Control Measurements of Escherichia coli Biofilm: A Review
Abstract
:1. Introduction
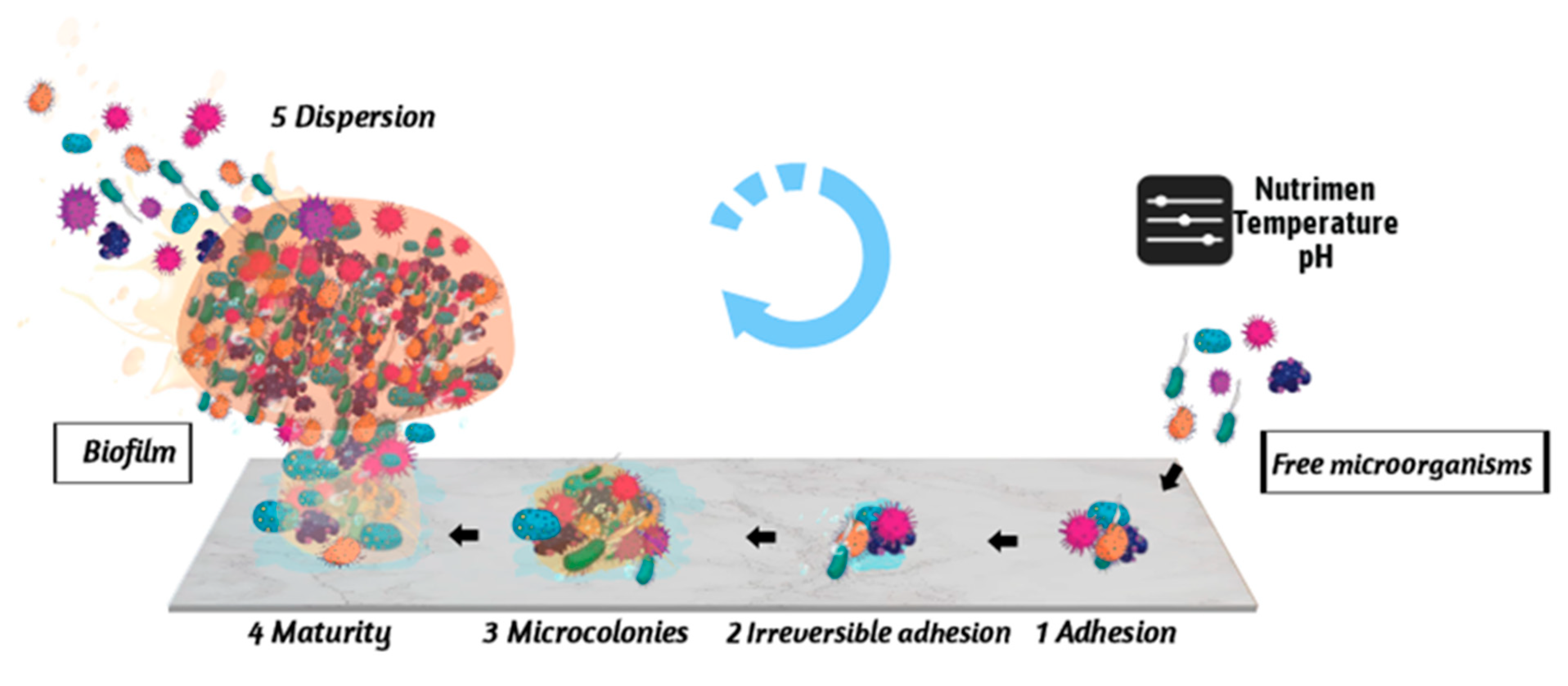
2. Stages of the Formation of E. coli Biofilm
2.1. Reversible Adhesion between Microorganisms and Carriers
2.2. Irreversible Adhesion between Microorganisms and Carriers
2.3. Colony Formation Stage
2.4. Biofilm Maturation Stage
2.5. Biofilm Dispersion Stage
3. Prevalence of E. coli Biofilm in the Food Industry
4. Control Measures for the Prevention of E. coli Biofilm Formation
4.1. Chemical Methods
4.2. Physical Methods
4.3. Biological Components
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friedmann, H. Escherich and Escherichia. Adv. Appl. Microbiol. 2006, 60, 133–196. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.G.; Davis, B.R.; Wachsmuth, I.K.; Riley, L.W.; Morris, G.K. Laboratory investigation of hemorrhagic colitis outbreaks associated with a rare Escherichia coli serotype. J. Clin. Microbiol. 1983, 18, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.P. Escherichia coli O157:H7 and its significance in foods. Int. J. Food Microbiol. 1991, 12, 289–301. [Google Scholar] [CrossRef]
- Shivaprasad, D.P.; Taneja, N.K.; Lakra, A.; Sachdev, D. In vitro and in situ abrogation of biofilm formation in E. coli by vitamin C through ROS generation, disruption of quorum sensing and exopolysaccharide production. Food Chem. 2020, 341, 128171. [Google Scholar] [CrossRef]
- Corzo-Ariyama, H.A.; García-Heredia, A.; Heredia, N.; García, S.; León, J.; Jaykus, L.; Solís-Soto, L. Phylogroups, pathotypes, biofilm formation and antimicrobial resistance of Escherichia coli isolates in farms and packing facilities of tomato, jalapeño pepper and cantaloupe from Northern Mexico. Int. J. Food Microbiol. 2019, 290, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.; Werber, D.; Cramer, J.; Askar, M.; Faber, M.; an der Heiden, M.; Bernard, H.; Fruth, A.; Prager, R.; Spode, A.; et al. Epidemic Profile of Shiga-Toxin-Producing Escherichia coli O104:H4 Outbreak in Germany. N. Engl. J. Med. 2011, 365, 1771–1780. [Google Scholar] [CrossRef]
- Buchholz, U.; Bernard, H.; Werber, D.; Böhmer, M.M.; Remschmidt, C.; Wilking, H.; Deleré, Y.; an der Heiden, M.; Adlhoch, C.; Dreesman, J.; et al. German Outbreak of Escherichia coli O104:H4 Associated with Sprouts. N. Engl. J. Med. 2011, 365, 1763–1770. [Google Scholar] [CrossRef]
- Mermin, J.H.; Griffin, P.M. Invited Commentary: Public Health in Crisis: Outbreaks of Escherichia coli O157: H7 Infections in Japan. Am. J. Epidemiol. 1999, 150, 797–803. [Google Scholar] [CrossRef]
- Branda, S.S.; Vik, Å.; Friedman, L.; Kolter, R. Biofilms: The matrix revisited. Trends Microbiol. 2005, 13, 20–26. [Google Scholar] [CrossRef]
- Flemming, H.C.; Neu, T.R.; Wozniak, D.J. The EPS Matrix: The “House of Biofilm Cells”. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef]
