Purification, Identification and Molecular Docking of Immunomodulatory Peptides from the Heads of Litopenaeus vannamei
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
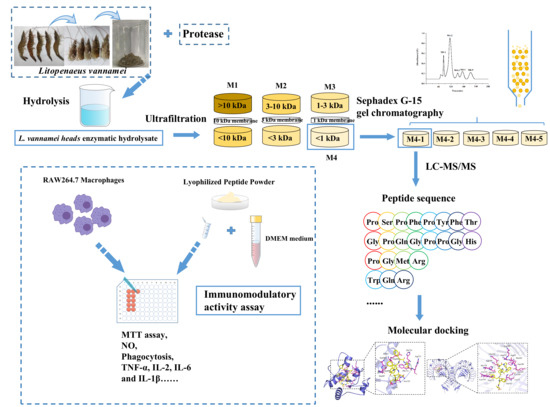
2.2. Preparation of L. vannamei Enzymatic Hydrolysate
2.3. Determination of Hydrolysis Degree (DH)
2.4. Determination of Molecular Weight (MW)
2.5. Ultrafiltration
2.6. Sephadex G-15 Separation
2.7. Identification by Liquid Chromatography–Mass Spectrometry (LC-MS/MS)
2.8. Determination of the Relative Proliferation Rate of Macrophages RAW 264.7 (MRPR)
2.9. Determination of Immune Activity Evaluation Index
2.9.1. Determination of Phagocytosis
2.9.2. Determination of Released NO and Various Cytokines
2.10. Peptide Synthesis
2.11. Stability Analysis of Immunomodulatory Peptides
2.12. Molecular Docking
2.13. Statistical Analysis
3. Results and Discussion
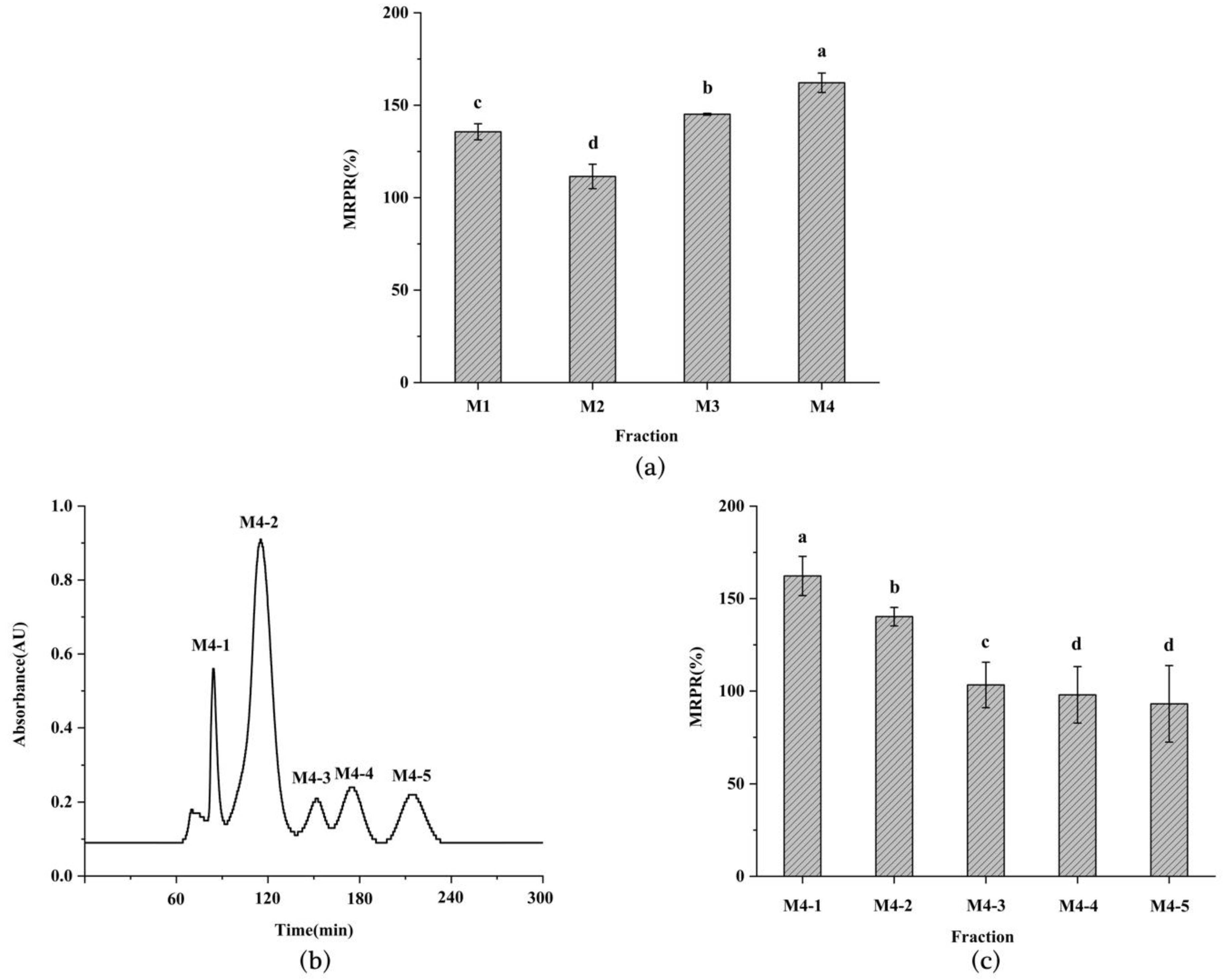
3.1. Immune Activity, DH, and MW of Enzymatic Hydrolysate from L. vannamei Heads
3.2. Purification of Immunomodulatory Peptide from L. vannamei Heads
3.3. Determination of Immune Activity of M4-1 Fraction
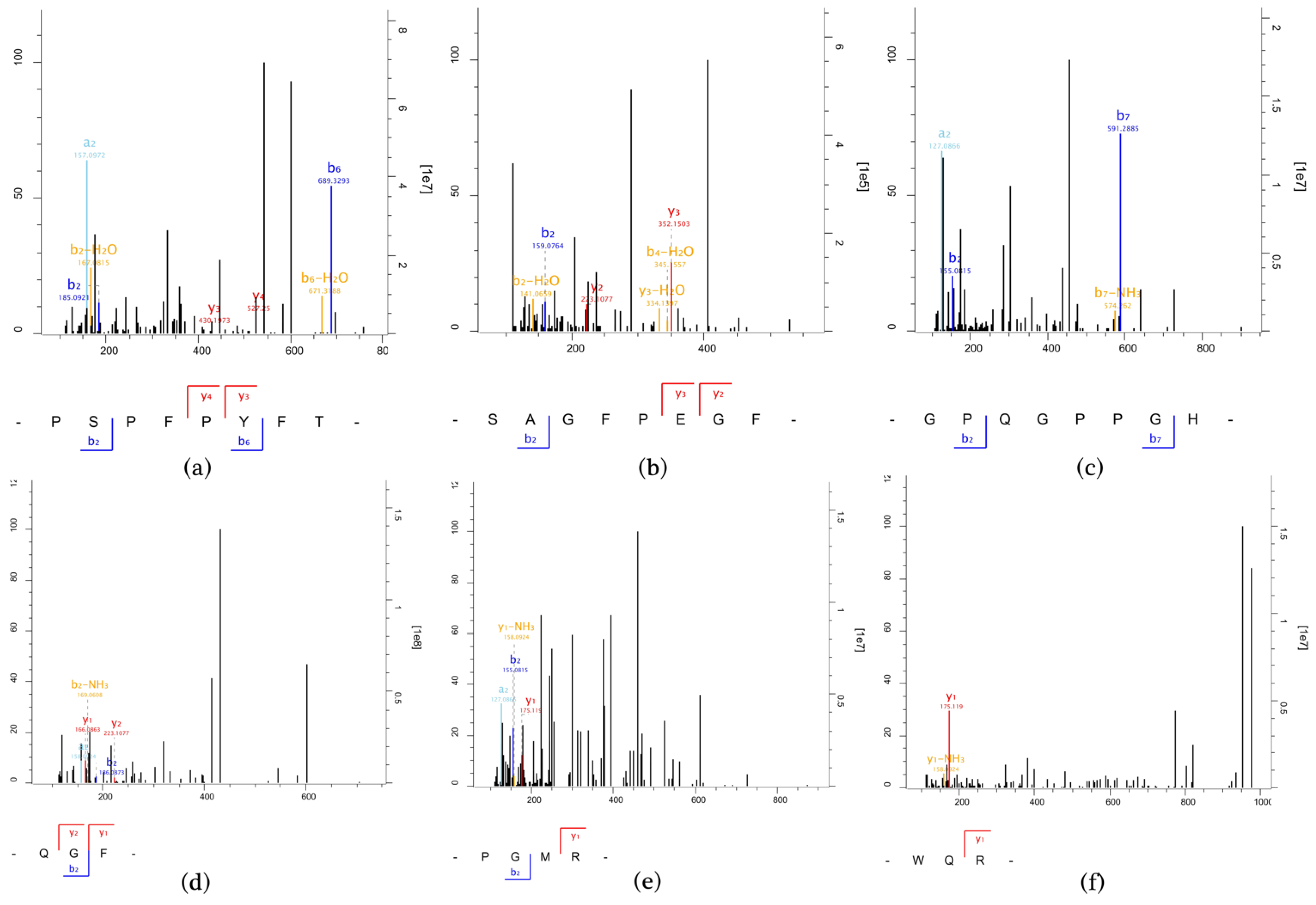
3.4. MS Identification of Immunomodulatory Peptides
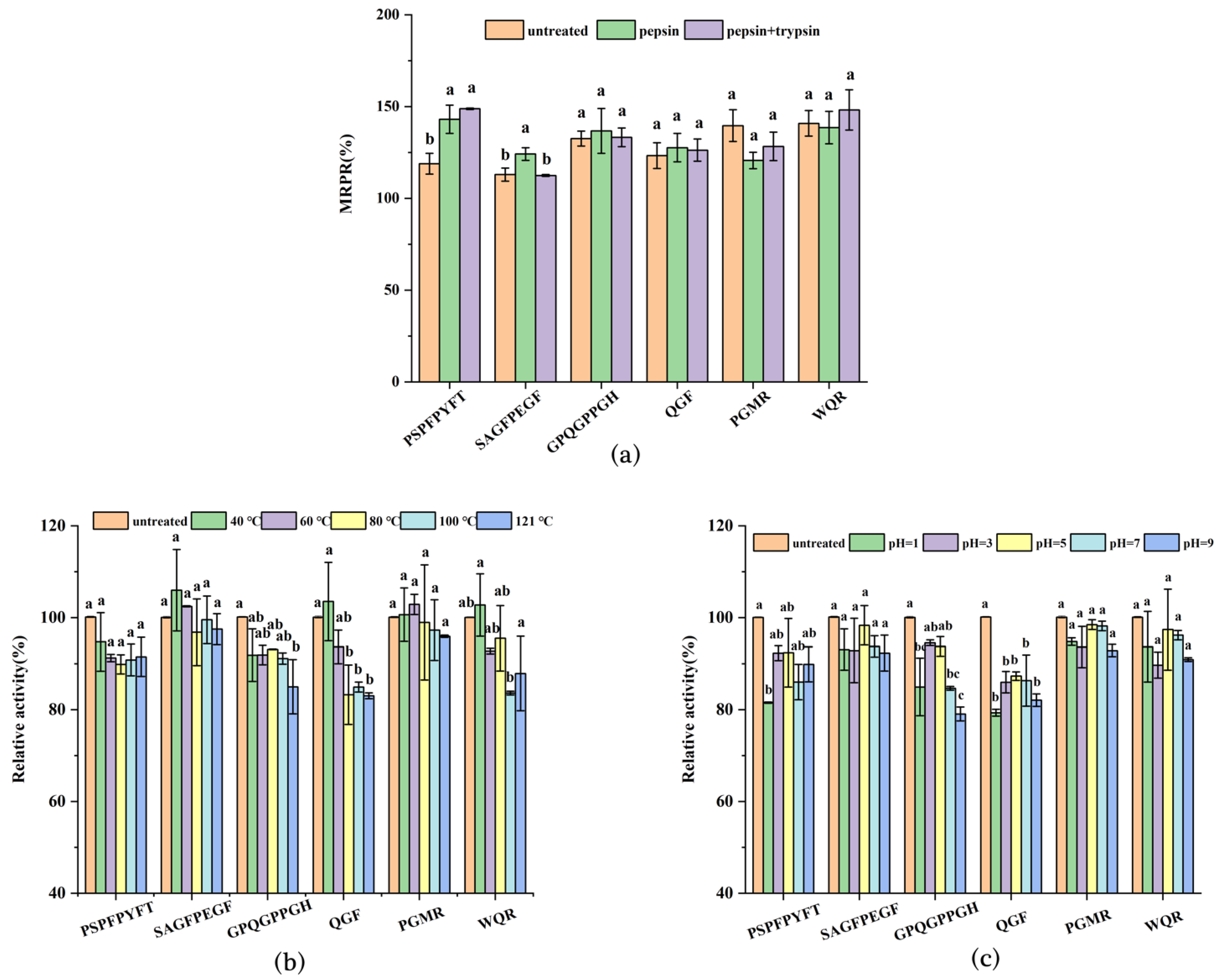
3.5. Immunoactivity Assay of Synthetic Peptides
3.6. Stability Analysis of Synthetic Peptides
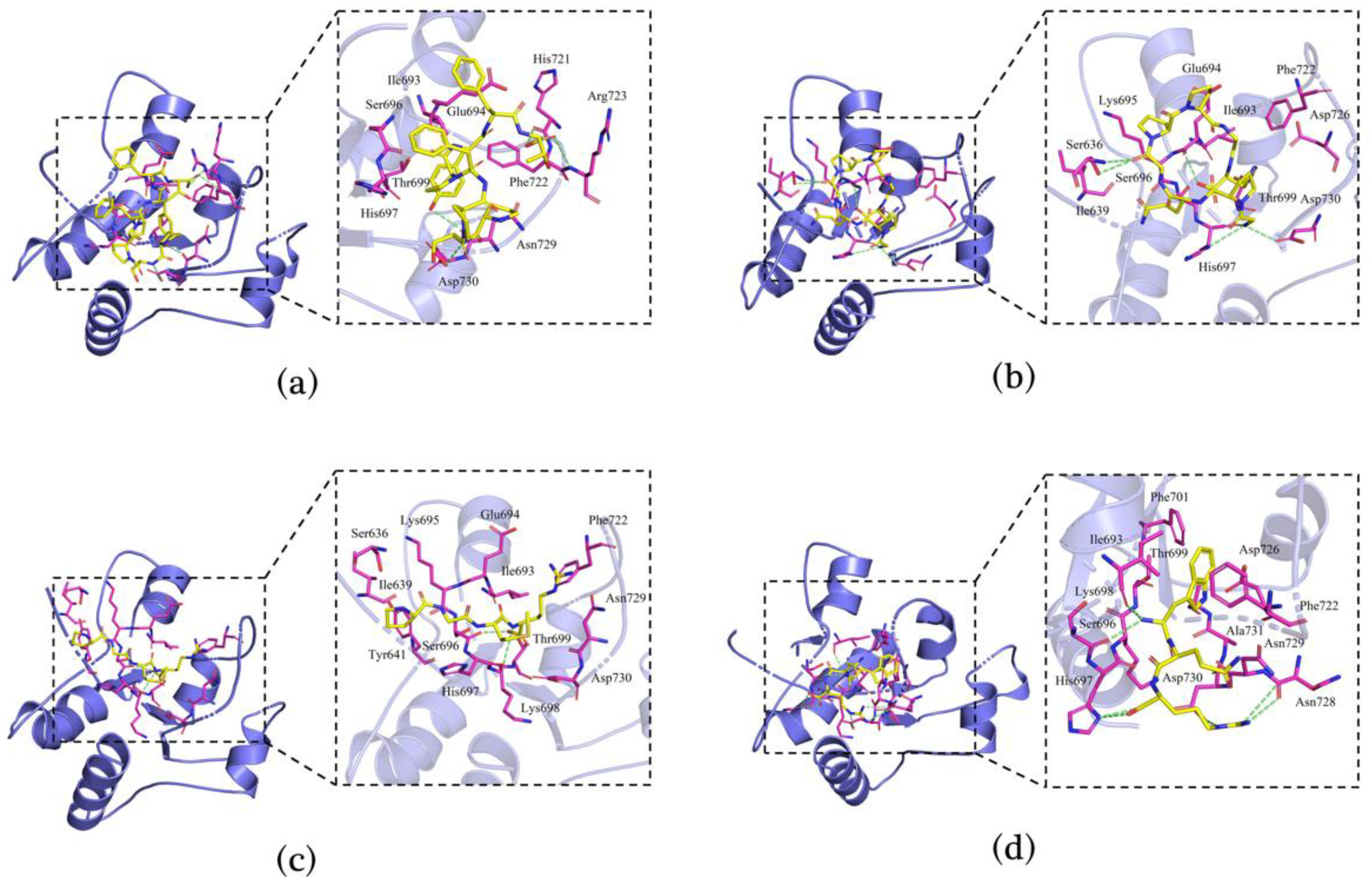
