Functional Properties and Extraction Techniques of Chicken Egg White Proteins
Abstract
:1. Introduction
2. Structures and Functional Properties of Egg White Proteins
2.1. Ovalbumin (OVA)
2.2. Ovotransferrin (OVT)
2.3. Ovomucoid (OVM)
2.4. Lysozyme (LYS)
2.5. Ovomucin (OVN)
2.6. Ovomacroglobulin
2.7. Avidin
| Protein | Percentage of Total Protein (%) | MW (kDa) | pI | Refs. |
|---|---|---|---|---|
| Ovalbumin | 54 | 45 | 4.5 | [6,7] |
| Ovotransferrin (conalbumin) | 12–13 | 77 | 6.0 | [14] |
| Ovomucoid | 11 | 28 | 4.1 | [24] |
| Lysozyme | 3.4–3.5 | 14.3 | 10.7 | [31] |
| Ovomucin | 1.5–3.5 | 0.22–270 × 103 | 4.5–5.0 | [43] |
| Ovomacroglobulin (ovostatin) | 0.5 | 7.6–9.0 × 102 | 4.5–4.7 | [46,47] |
| Avidin | 0.05 | 68.3 | 10.0 | [49] |
3. Common Techniques
3.1. Precipitation
3.2. Chromatography
3.2.1. Ion Exchange Chromatography
3.2.2. Gel Filtration Chromatography
3.2.3. Affinity Chromatography
3.2.4. Adsorption Chromatography
3.3. Membrane Separation Technology
4. Novel Methods
4.1. Electrophoresis
4.2. Membrane Chromatography
4.3. Aqueous Two-Phase Systems
4.4. Molecular Imprinting Technology
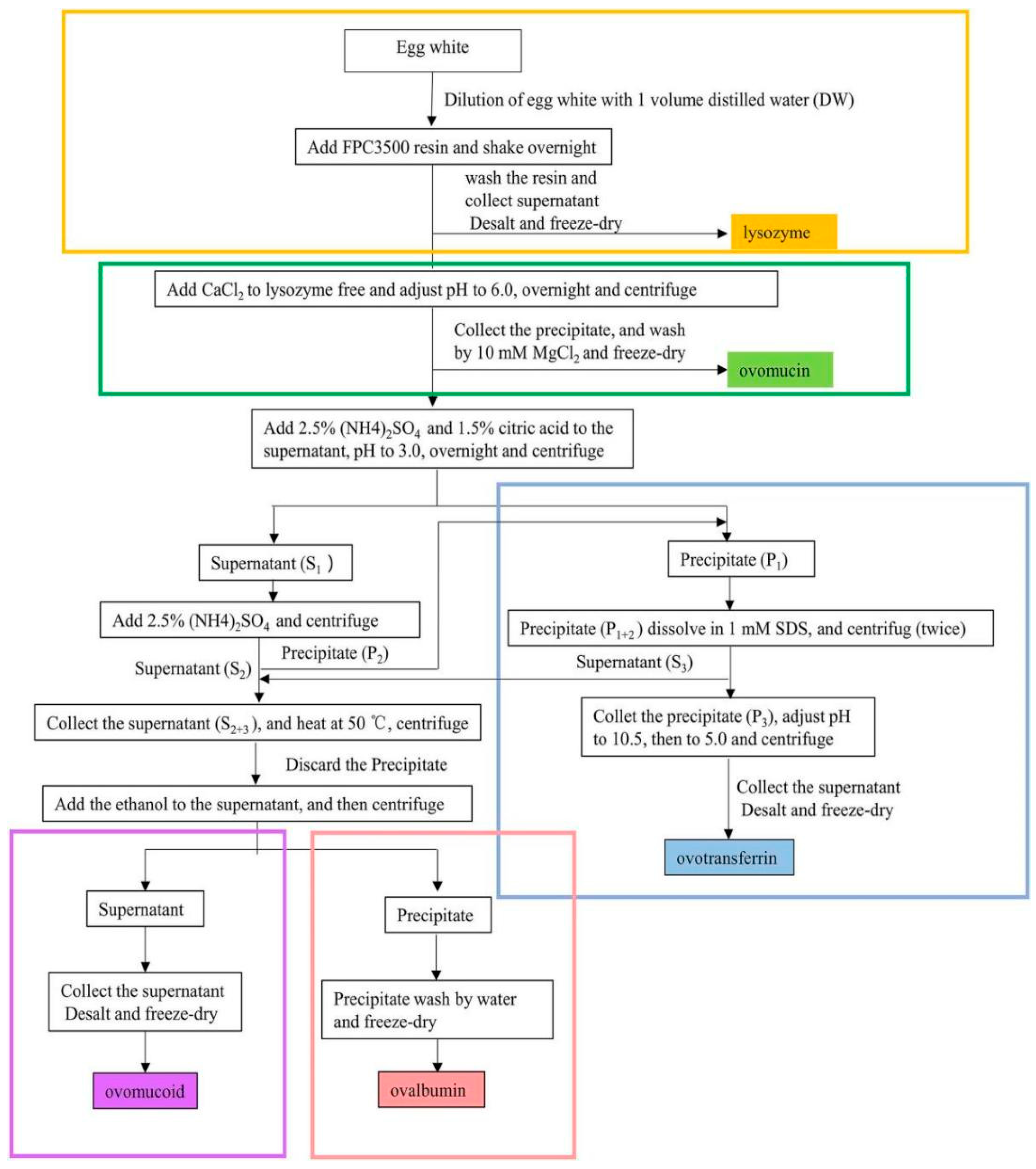
5. Co-Purification of Multiple Proteins
6. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, L.; Raikos, V.; Euston, S.R. Modification of functional properties of egg-white proteins. Nahrung 2003, 47, 369–376. [Google Scholar] [CrossRef]
- Benede, S.; Molina, E. Chicken Egg Proteins and Derived Peptides with Antioxidant Properties. Foods 2020, 9, 735. [Google Scholar] [CrossRef]
- Sun, Y.; Jin, H.B.; Sun, H.H.; Sheng, L. A Comprehensive Identification of Chicken Egg White Phosphoproteomics Based on a Novel Digestion Approach. J. Agric. Food Chem. 2020, 68, 9213–9222. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Khoiroh, I.; Ooi, C.W.; Ling, T.C.; Show, P.L. Recent Advances in Protein Extraction Using Ionic Liquid-based Aqueous Two-phase Systems. Sep. Purif. Rev. 2017, 46, 291–304. [Google Scholar] [CrossRef]
- Awade, A.C.; Efstathiou, T. Comparison of three liquid chromatographic methods for egg-white protein analysis. J. Chromatogr. B 1999, 723, 69–74. [Google Scholar] [CrossRef]
- Sheng, L.; Tang, G.Y.; Wang, Q.; Zou, J.; Ma, M.H.; Huang, X. Molecular characteristics and foaming properties of ovalbumin-pullulan conjugates through the Maillard reaction. Food Hydrocolloid 2020, 100, 8. [Google Scholar] [CrossRef]
- Guerin-Dubiard, C.; Pasco, M.; Molle, D.; Desert, C.; Croguennec, T.; Nau, F. Proteomic analysis of hen egg white. J. Agric. Food Chem. 2006, 54, 3901–3910. [Google Scholar] [CrossRef]
- Huntington, J.A.; Stein, P.E. Structure and properties of ovalbumin. J. Chromatogr. B 2001, 756, 189–198. [Google Scholar] [CrossRef]
- Sheng, L.; Liu, Q.; Dong, W.Y.; Cai, Z.X. Effect of high intensity ultrasound assisted glycosylation on the gel properties of ovalbumin: Texture, rheology, water state and microstructure. Food Chem. 2022, 372, 131215. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kato, E.; Ando, A. Increased antioxidative activity of ovalbumin by heat treating in an emulsion of linoleic acid. Biosci. Biotechnol. Biochem. 1996, 60, 1430–1433. [Google Scholar] [CrossRef]
- Pellegrini, A.; Hulsmeier, A.J.; Hunziker, P.; Thomas, U. Proteolytic fragments of ovalbumin display antimicrobial activity. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2004, 1672, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Suzuki, R.; Yokoyama, C.; Yano, S.; Konno, H. Antimicrobial activity and secondary structure of a novel peptide derived from ovalbumin. J. Pept. Sci. 2020, 26, e3276. [Google Scholar] [CrossRef] [PubMed]
