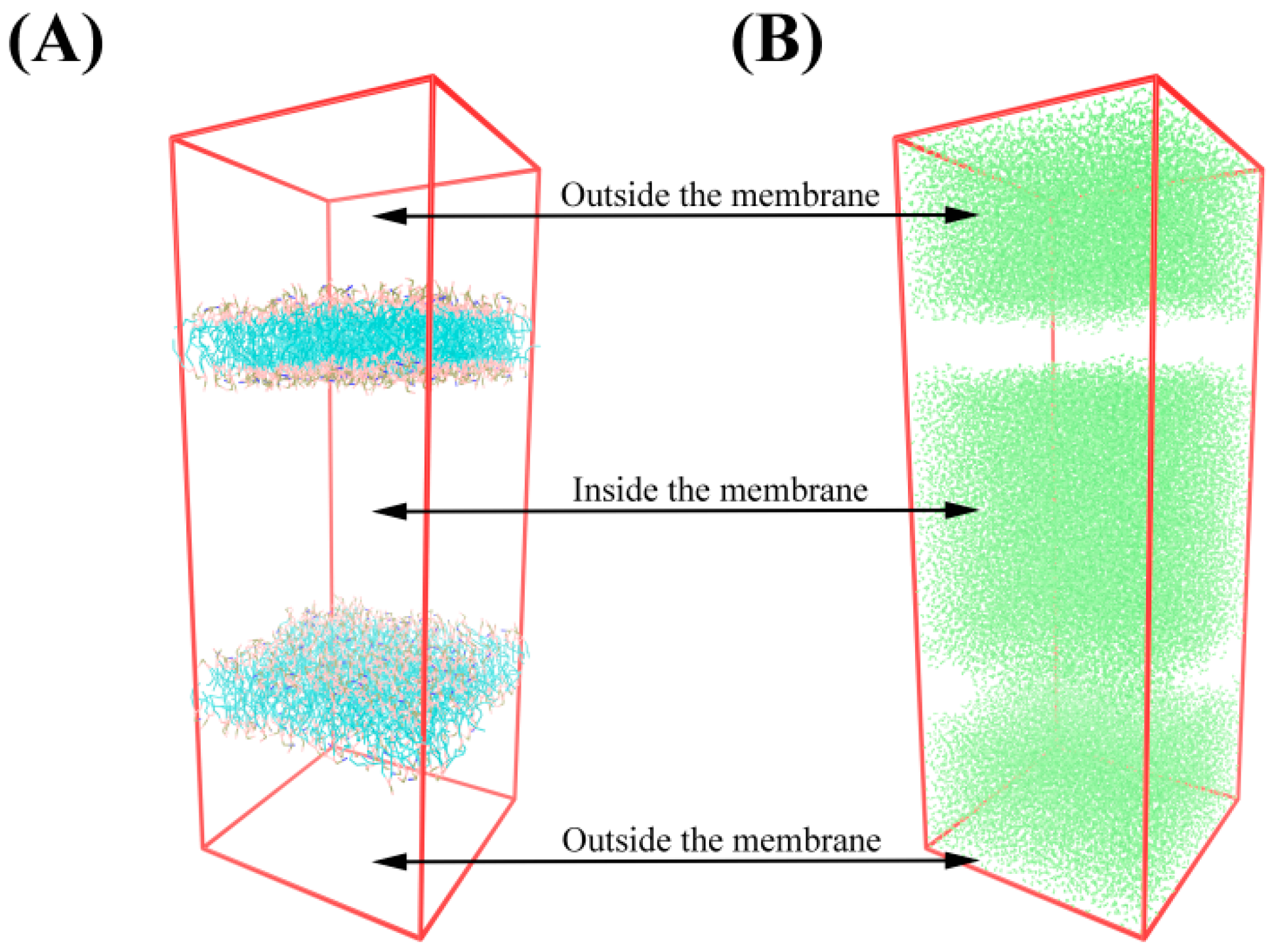
3.5.1. Schematic Diagram of Bacteriostatic Model of Bilayer Membrane
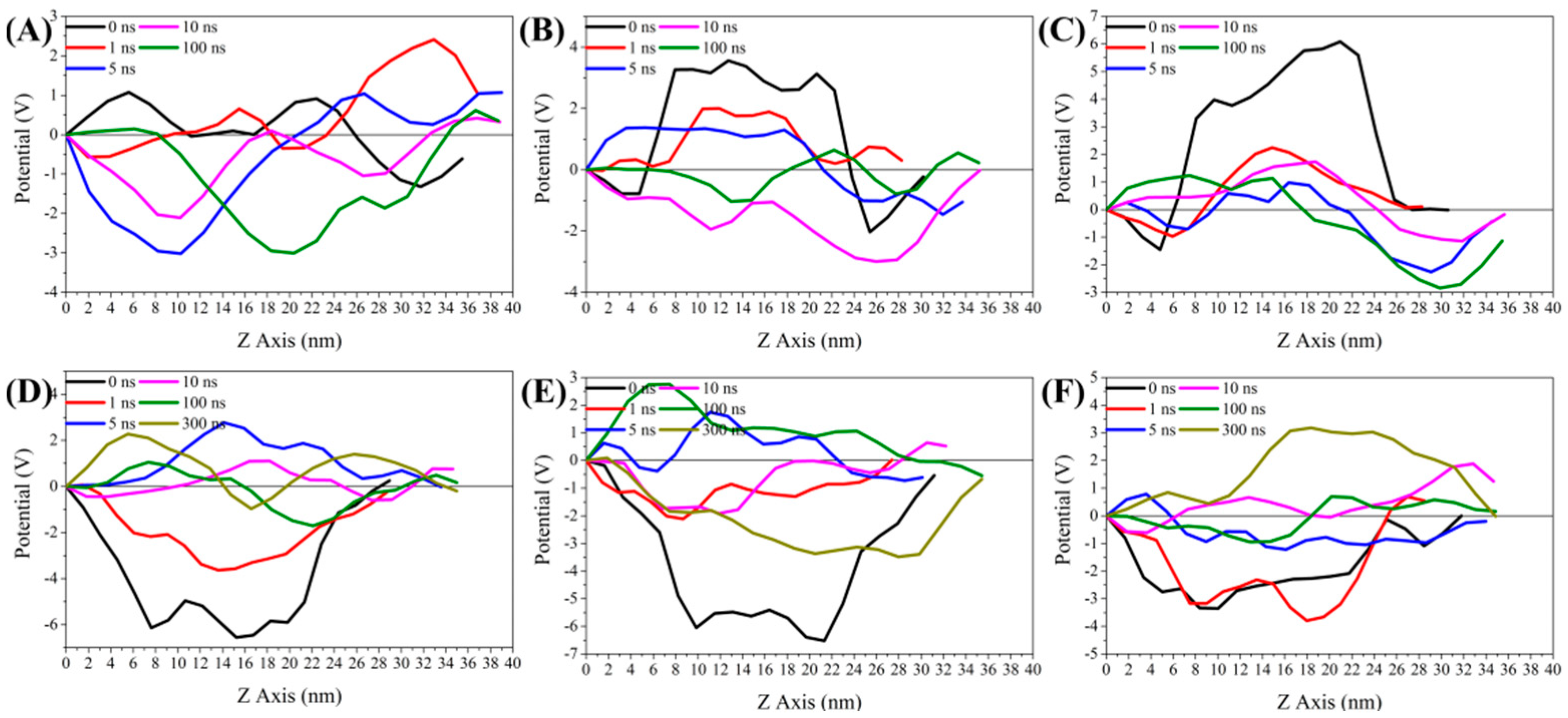
3.5.2. Analysis of Z-Axis Potential Difference of Bilayer Membrane
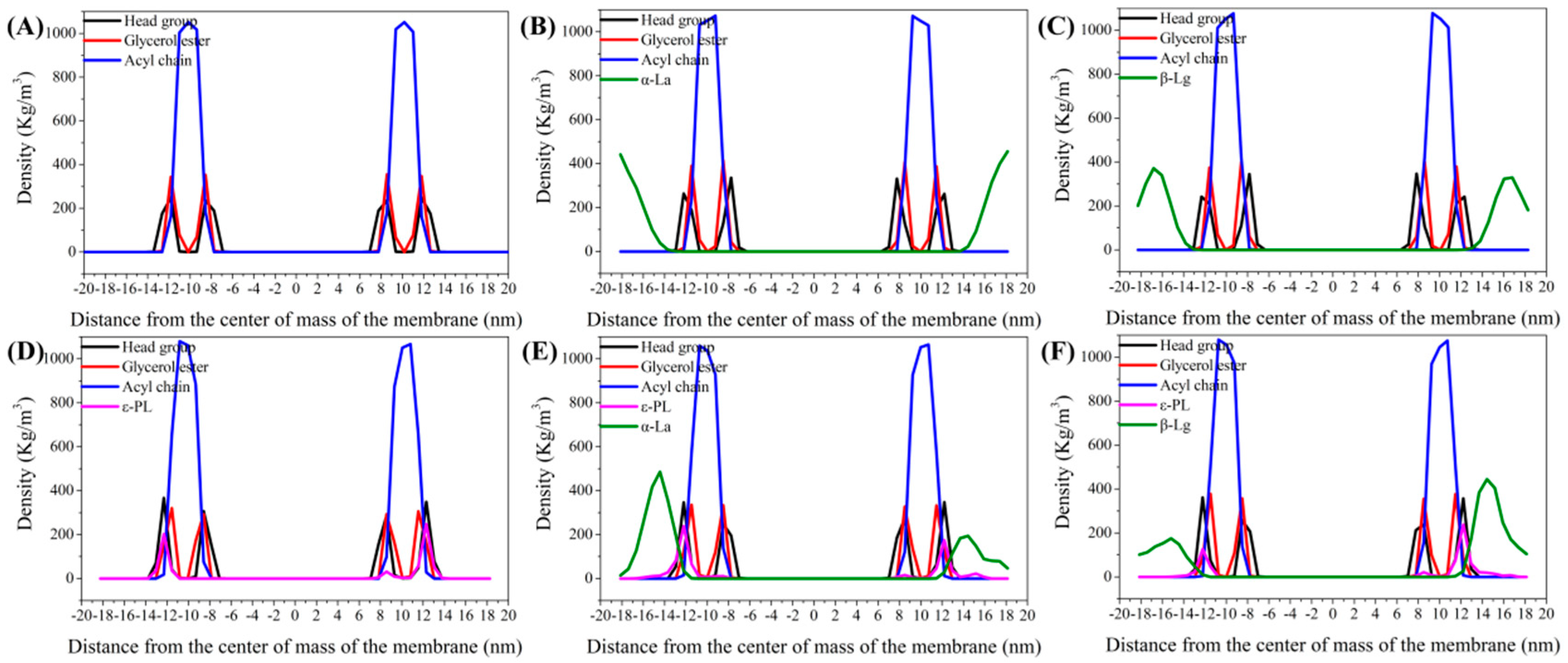
3.5.3. Density Distribution Curve of Components in Bilayer Membrane System
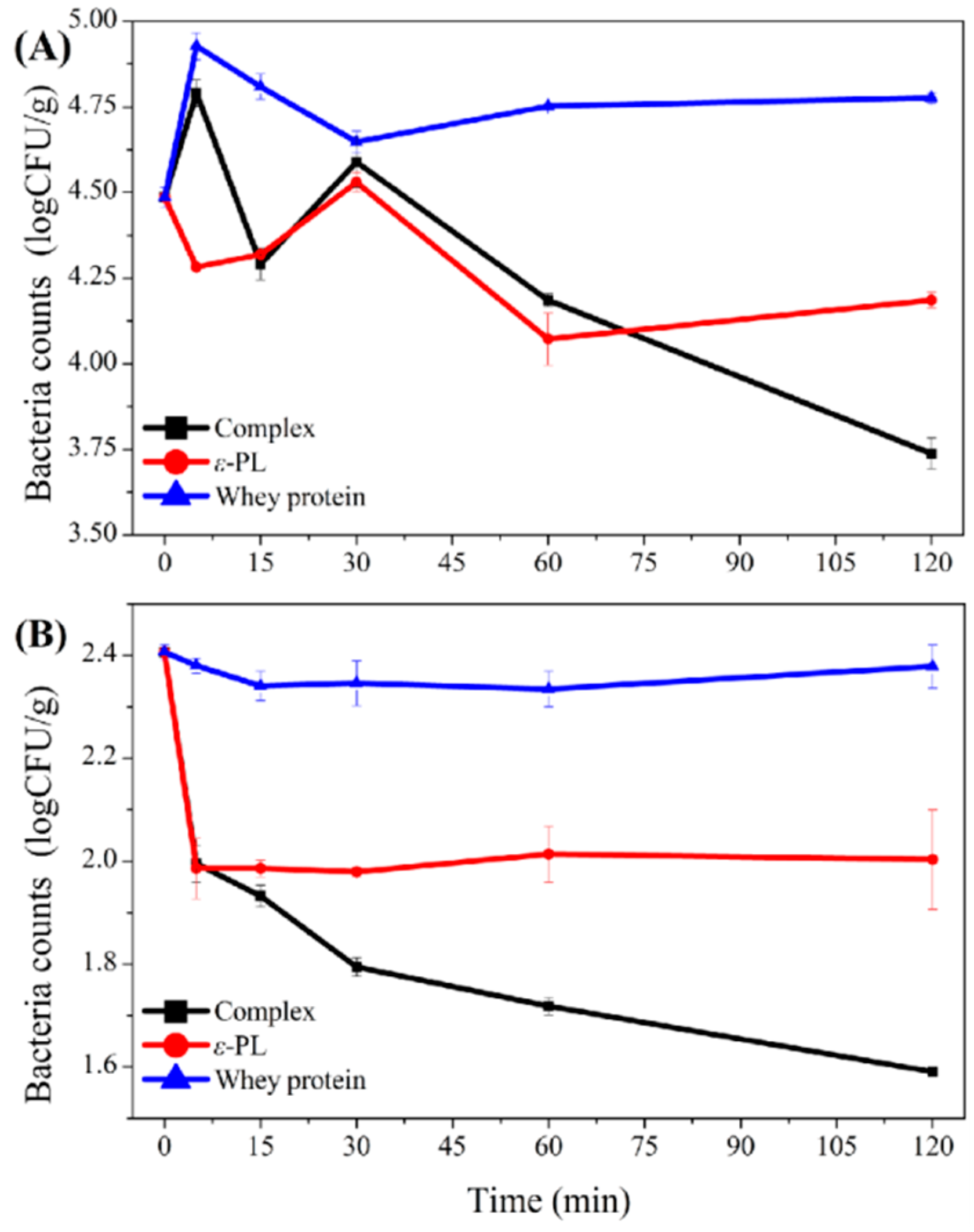
3.6. Antibacterial Effect of Whey Protein-ε-PL Complexes in Fresh Meat
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bo, T.; Han, P.-P.; Su, Q.-Z.; Fu, P.; Guo, F.-Z.; Zheng, Z.-X.; Tan, Z.-L.; Zhong, C.; Jia, S.-R. Antimicrobial ε-poly-l-lysine induced changes in cell membrane compositions and properties of Saccharomyces cerevisiae. Food Control 2016, 61, 123–134. [Google Scholar] [CrossRef]
- Cai, R.; Yuan, Y.; Wang, Z.; Guo, C.; Liu, B.; Pan, C.; Liu, L.; Yue, T. Effects of preservatives on Alicyclobacillus acidoterrestris growth and guaiacol production. Int. J. Food Microbiol. 2015, 214, 145–150. [Google Scholar] [CrossRef]
- Chang, S.-S.; Lu, W.-Y.W.; Park, S.-H.; Kang, D.-H. Control of foodborne pathogens on ready-to-eat roast beef slurry by ε-polylysine. Int. J. Food Microbiol. 2010, 141, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pei, H.; Han, Z.; Feng, G.; Li, D. The antimicrobial effects and synergistic antibacterial mechanism of the combination of ε-Polylysine and nisin against Bacillus subtilis. Food Control 2015, 47, 444–450. [Google Scholar] [CrossRef]
- Shima, S.; Sakai, H. Polylysine produced by Streptomyces. Agric. Biol. Chem. 1977, 41, 1807–1809. [Google Scholar] [CrossRef] [Green Version]
- Zahi, M.R.; El Hattab, M.; Liang, H.; Yuan, Q. Enhancing the antimicrobial activity of d-limonene nanoemulsion with the inclusion of ε-polylysine. Food Chem. 2017, 221, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Shima, S.; Sakai, H. Poly-l-lysine produced by Streptomyces. Part II. Taxonomy and fermentation studies. Agric. Biol. Chem. 1981, 45, 2497–2502. [Google Scholar] [CrossRef]