- Carpio, A.; Cebrián, E.; Vidal, P. Biofilms as poroelastic materials. Int. J. Non-Linear Mech. 2019, 109, 1–8. [Google Scholar] [CrossRef]
- Watnick, P.; Kolter, R. Biofilm, City of Microbes. J. Bacteriol. 2000, 182, 2675. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.W. The biofilm matrix-an immobilaized but dynamic microbial environment. Trends Microbiol. 2001, 9, 222–227. [Google Scholar] [CrossRef]
- Simes, M.; Bennett, R.N.; Rosa, E.A.S. Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Cheminform 2009, 26, 746–757. [Google Scholar] [CrossRef]
- Uneputty, A.; Dávila-Lezama, A.; Garibo, D.; Oknianska, A.; Bogdanchikova, N.; Hernández-Sánchez, J.F.; Susarrey-Arce, A. Strategies applied to modify structured and smooth surfaces: A step closer to reduce bacterial adhesion and biofilm formation. Colloid Interface Sci. Commun. 2022, 46, 100560. [Google Scholar] [CrossRef]
- Harmsen, M.; Yang, L.; Pamp, S.N.J.; Tolker-Nielsen, T. An update on Pseudomonas aeruginosa biofilm formation, tolerance, and dispersal. Pathog. Dis. 2010, 59, 253–268. [Google Scholar] [CrossRef]
- Simões, M.; Simões, L.C.; Vieira, M.J. A review of current and emergent biofilm control strategies. LWT-Food Sci. Technol. 2010, 43, 573–583. [Google Scholar] [CrossRef]
- Danese, N.P.; Pratt, A.L.; Kolter, R. Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J. Bacteriol. 2000, 182, 2399–3593. [Google Scholar] [CrossRef]
- Wille, J.; Coenye, T. Biofilm dispersion: The key to biofilm eradication or opening Pandora’s box? Biofilm 2020, 2, 100027. [Google Scholar] [CrossRef]
- Sofos, J.N.; Geornaras, I. Overview of current meat hygiene and safety risks and summary of recent studies on biofilms, and control of Escherichia coli O157:H7 in nonintact, and Listeria monocytogenes in ready-to-eat, meat products. Meat Ence 2010, 86, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.G.C. Assessing manufacturers’ recommended concentrations of commercial sanitizers on inactivation of Listeria monocytogenes. Food Control 2012, 26, 194–199. [Google Scholar] [CrossRef]
- Helgadóttir, S.; Pandit, S.; Mokkapati, V.R.S.S.; Westerlund, F.; Apell, P.; Mijakovic, I. Vitamin C Pretreatment Enhances the Antibacterial Effect of Cold Atmospheric Plasma. Front. Cell. Infect. Microbiol. 2017, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.; McBain, A.J.; Rickard, A.H. Formation of microbial biofilm in hygienic situations: A problem of control. Int. Biodeterior. Biodegrad. 2003, 51, 245–248. [Google Scholar] [CrossRef]
- Kang, J.W.; Lee, H.Y.; Kang, D.H. Synergistic bactericidal effect of hot water with citric acid against Escherichia coli O157:H7 biofilm formed on stainless steel. Food Microbiol. 2020, 95, 103676. [Google Scholar] [CrossRef]
- Ma, Z.; Bumunang, E.W.; Stanford, K.; Bie, X.; Niu, Y.D.; Mcallister, T.A. Biofilm Formation by Shiga Toxin-Producing Escherichia coli on Stainless Steel Coupons as Affected by Temperature and Incubation Time. Microorganisms 2019, 7, 95. [Google Scholar] [CrossRef]