- Mine, Y.; Rupa, P. Immunological and biochemical properties of egg allergens. World Poult. Sci. J. 2004, 60, 321–330. [Google Scholar] [CrossRef]
- Giansanti, F.; Leboffe, L.; Angelucci, F.; Antonini, G. The Nutraceutical Properties of Ovotransferrin and Its Potential Utilization as a Functional Food. Nutrients 2015, 7, 9105–9115. [Google Scholar] [CrossRef]
- Ko, K.Y.; Mendona, A.F.; Ahn, D.U. Influence of Zinc, Sodium Bicarbonate, and Citric Acid on the Antibacterial Activity of Ovotransferrin against Escherichia coli O157:H7 and Listeria monocytogenes in Model Systems and Ham. Poult. Sci. 2008, 87, 2660–2670. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Z.H.; Xue, C.H. The past and future of ovotransferrin: Physicochemical properties, assembly and applications. Trends Food Sci. Technol. 2021, 116, 47–62. [Google Scholar] [CrossRef]
- Rathnapala, E.C.N.; Ahn, D.U.; Abeyrathne, E.D.N.S. Functional properties of ovotransferrin from chicken egg white and its derived peptides: A review. Food Sci. Biotechnol. 2021, 30, 619–630. [Google Scholar] [CrossRef]
- Galla, R.; Grisenti, P.; Farghali, M.; Saccuman, L.; Ferraboschi, P.; Uberti, F. Ovotransferrin Supplementation Improves the Iron Absorption: An In Vitro Gastro-Intestinal Model. Biomedicines 2021, 9, 1543. [Google Scholar] [CrossRef]
- Srivastava, A.; Lall, R.; Talukder, J.; DuBourdieu, D.; Gupta, R.C. Iron Transport Tocopheryl Polyethylene Glycol Succinate in Animal Health and Diseases. Molecules 2019, 24, 4289. [Google Scholar] [CrossRef]
- Legros, J.; Jan, S.; Bonnassie, S.; Gautier, M.; Croguennec, T.; Pezennec, S.; Cochet, M.; Nau, F.; Andrews, S.; Baron, F. The Role of Ovotransferrin in Egg-White Antimicrobial Activity: A Review. Foods 2021, 10, 823. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Hoq, M.I.; Aoki, T. Ovotransferrin possesses SOD-like superoxide anion scavenging activity that is promoted by copper and manganese binding. Int. J. Biol. Macromol. 2007, 41, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Majumder, K.; Wu, J.P. Oxygen radical absorbance capacity of peptides from egg white protein ovotransferrin and their interaction with phytochemicals. Food Chem. 2010, 123, 635–641. [Google Scholar] [CrossRef]
- Moon, S.H.; Lee, J.H.; Kim, J.H.; Paik, H.D.; Ahn, D.U. In vitro cytotoxic and ACE-inhibitory activities of promod 278P hydrolysate of ovotransferrin from chicken egg white. Poult. Sci. 2017, 96, 1982–1987. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.C.; Coimbra, J.S.D.; da Silva, L.H.M.; Rojas, E.E.G.; da Silva, M.D.H. Ovomucoid partitioning in aqueous two-phase systems. Biochem. Eng. J. 2009, 47, 55–60. [Google Scholar] [CrossRef]
- Van der Plancken, I.; Van Remoortere, M.; Van Loey, A.; Hendrickx, M.E. Trypsin inhibition activity of heat-denatured ovomucoid: A kinetic study. Biotechnol. Prog. 2004, 20, 82–86. [Google Scholar] [CrossRef]
- Kido, J.; Matsumoto, T. Attenuated Allergenic Activity of Ovomucoid after Electrolysis. Allergy Asthma Immun. 2015, 7, 599–604. [Google Scholar] [CrossRef]