- Xiang, J.; Yang, Y.; Dabbour, M.; Mintah, B.K.; Zhang, Z.; Dai, C.; He, R.; Huang, G.; Ma, H. Metabolomic and genomic profiles of Streptomyces albulus with a higher ε-polylysine production through ARTP mutagenesis. Biochem. Eng. J. 2020, 162, 107720. [Google Scholar] [CrossRef]
- Shih, I.L.; Shen, M.H.; Van, Y.T. Microbial synthesis of poly (ε-lysine) and its various applications. Bioresour. Technol. 2006, 97, 1148–1159. [Google Scholar] [CrossRef]
- Hiraki, J.; Ichikawa, T.; Ninomiya, S.i.; Seki, H.; Uohama, K.; Seki, H.; Kimura, S.; Yanagimoto, Y.; Barnett, J.W. Use of ADME studies to confirm the safety of ε-polylysine as a preservative in food. Regul. Toxicol. Pharmacol. 2003, 37, 328–340. [Google Scholar] [CrossRef]
- Yoshida, T.; Nagasawa, T. ε-Poly-l-lysine: Microbial production, biodegradation and application potential. Appl. Microbiol. Biotechnol. 2003, 62, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Vad, B.S.; Stenvang, M.; Otzen, D.E.; Meyer, R.L. The antimicrobial mechanism of action of epsilon-poly-l-lysine. Appl. Environ. Microbiol. 2014, 80, 7758–7770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blin, T.; Purohit, V.; Leprin Ce, J.; Jouenne, T.; Glinel, K. Bactericidal microparticles decorated by an antimicrobial peptide for the easy disinfection of sensitive aqueous solutions. Biomacromolecules 2011, 12, 1259–1264. [Google Scholar] [CrossRef]