- Milho, C.; Silva, M.; Sillankorva, S.; Harper, D.R. Biofilm Applications of Bacteriophages. Bacteriophages 2019, 1–35. [Google Scholar] [CrossRef]
- Crecencio, R.B.; Brisola, M.C.; Bitner, D.; Frigo, A.; Stefani, L.M. Antimicrobial susceptibility, biofilm formation and genetic profiles of Escherichia coli isolated from retail chicken meat. Infect. Genet. Evol. 2020, 84, 104355. [Google Scholar] [CrossRef]
- Skandamis, P.N.; Stopforth, J.D.; Ashton, L.V.; Geornaras, I.; Kendall, P.A. Escherichia coli O157:H7 survival, biofilm formation and acid tolerance under simulated slaughter plant moist and dry conditions. Food Microbiol. 2009, 26, 112–119. [Google Scholar] [CrossRef]
- Hood, S.K.; Zottola, E.A. Adherence to stainless steel by foodborne microorganisms during growth in model food systems. Int. J. Food Microbiol. 1997, 37, 145–153. [Google Scholar] [CrossRef]
- Dourou, D.; Beauchamp, C.S.; Yoon, Y.; Geornaras, I.; Belk, K.E.; Smith, G.C.; Nychas, G.J.E.; Sofos, J.N. Attachment and biofilm formation by Escherichia coli O157:H7 at different temperatures, on various food-contact surfaces encountered in beef processing. Int. J. Food Microbiol. 2011, 149, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yang, L.; Chen, G.; Li, B.; Chen, D.; Li, L.; Xu, Z. Pathogenic features and characteristics of food borne pathogens biofilm: Biomass, viability and matrix. Microb. Pathog. 2017, 111, 285–291. [Google Scholar] [CrossRef]
- Liu, N.T.; Nou, X.; Lefcourt, A.M.; Shelton, D.R.; Lo, Y.M. Dual-species biofilm formation by Escherichia coli O157:H7 and environmental bacteria isolated from fresh-cut processing facilities. Int. J. Food Microbiol. 2014, 171, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Duc, H.M.; Son, H.M.; Honjoh, K.I.; Miyamoto, T. Isolation and application of bacteriophages to reduce Salmonella contamination in raw chicken meat. LWT-Food Sci. Technol. 2018, 91, 353–360. [Google Scholar] [CrossRef]
- Meireles, A.; Ferreira, C.; Melo, L.; Simes, M. Comparative stability and efficacy of selected chlorine-based biocides against Escherichia coli in planktonic and biofilm states. Food Res. Int. 2017, 102, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Pin-Ngoen, K.; Kositchaiyong, A.; Prapagdee, B.; Sombatsompop, N. Formation of Escherichia coli biofilm on LLDPE sheets by incorporation of 2-hydroxypropyl-3-piperazinyl-quinoline carboxylic acid methacrylate or silver-substituted zeolite. Int. Biodeterior. Biodegrad. 2016, 109, 211–222. [Google Scholar] [CrossRef]
- Bang, J.; Hong, A.; Kim, H.; Beuchat, L.R.; Rhee, M.S.; Kim, Y.; Ryu, J.-H. Inactivation of Escherichia coli O157:H7 in biofilm on food-contact surfaces by sequential treatments of aqueous chlorine dioxide and drying. Int. J. Food Microbiol. 2014, 191, 129–134. [Google Scholar] [CrossRef]