- Porta, R.; Giosafatto, C.V.L.; di Pierro, P.; Sorrentino, A.; Mariniello, L. Transglutaminase-mediated modification of ovomucoid: Effects on its trypsin inhibitory activity and antigenic properties. Amino Acids 2013, 44, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.W.; Tu, Z.C.; Hu, Y.M.; Wang, H. Effects of Superheated Steam Treatment on the Allergenicity and Structure of Chicken Egg Ovomucoid. Foods 2022, 11, 238. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Ahn, D.U. Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents-A review. Poult. Sci. 2013, 92, 3292–3299. [Google Scholar] [CrossRef]
- Hu, X.X.; Liu, Y.F.; Zhu, D.D.; Jin, Y.G.; Jin, H.B.; Sheng, L. Preparation and characterization of edible carboxymethyl cellulose films containing natural antibacterial agents: Lysozyme. Food Chem. 2022, 385, 132708. [Google Scholar] [CrossRef]
- Jin, H.B.; Li, P.S.; Jin, Y.G.; Sheng, L. Effect of sodium tripolyphosphate on the interaction and aggregation behavior of ovalbumin-lysozyme complex. Food Chem. 2021, 352, 9. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.B.; Chen, J.H.; Zhang, J.; Sheng, L. Impact of phosphates on heat-induced egg white gel properties: Texture, water state, micro-rheology and microstructure. Food Hydrocoll. 2021, 110, 106200. [Google Scholar] [CrossRef]
- Lesnierowski, G.; Yang, T.Y. Lysozyme and its modified forms: A critical appraisal of selected properties and potential. Trends Food Sci. Technol. 2021, 107, 333–342. [Google Scholar] [CrossRef]
- Sheng, L.; Su, P.; Han, K.; Chen, J.H.; Cao, A.Q.; Zhang, Z.L.; Jin, Y.G.; Ma, M.H. Synthesis and structural characterization of lysozyme-pullulan conjugates obtained by the Maillard reaction. Food Hydrocolloid 2017, 71, 1–7. [Google Scholar] [CrossRef]
- Aminlari, L.; Hashemi, M.M.; Aminlari, M. Modified Lysozymes as Novel Broad Spectrum Natural Antimicrobial Agents in Foods. J. Food Sci. 2014, 79, R1077–R1090. [Google Scholar] [CrossRef]
- Evran, S.; Yasa, I.; Telefoncu, A. Modification of lysozyme with oleoyl chloride for broadening the antimicrobial specificity. Prep. Biochem. Biotechnol. 2010, 40, 316–325. [Google Scholar] [CrossRef]
- Liu, J.H.; Wang, N.; Liu, Y.P.; Jin, Y.G.; Ma, M.H. The antimicrobial spectrum of lysozyme broadened by reductive modification. Poult. Sci. 2018, 97, 3992–3999. [Google Scholar] [CrossRef]
- Masschalck, B.; Van Houdt, R.; Van Haver, E.G.R.; Michiels, C.W. Inactivation of gram-negative bacteria by lysozyme, denatured lysozyme, and lysozyme-derived peptides under high hydrostatic pressure. Appl. Environ. Microb. 2001, 67, 339–344. [Google Scholar] [CrossRef]
- Mine, Y.; Ma, F.P.; Lauriau, S. Antimicrobial peptides released by enzymatic hydrolysis of hen egg white lysozyme. J. Agric. Food Chem. 2004, 52, 1088–1094. [Google Scholar] [CrossRef]
- Juneja, V.K.; Dwivedi, H.P.; Yan, X. Novel natural food antimicrobials. Annu. Rev. Food Sci. Technol. 2012, 3, 381–403. [Google Scholar] [CrossRef]
- Hartono, Y.D.; Lee, A.N.; Lee-Huang, S.; Zhang, D.W. Computational study of bindings of HL9, a nonapeptide fragment of human lysozyme, to HIV-1 fusion protein gp41. Bioorg. Med. Chem. Lett. 2011, 21, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.K.; Ndung’u, T. The potential of lactoferrin, ovotransferrin and lysozyme as antiviral and immune-modulating agents in COVID-19. Future Virol. 2020, 15, 609–624. [Google Scholar] [CrossRef]