3.7. Molecular Docking of Immunomodulatory Peptides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmermann, P.; Ziesenitz, V.C.; Curtis, N.; Ritz, N. The immunomodulatory effects of macrolides-a systematic review of the underlying mechanisms. Front. Immunol. 2018, 9, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bascones-Martinez, A.; Mattila, R.; Gomez-Font, R.; Meurman, J.H. Immunomodulatory drugs: Oral and systemic adverse effects. Med. Oral Patol. Oral Y Cir. Bucal 2014, 19, e24–e31. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Teng, X.; Shang, J.; Wang, D.; Liu, N. Identification of novel DPP-IV inhibitory peptides from Atlantic salmon (Salmo salar) skin. Food Res. Int. 2020, 133, 109161. [Google Scholar] [CrossRef] [PubMed]
- Pujiastuti, D.Y.; Ghoyatul Amin, M.N.; Alamsjah, M.A.; Hsu, J.L. Marine organisms as potential sources of bioactive peptides that inhibit the activity of angiotensin I-converting enzyme: A review. Molecules 2019, 24, 2541. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.K.; Lee, H.H.; Seo, C.H.; Park, Y. Antimicrobial and immunomodulatory properties and applications of marine-derived proteins and peptides. Mar. Drugs 2019, 17, 350. [Google Scholar] [CrossRef] [Green Version]
- Pavlicevic, M.; Marmiroli, N.; Maestri, E. Immunomodulatory peptides-A promising source for novel functional food production and drug discovery. Peptides 2022, 148, 170696. [Google Scholar] [CrossRef]