- Brogden, K. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria. Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; McLandsborough, L.; McClements, D.J. Interaction of cationic antimicrobial (ε-polylysine) with food-grade biopolymers: Dextran, chitosan, carrageenan, alginate, and pectin. Food Res. Int. 2014, 64, 396–401. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, J.; Li, F.; Shi, Y.; Li, D.; Huang, Q. Chitosan-sodium alginate nanoparticle as a delivery system for ε-polylysine: Preparation, characterization and antimicrobial activity. Food Control 2018, 91, 302–310. [Google Scholar] [CrossRef]
- Karimirad, R.; Behnamian, M.; Dezhsetan, S. Application of chitosan nanoparticles containing cuminum cyminum oil as a delivery system for shelf life extension of agaricus bisporus. LWT 2019, 106, 218–228. [Google Scholar] [CrossRef]
- Hiraki, J. Basic and applied studies on ε-Polylysine. J. Antibact. Antifung. Agents 1995, 23, 349–354. [Google Scholar]
- Chang, Y.; McLandsborough, L.; McClements, D. Cationic antimicrobial (ε-Polylysine) -anionic polysaccharide (pectin) interactions: Influence of polymer charge on physical stability and antimicrobial efficacy. J. Agric. Food Chem. 2012, 60, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Zhao, X.; Liu, Y.; Liang, X.; Yang, Y. Fabricating multilayer emulsions by using OSA starch and chitosan suitable for spray drying: Application in the encapsulation of β-carotene. Food Hydrocoll. 2019, 93, 102–110. [Google Scholar] [CrossRef]
- Chang, Y.; McLandsborough, L.; McClements, D. Physicochemical properties and antimicrobial efficacy of electrostatic complexes based on cationic ε-Polylysine and anionic pectin. J. Agric. Food Chem. 2011, 59, 6776–6782. [Google Scholar] [CrossRef]
- Ho, Y.; Ishizaki, S.; Tanaka, M. Improving emulsifying activity of ε-polylysine by conjugation with dextran through the Maillard reaction. Food Chem. 2000, 68, 449–455. [Google Scholar] [CrossRef]