- Omana, D.A.; Wang, J.P.; Wu, J.P. Ovomucin—A glycoprotein with promising potential. Trends Food Sci. Technol. 2010, 21, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Tang, D.; Wang, R.; Tu, A.; Yi, Y.; Wang, X.; Liu, B.; Zhou, Y.; Huang, Q.; Lu, X. Rheological and structural properties of ovomucin from chicken eggs with different interior quality. Food Hydrocolloid 2020, 100, 10. [Google Scholar] [CrossRef]
- Kato, A.; Ogata, S.; Matsudomi, N.; Kobayashi, K. A comparative-study of aggregated and disaggregated ovomucin during egg-white thinning. J. Agric. Food Chem. 1981, 29, 821–823. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Huang, X.; Ahn, D.U. Antioxidant, angiotensin-converting enzyme inhibitory activity and other functional properties of egg white proteins and their derived peptides—A review. Poult. Sci. 2018, 97, 1462–1468. [Google Scholar] [CrossRef]
- Kitamoto, T.; Nakashima, M.; Ikai, A. Hen egg-white ovomacroglobulin has a protease inhibitory activity. J. Biochem. 1982, 92, 1679–1682. [Google Scholar] [CrossRef]
- Geng, F.; Huang, X.; Ma, M.-H.; Li, Z.; Zhang, X.-W. Simple Two-step Chromatographic Method for Purification of Ovomacroglobulin. Asian J. Chem. 2013, 25, 2683–2686. [Google Scholar] [CrossRef]
- Jain, A.; Cheng, K. The principles and applications of avidin-based nanoparticles in drug delivery and diagnosis. J. Control Release 2017, 245, 27–40. [Google Scholar] [CrossRef]
- Chen, G.N.; Guo, P.Q.; Wang, Y.; Wang, L.; Shu, H.; Li, Y.Z.; Jing, W.H.; Chang, C.; Fu, Q. Preparation of molecularly imprinted polymers and application in a biomimetic biotin-avidin-ELISA for the detection of bovine serum albumin. Talanta 2019, 198, 55–62. [Google Scholar]
- Wilchek, M.; Bayer, E.A. The avidin biotin complex in bioanalytical applications. Anal. Biochem. 1988, 171, 1–32. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Ham, J.S.; Ahn, D.U. Separation of ovotransferrin from chicken egg white without using organic solvents. Poult. Sci. 2013, 92, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Xie, Y.; Wang, J.; Li, S.; Jin, Y.; Ma, M. Large-scale purification of ovalbumin using polyethylene glycol precipitation and isoelectric precipitation. Poult. Sci. 2019, 98, 1545–1550. [Google Scholar] [CrossRef]
- Coskun, O. Separation techniques: Chromatography. North Clin. Istanb. 2016, 3, 156–160. [Google Scholar] [PubMed]
- Cummins, P.M.; Rochfort, K.D.; O’Connor, B.F. Ion-Exchange Chromatography: Basic Principles and Application. MIMB 2017, 1485, 209–223. [Google Scholar]
- Wen, W.; Wan, J.; Cao, X.; Xia, J. Preparation of a light-sensitive and reversible dissolution copolymer and its application in lysozyme purification. Biotechnol. Prog. 2007, 23, 1124–1129. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Tekinay, T.; Ozalp, V.C.; Arica, M.Y. Fibrous polymer grafted magnetic chitosan beads with strong poly(cation-exchange) groups for single step purification of lysozyme. J. Chromatogr. B 2015, 990, 84–95. [Google Scholar] [CrossRef]
- Hirsch, D.B.; Baieli, M.F.; Urtasun, N.; Lazaro- Martinez, J.M.; Glisoni, R.J.; Miranda, M.V.; Cascone, O.; Wolman, F.J. Sulfanilic Acid-Modified Chitosan Mini-Spheres and Their Application for Lysozyme Purification from Egg White. Biotechnol. Prog. 2018, 34, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.-F.; La, S.-Y.; Lin, C.-S.; Suen, S.-Y.; Wang, M.-Y. Purification of lysozyme from chicken egg white using diatom frustules. Food Chem. 2019, 286, 483–490. [Google Scholar] [CrossRef]