- Hu, H.; Yan, F.; Shikai, W.; Leilei, S.; Bafang, L. Immunomodulatory activity of Alaska pollock hydrolysates obtained by glutamic acid biosensor-artificial neural network and the identification of its active central fragment. J. Funct. Foods 2016, 24, 37–47. [Google Scholar] [CrossRef]
- Mallet, J.F.; Duarte, J.; Vinderola, G.; Anguenot, R.; Beaulieu, M.; Matar, C. The immunopotentiating effects of shark-derived protein hydrolysate. Nutrition 2014, 30, 706–712. [Google Scholar] [CrossRef]
- Ren, S.; Mather, P.B.; Tang, B.; Hurwood, D.A. Levels of genetic diversity and inferred origins of Penaeus vannamei culture resources in China: Implications for the production of a broad synthetic base population for genetic improvement. Aquaculture 2018, 491, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Dayakar, B.; Xavier, K.A.M.; Ngasotter, S.; Layana, P.; Balange, A.K.; Priyadarshini, B.; Nayak, B.B. Characterization of spray-dried carotenoprotein powder from Pacific white shrimp (Litopenaeus vannamei) shells and head waste extracted using papain: Antioxidant, spectroscopic, and microstructural properties. LWT 2022, 159, 113188. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Santivarangkna, C.; Rajput, M.S.; Benjakul, S. Trends in shrimp processing waste utilization: An industrial prospective. Trends Food Sci. Technol. 2020, 103, 20–35. [Google Scholar] [CrossRef]
- Jianping, L.; Wei, S.; Hosahalli, S.R.; Yong, Y.; Songming, Z.; Jing, W.; Huanhuan, L. High pressure extraction of astaxanthin from shrimp waste (Penaeus vannamei Boone): Effect on yield and antioxidant activity. J. Food Process Eng. 2017, 40, e12353. [Google Scholar] [CrossRef]