- Islam, M.T.; Ogura, A.; Machida, C.; Morinaga, N.; Honjoh, K.I.; Miyamoto, T. Effects of ε-polylysine and milk serum protein on the attachment and decontamination of Salmonella enteritidis on lettuce and radish sprouts. Food Sci. Technol. Res. 2016, 22, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Shao, Z.; Yang, Y.; Fang, S.; Li, Y.; Chen, J.; Meng, Y. Mechanism of the antimicrobial activity of whey protein-ε-polylysine complexes against Escherichia coli and its application in sauced duck products. Int. J. Food Microbiol. 2020, 328, 108663. [Google Scholar] [CrossRef]
- Meng, Y.; Xue, Q.; Chen, J.; Li, Y.; Shao, Z. Structure, stability, rheology and texture properties of ε-polylysine-whey protein complexes. J. Dairy Sci. 2022, 105, 3746–3757. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Geng, X.; Yang, R.; Huang, J.; Zhang, X.; Wang, X. Evaluation antibacterial activity of quaternary-based chitin/chitosan derivatives in vitro. J. Food Sci. 2013, 78, 90–97. [Google Scholar] [CrossRef]
- Qian, J.; Wang, Y.; Zhuang, H.; Yan, W.; Zhang, J.; Luo, J. Plasma activated water-induced formation of compact chicken myofibrillar protein gel structures with intrinsically antibacterial activity. Food Chem. 2021, 351, 129278. [Google Scholar] [CrossRef] [PubMed]
- Ukuku, D.O.; Niemira, B.A.; Ukanalis, J. Nisin-based antimicrobial combination with cold plasma treatment inactivate Listeria monocytogenes on granny smith apples. LWT 2019, 104, 120–127. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Marrink, S.; Risselada, H.; Yefimov, S.; Tieleman, D.; Vries, A. The MARTINI force field: Coarse grained model for biomolecular simulations. J. Phys. Chem. B 2007, 111, 7812–7824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pike, A.C.W.; Brew, K.; Acharya, K.R. Crystal structures of guinea-pig, goat and bovine α-lactalbumin highlight the enhanced conformational flexibility of regions that are significant for its action in lactose synthase. Structure 1996, 4, 691–703. [Google Scholar] [CrossRef] [Green Version]