- Duong-Ly, K.C.; Gabelli, S.B. Gel Filtration Chromatography (Size Exclusion Chromatography) of Proteins. Method Enzymol. 2014, 541, 105–114. [Google Scholar]
- O’Fagain, C.; Cummins, P.M.; O’Connor, B.F. Gel-Filtration Chromatography. MIMB 2016, 1485, 15–25. [Google Scholar]
- Wang, Z.H.; Tu, A.B.; Tang, D.Y.; Shan, Y.Y. Effectively preparing soluble ovomucin with high antiviral activity from egg white. Int. J. Biol. Macromol. 2018, 118, 504–510. [Google Scholar] [CrossRef]
- Geng, F.; Huang, X.; Yan, N.; Jia, L.; Ma, M. Purification of hen egg white ovomacroglobulin using one-step chromatography. J. Sep. Sci. 2013, 36, 3717–3722. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, E.S.P.; Koza, S.M. Advances in size-exclusion separations of proteins and polymers by UHPLC. TrAC-Trend Anal. Chem. 2014, 63, 85–94. [Google Scholar] [CrossRef]
- Wolman, F.J.; Copello, G.J.; Mebert, A.M.; Targovnik, A.M.; Miranda, M.V.; Navarro del Canizo, A.A.; Diaz, L.E.; Cascone, O. Egg white lysozyme purification with a chitin-silica-based affinity chromatographic matrix. Eur. Food Res. Technol. 2010, 231, 181–188. [Google Scholar] [CrossRef]
- Lacki, K.M.; Riske, F.J. Affinity Chromatography: An Enabling Technology for Large-Scale Bioprocessing. Biotechnol. J. 2020, 15, 1800397. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-W.; Yang, T.; Chen, S.; Chen, X.-W.; Wang, J.-H. Nickel chelating functionalization of graphene composite for metal affinity membrane isolation of lysozyme. J. Mater. Chem. B 2013, 1, 810–818. [Google Scholar] [CrossRef]
- Li, Z.; Cao, M.; Zhang, W.; Liu, L.; Wang, J.; Ge, W.; Yuan, Y.; Yue, T.; Li, R.; Yu, W. Affinity adsorption of lysozyme with Reactive Red 120 modified magnetic chitosan microspheres. Food Chem. 2014, 145, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Baydemir, G.; Turkoglu, E.A.; Andac, M.; Percin, I.; Denizli, A. Composite cryogels for lysozyme purification. Biotechnol. Appl. Biochem. 2015, 62, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-H.; Chou, S.-Y.; Chang, Y.-K. Rapid purification of lysozyme by mixed-mode adsorption chromatography in stirred fluidized bed. Food Chem. 2019, 272, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Feng, X.; Zhong, T.; Zhang, X. Preparation of supermacroporous cryogels with improved mechanical strength for efficient purification of lysozyme from chicken egg white. J. Sep. Sci. 2020, 43, 3315–3326. [Google Scholar] [CrossRef] [PubMed]
- Show, P.L.; Ooi, C.W.; Song, C.P.; Chai, W.S.; Lin, G.-T.; Liu, B.-L.; Chang, Y.-K. Purification of lysozyme from chicken egg white by high-density cation exchange adsorbents in stirred fluidized bed adsorption system. Food Chem. 2021, 343, 128543. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, T.; Wu, N.; Tu, Y.; Huang, X.; Ahn, D.U. An efficient, scalable and environmentally friendly separation method for ovoinhibitor from chicken egg white. LWT-Food Sci. Technol. 2020, 127, 109367. [Google Scholar] [CrossRef]
- Shimazaki, Y.; Ochi, Y.; Fujimura, K. Microscale isolation of native forms of lysozyme from chicken egg white by gel isoelectric focusing. Electrophoresis 2018, 39, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, Y.; Takahashi, A. Antibacterial activity of lysozyme-binding proteins from chicken egg white. J. Microbiol. Methods 2018, 154, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Jiang, Z.; Liu, Z.; Chen, L.; Zhang, Q.; Tian, Y.; Sohail, A.; Khan, M.I.; Xiao, H.; Liu, X.; et al. Purification of low-abundance lysozyme in egg white via free-flow electrophoresis with gel-filtration chromatography. Electrophoresis 2020, 41, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, L.; Sun, H.; Chu, Z.; Chen, H.; Zhao, Y.; Zhang, W. Carrier ampholyte-free free-flow isoelectric focusing for separation of protein. Electrophoresis 2019, 40, 2610–2617. [Google Scholar] [CrossRef]