- Fu, Y.; Deering, A.J.; Bhunia, A.K.; Yao, Y. Biofilm of Escherichia coli O157:H7 on cantaloupe surface is resistant to lauroyl arginate ethyl and sodium hypochlorite. Int. J. Food Microbiol. 2017, 260, 11–16. [Google Scholar] [CrossRef]
- Banerjee, S.; Ghosh, D.; Vishakha, K.; Das, S.; Mondal, S.; Ganguli, A. Photodynamic antimicrobial chemotherapy (PACT) using riboflavin inhibits the mono and dual species biofilm produced by antibiotic resistant Staphylococcus aureus and Escherichia coli. Photodiagnosis Photodyn. Ther. 2020, 32, 102002. [Google Scholar] [CrossRef]
- Ban, G.-H.; Yoon, H.; Kang, D.-H. A comparison of saturated steam and superheated steam for inactivation of Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes biofilms on polyvinyl chloride and stainless steel. Food Control 2014, 40, 344–350. [Google Scholar] [CrossRef]
- Kovalova, Z.; Leroy, M.; Kirkpatrick, M.J.; Odic, E.; Machala, Z. Corona discharges with water electrospray for Escherichia coli biofilm eradication on a surface. Bioelectrochemistry 2016, 112, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Ban, G.H.; Park, S.H.; Kim, S.O.; Ryu, S.; Kang, D.H. Synergistic effect of steam and lactic acid against Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes biofilms on polyvinyl chloride and stainless steel. Int. J. Food Microbiol. 2012, 157, 218–223. [Google Scholar] [CrossRef]
- Cui, H.; Ma, C.; Lin, L. Synergetic antibacterial efficacy of cold nitrogen plasma and clove oil against Escherichia coli O157:H7 biofilms on lettuce. Food Control 2016, 66, 8–16. [Google Scholar] [CrossRef]
- Bodur, T.; Cagri-Mehmetoglu, A. Removal of Listeria monocytogenes, Staphylococcus aureus and Escherichia coli O157:H7 biofilms on stainless steel using scallop shell powder. Food Control 2012, 25, 1–9. [Google Scholar] [CrossRef]
- Wang, C.; Hang, H.; Zhou, S.B.; Niu, Y.D.; Du, H.C.; Kim, S.; Tim, A.M. Bacteriophage biocontrol of Shiga toxigenic Escherichia coli (STEC) O145 biofilms on stainless steel reduces the contamination of beef. Food Microbiol. 2020, 92, 103572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shigemura, K.; Duc, H.M.; Shen, C.; Huang, H.-H.; Sato, J.; Masuda, Y.; Honjoh, K.-I.; Miyamoto, T. Effects of bacteriophage on inhibition and removal of mixed biofilm of enterohemorrhagic Escherichia coli O157:H7 and O91:H. LWT-Food Sci. Technol. 2020, 134, 109945. [Google Scholar] [CrossRef]
- Montso, P.K.; Mlambo, V.; Ateba, C.N. Efficacy of novel phages for control of multi-drug resistant Escherichia coli O177 on artificially contaminated beef and their potential to disrupt biofilm formation. Food Microbiol. 2021, 94, 103647. [Google Scholar] [CrossRef]
- Lou, Z.; Song, X.; Hong, Y.; Wang, H.; Lin, Y. Separation and enrichment of burdock leaf components and their inhibition activity on biofilm formation of E. coli. Food Control 2013, 32, 270–274. [Google Scholar] [CrossRef]