- Loch, J.; Polit, A.; Górecki, A.; Bonarek, P.; Kurpiewska, K.; Dziedzicka Wasylewska, M.; Lewiński, K. Two modes of fatty acid binding to bovine β-lactoglobulin-crystallographic and spectroscopic studies. J. Mol. Recognit. 2011, 24, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J. Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Shao, Z.; Fang, S.; Li, Y.; Chen, J.; Meng, Y. Physicochemical properties and formation mechanism of electrostatic complexes based on ε-polylysine and whey protein: Experimental and molecular dynamics simulations study. Int. J. Biol. Macromol. 2018, 118, 2208–2215. [Google Scholar] [CrossRef]
- Goresline, H.E.; Bartram, M.T. Compendium of methods for the microbiological examination of foods. Am. J. Public Health Nations Health 1976, 39, 83–85. [Google Scholar] [CrossRef]
- Li, Y.; Han, Q.; Feng, J.; Tian, W.; Mo, H. Antibacterial characteristics and mechanisms of ε-poly-lysine against Escherichia coli and Staphylococcus aureus. Food Control 2014, 43, 22–27. [Google Scholar] [CrossRef]
- Tan, Z.; Shi, Y.; Xing, B.; Hou, Y.; Jia, S. The antimicrobial effects and mechanism of ε-poly-lysine against Staphylococcus aureus. Bioresour. Bioprocess. 2019, 6, 11. [Google Scholar] [CrossRef]
- Hancock, R.E.W. Cationic peptides: Effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 2001, 1, 156–164. [Google Scholar] [CrossRef]
- Geornaras, I.; Yoon, Y.; Belk, K.E.; Smith, G.C.; Sofos, J.N. Antimicrobial activity of epsilon-polylysine against Escherichia coli O157: H7, Salmonella typhimurium, and Listeria monocytogenes in various food extracts. J. Food Sci. 2007, 72, M330–M334. [Google Scholar] [CrossRef] [PubMed]