- Rathore, A.S.; Shirke, A. Recent developments in membrane-based separations in biotechnology processes: Review. Prep. Biochem. Biotechnol. 2011, 41, 398–421. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.-S.; Yang, S.-M.; Hsin, A.; Chang, Y.-K. Dye-Affinity Nanofibrous Membrane for Adsorption of Lysozyme: Preparation and Performance Evaluation. Food Technol. Biotechnol. 2018, 56, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.L.; Ooi, C.W.; Ng, I.S.; Show, P.L.; Lin, K.J.; Chang, Y.K. Effective purification of lysozyme from chicken egg white by tris (hydroxymethyl)aminomethane affinity nanofiber membrane. Food Chem. 2020, 327, 12. [Google Scholar] [CrossRef]
- Wang, C.Z.; Shen, J.W.; Zhu, J.T.; Bo, C.M.; Wei, Y.M. Tetrazole-functionalized cation-exchange membrane adsorbers with high binding capacity and unique separation feature for protein. J. Chromatogr. B 2018, 1097, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Madadkar, P.; Sadavarte, R.; Ghosh, R. Performance Comparison of a Laterally-Fed Membrane Chromatography (LFMC) Device with a Commercial Resin Packed Column. Membranes 2019, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Diederich, P.; Amrhein, S.; Haemmerling, F.; Hubbuch, J. Evaluation of PEG/phosphate aqueous two-phase systems for the purification of the chicken egg white protein avidin by using high-throughput techniques. Chem. Eng. Sci. 2013, 104, 945–956. [Google Scholar] [CrossRef]
- Pereira, M.M.; Cruz, R.A.P.; Almeida, M.R.; Lima, A.S.; Coutinho, J.A.P.; Freire, M.G. Single-step purification of ovalbumin from egg white using aqueous biphasic systems. Process Biochem. 2016, 51, 781–791. [Google Scholar] [CrossRef]
- Belchior, D.C.V.; Quental, M.V.; Pereira, M.M.; Mendonca, C.M.N.; Duarte, I.F.; Freire, M.G. Performance of tetraalkylammonium-based ionic liquids as constituents of aqueous biphasic systems in the extraction of ovalbumin and lysozyme. Sep. Purif. Technol. 2020, 233, 116019. [Google Scholar] [CrossRef]
- Zhang, N.; Xu, Y.; Li, Z.; Yan, C.; Mei, K.; Ding, M.; Ding, S.; Guan, P.; Qian, L.; Du, C.; et al. Molecularly Imprinted Materials for Selective Biological Recognition. Macromol. Rapid Commun. 2019, 40, 1900096. [Google Scholar] [CrossRef]
- Chen, Y.; He, X.-W.; Mao, J.; Li, W.-Y.; Zhang, Y.-K. Preparation and application of hollow molecularly imprinted polymers with a super-high selectivity to the template protein. J. Sep. Sci. 2013, 36, 3449–3456. [Google Scholar] [CrossRef]
- Wang, C.; Hu, X.; Guan, P.; Wu, D.; Yang, L.; Du, C. Preparation of Molecularly Imprinted Regenerated Cellulose Composite Membranes by Surface-Initiated Atom Transfer Radical Polymerization Method for Selective Recognition of Lysozyme. Adsorpt. Sci. Technol. 2015, 33, 411–425. [Google Scholar] [CrossRef]