- Xiang, X.; Lang, M.; Li, Y.; Zhao, X.; Sun, H.; Jiang, W.; Ni, L.; Song, Y. Purification, identification and molecular mechanism of dipeptidyl peptidase IV inhibitory peptides from discarded shrimp (Penaeus vannamei) head. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021, 1186, 122990. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Hu, X.; Pan, C.; Cai, M.; Li, L.; Yang, X.; Xiang, H.; Chen, S. Novel antioxidant peptides from Grateloupia livida hydrolysates: Purification and identification. Foods 2022, 11, 1498. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Ye, L.; Tang, Y.; Zheng, J.; Tian, X.; Yang, Y.; Yang, Z. Preparation and purification of an immunoregulatory peptide from Stolephorus chinensis of the East Sea of China. Process Biochem. 2020, 98, 151–159. [Google Scholar] [CrossRef]
- Xu, F.; Yao, Y.; Xu, X.; Wang, M.; Pan, M.; Ji, S.; Wu, J.; Jiang, D.; Ju, X.; Wang, L. Identification and quantification of DPP-IV-Inhibitory peptides from hydrolyzed-rapeseed-protein-derived Napin with analysis of the interactions between key residues and protein domains. J. Agric. Food Chem. 2019, 67, 3679–3690. [Google Scholar] [CrossRef]
- Yang, F.J.; Chen, X.; Huang, M.C.; Yang, Q.; Cai, X.X.; Chen, X.; Du, M.; Huang, J.L.; Wang, S.Y. Molecular characteristics and structure-activity relationships of food-derived bioactive peptides. J. Integr. Agric. 2021, 20, 2313–2332. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Hemalatha, R.; Jyothirmayi, T.; Diwan, P.V.; Bhaskarachary, K.; Vajreswari, A.; Ramesh Kumar, R.; Dinesh Kumar, B. Chemical composition and immunomodulatory effects of enzymatic protein hydrolysates from common carp (Cyprinus carpio) egg. Nutrition 2015, 31, 388–398. [Google Scholar] [CrossRef]
- Tian, S.; Yu, B.; Du, K.; Li, Y. Purification of wheat germ albumin hydrolysates by membrane separation and gel chromatography and evaluating their antioxidant activities. LWT 2022, 161, 113365. [Google Scholar] [CrossRef]
- Hsieh, L.S.; Lu, M.S.; Chiang, W.D. Identification and characterization of immunomodulatory peptides from pepsin-soy protein hydrolysates. BIOB 2022, 9, 39. [Google Scholar] [CrossRef]
- Cai, B.; Chen, H.; Wan, P.; Luo, L.; Ye, Z.; Huang, J.; Chen, D.; Pan, J. Isolation and identification of immunomodulatory peptides from the protein hydrolysate of tuna trimmings (Thunnas albacares). LWT 2022, 164, 113614. [Google Scholar] [CrossRef]
- Ping, H.; Leiman, P.; Hui, W.; Lina, Z.; Yi, Z.; Yizhe, Z.; Jinxi, Y.; Zhengli, L.; Mengmeng, Z. Isolation, identification, and immunomodulatory mechanism of peptides from Lepidium meyenii (Maca)protein hydrolysate. J. Agric. Food Chem. 2022, 70, 4328–4341. [Google Scholar] [CrossRef]