- Baptista, J.; Simes, M.; Borges, A. Effect of plant-based catecholic molecules on the prevention and eradication of Escherichia coli biofilms: A structure activity relationship study. Int. Biodeterior. Biodegrad. 2018, 141, 101–113. [Google Scholar] [CrossRef]
- Campana, R.; Baffone, W. Carvacrol efficacy in reducing microbial biofilms on stainless steel and in limiting re-growth of injured cells. Food Control 2018, 90, 10–17. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Cho, H.S.; Ryu, S.Y.; Cho, M.H.; Lee, J. Coumarins reduce biofilm formation and the virulence of Escherichia coli O157:H7. Phytomed. Int. J. Phytother. Phytopharm. 2014, 21, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Inhibition of Escherichia coli O157:H7 biofilm on vegetable surface by solid liposomes of clove oil. LWT-Food Sci. Technol. 2019, 117, 108656. [Google Scholar] [CrossRef]
- Silva, P.M.; Baldry, M.; Peng, P.; Oliveira Silva, J.N.; Soares, T.; Brayner, F.A.; Alves, L.C.; Feitosa, A.P.S.; Paiva, P.M.G.; Ingmer, H.; et al. Punica granatum sarcotesta lectin (PgTeL) impairs growth, structure, viability, aggregation, and biofilm formation ability of Staphylococcus aureus clinical isolates. Int. J. Biol. Macromol. 2019, 123, 600–608. [Google Scholar] [CrossRef]
- Mohammadi, M.; Masoumipour, F.; Hassanshahian, M.; Jafarinasab, T. Study the antibacterial and antibiofilm activity of Carum copticum against antibiotic-resistant bacteria in planktonic and biofilm forms. Microb. Pathog. 2019, 129, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Vidács, A.; Kerekes, E.; Rajkó, R.; Petkovits, T.; Alharbi, N.S.; Khaled, J.M.; Vágvölgyi, C.; Krisch, J. Optimization of essential oil-based natural disinfectants against Listeria monocytogenes and Escherichia coli biofilms formed on polypropylene surfaces. J. Mol. Liq. 2018, 255, 257–262. [Google Scholar] [CrossRef]
- Wang, R.; Kalchayanand, N.; King, D.A.; Luedtke, B.E.; Bosilevac, J.M.; Arthur, T.M. Biofilm Formation and Sanitizer Resistance of Escherichia coli O157:H7 Strains Isolated from “High Event Period” Meat Contamination. J. Food Prot. 2014, 77, 1982–1987. [Google Scholar] [CrossRef]
- Lin, F.; Yuan, S.; Han, W. Effective prevention of Escherichia coli biofilm on materials by nano-vibration. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125610. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Feasibility of cold plasma for the control of biofilms in food industry. Trends Food Sci. Technol. 2020, 99, 142–151. [Google Scholar] [CrossRef]
- Kadri, H.; Costello, K.M.; Thomas, P.; Wantock, T.; Velliou, E.G. The antimicrobial efficacy of remote cold atmospheric plasma effluent against single and mixed bacterial biofilms of varying age. Food Res. Int. 2021, 141, 110126. [Google Scholar] [CrossRef]