- Wang, X.; Dong, S.; Bai, Q. Preparation of lysozyme molecularly imprinted polymers and purification of lysozyme from egg white. Biomed. Chromatogr. 2014, 28, 907–912. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, L.; Hao, Y.; Cui, X.; Liu, D.; Zhang, M.; Tang, Y. Novel polydopamine imprinting layers coated magnetic carbon nanotubes for specific separation of lysozyme from egg white. Talanta 2015, 144, 1125–1132. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Y.; Wei, X.; Chen, J.; Xu, P.; Zhou, Y. Preparation of magnetic molecularly imprinted polymers based on a deep eutectic solvent as the functional monomer for specific recognition of lysozyme. Microchim. Acta 2018, 185, 146. [Google Scholar] [CrossRef] [PubMed]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Ahn, D.U. Separation of ovotransferrin and ovomucoid from chicken egg white. Poult. Sci. 2014, 93, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Ahn, D.U. Sequential separation of lysozyme, ovomucin, ovotransferrin, and ovalbumin from egg white. Poult. Sci. 2014, 93, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Ahn, D.U.; Zhao, Y.; Li, K.; Li, S.; Huang, X. An easy and rapid separation method for five major proteins from egg white: Successive extraction and MALDI-TOF-MS identification. Food Chem. 2020, 315, 126207. [Google Scholar] [CrossRef]
- Geng, F.; Huang, Q.; Wu, X.; Ren, G.; Shan, Y.; Jin, G.; Ma, M. Co-purification of chicken egg white proteins using polyethylene glycol precipitation and anion-exchange chromatography. Sep. Purif. Technol. 2012, 96, 75–80. [Google Scholar] [CrossRef]
- Brand, J.; Dachmann, E.; Pichler, M.; Lotz, S.; Kulozik, U. A novel approach for lysozyme and ovotransferrin fractionation from egg white by radial flow membrane adsorption chromatography: Impact of product and process variables. Sep. Purif. Technol. 2016, 161, 44–52. [Google Scholar] [CrossRef]
- Ma, X.; Liang, R.; Yang, X.; Gou, J.; Li, Y.; Lozano-Ojalvo, D. Simultaneous separation of the four major allergens of hen egg white. J. Chromatogr. B 2020, 1152, 122231. [Google Scholar] [CrossRef]

| Method | Mechanism | Type | Target Protein | Yield | Purity | Activity | Refs. |
|---|---|---|---|---|---|---|---|
| Ion Exchange Chromatography | Differences in the ability of protein ions to compete with mobile phase for stationary phase surface charge positions | PNBCC | LYS | 81.3% | - | Keep | [56] |
| Magnetic chitosan (MCHT) beads | LYS | - | 93% | Keep | [57] | ||
| A cation exchange matrix with zwitterionic and multimodal properties | LYS | 81.9% | 86.5% | - | [58] | ||
| AQ1 and NP | LYS | With AQ1 was 86%, and with NP was 82% | With AQ1 was 95%, and with NP was 90% | Keep | [59] | ||
| Gel Filtration Chromatography | Differences in molecular weight or molecular shape of proteins | Sephacryl S-300 HR gel column | OVN | 3.02 g/kg fresh egg white | 99.13% | Keep | [62] |
| Q Sepharose Fast Flow anion-exchange column and Sephacryl S- 200 HR gel column | Ovomacroglobulin | 37.76% | 100% | - | [48] | ||
| Sephacryl S-200 gel column | Ovomacroglobulin | 62.5% | 97.0 ± 0.3% | - | [63] | ||
| Affinity Chromatography | Differences in affinity between the substance to be separated and others with specific ligands | GO–PBA–IDA–Ni composite | LYS | 90% | Electrophoresis pure | - | [67] |
| Reactive Red 120 | LYS | 89.1% | 80.7% | - | [68] | ||
| Adsorption Chromatography | Differences in the adsorption capacity of substances to be separated on the active adsorption center of the stationary phase surface | PHEMAPA BEC | LYS | - | Electrophoresis pure | - | [69] |
| STREAMLINE Direct HST | LYS | 94.3% | Purification factor of 15.7 | Keep | [70] | ||