- Leontiadou, H.; Mark, A.E.; Marrink, S.J. Antimicrobial peptides in action. J. Am. Chem. Soc. 2006, 128, 12156–12161. [Google Scholar] [CrossRef] [Green Version]
- Jean-François, F.; Elezgaray, J.; Berson, P.; Vacher, P.; Dufourc, E.J. Pore formation induced by an antimicrobial peptide: Electrostatic effects. Biophys. J. 2008, 95, 5748–5756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Meng, Y.; Fang, S. Physicochemical and antimicrobial properties of ε-polylysine/carboxymethyl chitosan polyelectrolyte complexes and their effect against spoilage microorganisms in raw pork. Food Funct. 2017, 8, 2243–2248. [Google Scholar] [CrossRef]
- Meng, Y.; He, X.; Guo, L.; Xu, Y.; Fang, S.; Li, Y.; Chen, J. Physicochemical and antibacterial properties of sodium tripolyphosphate/ε-Polylysine complexes and their application in cooked sausage. Food Biophys. 2021, 16, 415–425. [Google Scholar] [CrossRef]

| Composition | Typical | Specification | Test Method |
|---|---|---|---|
| Protein (% dry basis) | 93.0 | 91.0 min | Calculation |
| Protein (% as is) | 89.0 | 87.0 min | AOAC |
| Lactose (%) | 0.1 | / | AOAC |
| Fat (%) | 1.3 | 1.8 max | AOAC |
| Moisture (%) | 4.7 | 6.0 max | AOAC |
| Ash (%) | 2.7 | 3.5 max | AOAC |
| pH | / | 6.2–7.0 | 10% Sol. At 20 °C |
| System a | ε-PL (Number) | Protein (Number) | POPE/POPG | Temperature (°C) | Time (ns) |
|---|---|---|---|---|---|
| POPE/POPG | 0 | 0 | 1024 | 37 °C | 100 |
| α-La-POPE/POPG | 0 | 3 | 1024 | 37 °C | 100 |
| β-Lg-POPE/POPG ε-PL-POPE/POPG | 0 10 | 10 0 | 1024 1024 | 37 °C 37 °C | 100 300 |
| ε-PL-α-La-POPE/POPG | 12 | 3 | 1024 | 37 °C | 300 |
| ε-PL-β-Lg-POPE/POPG | 10 | 10 | 1024 | 37 °C | 300 |
| Samples | Mass Ratios (Whey Protein-to-ε-PL) | MIC (μg/mL) | |
|---|---|---|---|
| Staphylococcus aureus | Bacillus subtilis | ||
| whey protein | / | - | - |
| ε-PL | / | 19.53 | 4.88 |
| complexes | 50:1 | 25.00 | 6.25 |