- Adator, E.H.; Cheng, M.; Holley, R.; Mcallister, T.; Narvaez-Bravo, C. Ability of Shiga toxigenic Escherichia coli to survive within dry-surface biofilms and transfer to fresh lettuce. Int. J. Food Microbiol. 2018, 269, 52–59. [Google Scholar] [CrossRef]
- Cagri-Mehmetoglu, A. Inhibition of Listeria monocytogenes and Salmonella enteritidis on chicken wings using scallop-shell powder. Poultry Science 2011, 20, 2600–2605. [Google Scholar] [CrossRef] [PubMed]
- Bae, D.H.; Yeon, J.H.; Park, S.Y.; Lee, D.H.; Ha, S.D. Bactericidal effects of CaO (scallop-shell powder) on foodborne pathogenic bacteria. Arch. Pharmacal Res. 2006, 29, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.A.; Huang, Z.; Rathinavelu, S.; Hu, J.-F.; Garo, E.; Ellis, M.; Norman, V.L.; Buckle, R.; Williams, R.B.; Starks, C.M.; et al. Optimized plant compound with potent anti-biofilm activity across gram-negative species. Bioorg. Med. Chem. 2020, 28, 115229. [Google Scholar] [CrossRef] [PubMed]
| Bacteriostatic Mode | Bacteria | Carrier Material | Antibacterial Substance | Concentration | Process Time | Reduction (logCFU·cm−2) or Bacteriostatic Rate | Reference |
|---|---|---|---|---|---|---|---|
| Chemical methods | E. coli CECT 434 | Stainless steel AISI 316 | Neutral oxygen potential water | 50 ppm | 20 min | 3.26 | [35] |
| Chlorine dioxide | 50 ppm | 20 min | 3.20 | ||||
| Sodium dichloroisocyanurate | 50 ppm | 20 min | 3.20 | ||||
| Sodium hypochlorite | 50 ppm | 20 min | 2.46 | ||||
| E. coli ATCC 25922 | Linear low-density poly-ethylene | 2-hydroxypropyl-3-piperazinyl-quinoline carboxylic acid methacrylate | 1500–2500 ppm | 3-5 day | 99% | [36] | |
| E. coli O157:H7 | Stainless steel | Sodium hypochlorite (NaOCl) | 200 μg/mL | 15 min | 7.7 | [37] | |
| Aqueous chlorine dioxide (ClO2) | 200 μg/mL | 15 min | ND | ||||
| Glass | NaOCl | 200 μg/mL | 15 min | 8.2 | |||
| ClO2 | 200 μg/mL | 15 min | ND | ||||
| Plastic | NaOCl | 200 μg/mL | 15 min | 3.3 | |||
| ClO2 | 200 μg/mL | 15 min | ND | ||||
| Wood | NaOCl | 200 μg/mL | 15 min | 1.3 | |||
| ClO2 | 200 μg/mL | 15 min | 1.5 | ||||
| E. coli O157:H7 B6-914 | Glass cover Slides | Lauroyl arginate ethyl | 200 μg/mL | 24 h | 0.46 | [38] | |
| Sodium hypochlorite | 200 μg/mL | 24 h | 0.59 | ||||
| Multi drug resistant Escherichia coli | glass slides | Photodynamic antimicrobial chemotherapy | 50 μM | 10min | 34% | [39] | |
| Physical methods | E. coli O157:H7 | Polyvinyl chloride | Saturated steam | / | 5 s | 1.21 | [40] |
| Superheated steam | / | 5 s | 1.26 | ||||
| Stainless steel | Saturated steam | / | 5 s | 1.52 | |||
| Superheated steam | / | 5 s | 1.84 | ||||
| E. coli strain BW25113 F+ | Glass cover slides | Positive corona | / | 15 min | 5.28 | [41] | |
| Negative corona | 5.4 | ||||||
| E. coli O157:H7 (ATCC 35150, ATCC 43889, ATCC 43890) | Polyvinyl chloride | Lactic acid and water vapors | 0.5%–2% | 5 s | 0.76–3.78 | [42] | |
| Stainless steel | Lactic acid and water vapors | 0.5%–2% | 5 s | 1.64–3.92 | |||