| Low temperature copolymer gel | LYS | 100% | - | - | [71] | ||
| STREAMLINE SP and SP-XL | LYS | 100% by SP vs. 93.78% by SP-XL | Purification factor of 26-fold by SP vs. 40-fold by SP-XL | Keep | [72] |
| Method | Mechanism | Type | Target Protein | Yield | Purity | Activity | Refs. |
|---|---|---|---|---|---|---|---|
| Differences in mobility of proteins in electric fields due to different charging of proteins when pH is at the isoelectric point or the non-isoelectric point | Non-denatured gel isoelectric focusing | LYS | - | - | Keep | [74] | |
| Free-flow electrophoresis | LYS | 53.3% | 80% | Keep | [76] | ||
| Homemade carrier ampholyte free-flow isoeletric focusing system | OVM, OVA and OVT | - | - | - | [77] | ||
| Membrane chromatography | Using membranes as substrates to bind ligands, then separating proteins by adsorption, washing, elution, and regeneration | Polyacrylonitrile nanofiber membranes | LYS | 87% | Purification factor of 47-fold | - | [79] |
| Polyacrylonitrile nanofiber membranes functionalized with P-Tris | LYS | 93.3% | Purification factor of 103.98-fold | Keep | [80] | ||
| Novel high-capacity tetrazolium-functionalized weak cation exchange membranes | LYS and OVT | 93% | - | - | [81] | ||
| Laterally-fed membrane chromatography (LFMC) devices | The protein mixture consisting of OVA, OVT and LYS | - | - | - | [82] | ||
| Aqueous two-phase system | Differences in partition coefficients of substances between mutually immiscible two-aqueous phases. The partition coefficients depend on various interactions between the solute and the aqueous two-phase system, mainly electrostatic, hydrophobic and bio-affinity interactions | The PEG/phosphate system | Avidin | 92% | Purification factor of 5.7 | - | [83] |
| The PEG/potassium citric acid buffer | OVA | 65% | No other peaks in HPLC | Keep | [84] | ||
| The tetraalkylammonium-based ionic liquid/potassium phosphate solution | LYS | 99% | - | Keep | [85] | ||
| Molecular imprinting technology | Preparing specific molecularly imprinted polymers by simulating enzyme-substrate or antibody-antigen interactions for specific recognition of target protein | Hollow imprinted silica polymers | LYS | - | - | - | [87] |
| Novel types of polymeric membranes | LYS | - | Separation factor of 23.08 | - | [88] | ||
| Molecularly imprinted polymers | LYS | 98.2% | 100% | - | [89] | ||
| Novel core-shell nanocomposites | LYS | - | - | - | [90] | ||
| Magnetized molecularly imprinted polymers | LYS | - | - | - | [91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Huang, X.; Tang, Q.; Ma, M.; Jin, Y.; Sheng, L. Functional Properties and Extraction Techniques of Chicken Egg White Proteins. Foods 2022, 11, 2434. https://doi.org/10.3390/foods11162434
Li Z, Huang X, Tang Q, Ma M, Jin Y, Sheng L. Functional Properties and Extraction Techniques of Chicken Egg White Proteins. Foods. 2022; 11(16):2434. https://doi.org/10.3390/foods11162434
Chicago/Turabian StyleLi, Zhe, Xi Huang, Qinyue Tang, Meihu Ma, Yongguo Jin, and Long Sheng. 2022. "Functional Properties and Extraction Techniques of Chicken Egg White Proteins" Foods 11, no. 16: 2434. https://doi.org/10.3390/foods11162434
APA StyleLi, Z., Huang, X., Tang, Q., Ma, M., Jin, Y., & Sheng, L. (2022). Functional Properties and Extraction Techniques of Chicken Egg White Proteins. Foods, 11(16), 2434. https://doi.org/10.3390/foods11162434