| complexes | 50:2 | 25.00 | 6.25 |
| complexes | 50:10 | 31.26 | 7.81 |
| complexes | 50:20 | 31.26 | 3.90–7.81 |
| complexes | 50:30 | 23.44 | 5.86 |
| complexes | 50:40 | 31.26 | 3.90 |
| complexes | 50:50 | 19.53 | 4.88 |
| Samples | Mass Ratios (Whey Protein-to-ε-PL) | MBC (μg/mL) | |
|---|---|---|---|
| Staphylococcus aureus | Bacillus subtilis | ||
| whey protein | / | - | - |
| ε-PL | / | 39.06 | 9.77 |
| complexes | 50:1 | 50.00–100.00 | 12.50–25.00 |
| complexes | 50:2 | 50.00–100.00 | 12.50–25.00 |
| complexes | 50:10 | 62.50 | 15.63 |
| complexes | 50:20 | 62.50 | 15.63 |
| complexes | 50:30 | 93.75 | 11.72–23.43 |
| complexes | 50:40 | 62.50 | 15.63 |
| complexes | 50:50 | 39.06–78.13 | 9.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Lou, L.; Shao, Z.; Chen, J.; Li, Y.; Zhang, T. Antibacterial Activity and Mechanism of Action of Whey Protein-ε-Polylysine Complexes against Staphylococcus aureus and Bacillus subtilis. Foods 2022, 11, 2311. https://doi.org/10.3390/foods11152311
Meng Y, Lou L, Shao Z, Chen J, Li Y, Zhang T. Antibacterial Activity and Mechanism of Action of Whey Protein-ε-Polylysine Complexes against Staphylococcus aureus and Bacillus subtilis. Foods. 2022; 11(15):2311. https://doi.org/10.3390/foods11152311
Chicago/Turabian StyleMeng, Yuecheng, Li Lou, Zhipeng Shao, Jie Chen, Yanhua Li, and Tianqi Zhang. 2022. "Antibacterial Activity and Mechanism of Action of Whey Protein-ε-Polylysine Complexes against Staphylococcus aureus and Bacillus subtilis" Foods 11, no. 15: 2311. https://doi.org/10.3390/foods11152311
APA StyleMeng, Y., Lou, L., Shao, Z., Chen, J., Li, Y., & Zhang, T. (2022). Antibacterial Activity and Mechanism of Action of Whey Protein-ε-Polylysine Complexes against Staphylococcus aureus and Bacillus subtilis. Foods, 11(15), 2311. https://doi.org/10.3390/foods11152311