| E. coli EHEC O157:H7 CICC 21530 | Stainless steel | Clove oil | 1 mg/mL | 30 min | 3.32 | [43] | |
| Cold nitrogen plasma | / | 3 min | 2.23 | ||||
| Biological components components | E. coli O157:H7 NCTC 12900 | Stainless steel | Scallop shell powder | 0.25% | 1 min | 4-6 | [44] |
| 0.5% | 3-5 | ||||||
| STEC O145:H25 | Stainless steel | Bacteriophage AZO145A | 2 × 1010 pfu/mL | 3 h | 3.1 | [45] | |
| Escherichia coli O157:H7 and O91:H- | 96-well plates | Bacteriophage FP43 | 1010 pfu/mL | 6 h | 2.85 | [46] | |
| E. coli O177 | 96-well polystyrene plates | Phage cocktail stock | 1 × 108 pfu/mL | 24 h | ND | [47] | |
| E. coli ATCC25922 | Silicone disks | Components of burdock leaves | 0.017 mg/mL | 24 h | 50% | [48] | |
| E. coli CECT434 | 96-well microtiter plates | 2-ethoxyphenol | 7 mM | 24 h | 58.0 ± 15.0% | [49] | |
| 4-methylcatechol | 3.5 mM | 24 h | 61.0 ± 10.0% | ||||
| 4-tert-butyl catechol | 1.6 mM | 24 h | 77.0 ± 0.0% | ||||
| pyrogallol | 5 mM | 24 h | 73.0 ± 4.0% | ||||
| E. coli O157:H7 ATCC 35150 | Stainless steel | Carvacrol | 1% | 5 min | 6.04 | [50] | |
| E. coli O157:H7 ATCC43895 | 96-well polystyrene plates | Coumarins | 50 µg/mL | 24 h | above 80% | [51] | |
| E. coli O157:H7 | 48-well plate | Solid liposomes | 0.5 mg/mL | 24 h | 65.74% | [52] | |
| E. coli 2011-60-1493-3, 2011/25/62, C24716 C-26036 | 96-well microtiter plate | Punica granatum sarcotesta lectin | ≥6.25 g/mL | 24 h | ≥50% | [53] | |
| Escherichia coli (ATCC 35218) | 96-well poly- styrene microtiter plate | Ethanol extract of Carum coptis chinensis | 25 mg/mL | 24 h | ≥70% | [54] | |
| E. coli EMC17 | 96-well polystyrene plate | Vitamin C | 30 mM | 24 h | 50% | [4] | |
| Escherichia coli SZMC 0582 | Polypropylene spatula | Cinnamomum Zeylanicum | 1.2 mg/mL | 10 min | 9 ± 5.45% | [55] | |
| Origanum majorana | 4.5 mg/mL | 10 min | 100 ± 0.00% | ||||
| Thymus vulgaris | 3.8 mg/mL | 10 min | 100 ± 0.00% | ||||
| HC-DPE (active ingredients: 15% peracetic-acid, 20% total peroxide) | 0.1% | 10 min | 100 ± 0.00% | ||||
| Sodium hypochlorite | 0.84% | 10 min | 100 ± 0.00% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, F.; Wang, D.; Hu, J.; Zhang, Y.; Tan, B.K.; Lin, S. Control Measurements of Escherichia coli Biofilm: A Review. Foods 2022, 11, 2469. https://doi.org/10.3390/foods11162469
Zhou F, Wang D, Hu J, Zhang Y, Tan BK, Lin S. Control Measurements of Escherichia coli Biofilm: A Review. Foods. 2022; 11(16):2469. https://doi.org/10.3390/foods11162469
Chicago/Turabian StyleZhou, Feng, Dehua Wang, Jiamiao Hu, Yi Zhang, Bee K. Tan, and Shaoling Lin. 2022. "Control Measurements of Escherichia coli Biofilm: A Review" Foods 11, no. 16: 2469. https://doi.org/10.3390/foods11162469
APA StyleZhou, F., Wang, D., Hu, J., Zhang, Y., Tan, B. K., & Lin, S. (2022). Control Measurements of Escherichia coli Biofilm: A Review. Foods, 11(16), 2469. https://doi.org/10.3390/foods11162469