- Yang, X.; Wei, S.; Lu, X.; Qiao, X.; Simal-Gandara, J.; Capanoglu, E.; Wozniak, L.; Zou, L.; Cao, H.; Xiao, J.; et al. A neutral polysaccharide with a triple helix structure from ginger: Characterization and immunomodulatory activity. Food Chem. 2021, 350, 129261. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, C.; Zhang, C.; Cao, W.; Qin, X.; Gao, J.; Zheng, H. The purification and identification of immunoregulatory peptides from oyster (Crassostrea hongkongensis) enzymatic hydrolysate. RSC Adv. 2019, 9, 32854–32863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Ye, S.; Zhang, Z.; Tang, J.; Jin, H.; Huang, F.; Yang, Z.; Tang, Y.; Chen, Y.; Ding, G.; et al. Purification and characterization of a novel pentadecapeptide from protein hydrolysates of Cyclina sinensis and its immunomodulatory effects on RAW264.7 cells. Mar. Drugs 2019, 17, 30. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Hu, X.; Lin, L.; Ding, G.; Yu, F. Immunomodulatory activity of low molecular-weight peptides from Nibea japonica in RAW264.7 cells via NF-kappaB pathway. Mar. Drugs 2019, 17, 404. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Cai, X.; Huang, M.; Wang, S. A specific peptide with immunomodulatory activity from Pseudostellaria heterophylla and the action mechanism. J. Funct. Foods 2020, 68, 103887. [Google Scholar] [CrossRef]
- He, K.; Zeng, Y.; Tian, H.; Zhang, Z.; Zhang, H.; Huang, F.; Yu, F. Macrophage immunomodulatory effects of low molecular weight peptides from Mytilus coruscus via NF-κB/MAPK signaling pathways. J. Funct. Foods 2021, 83, 104562. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef]
- Fang, J.; Lu, J.; Zhang, Y.; Wang, J.; Wang, S.; Fan, H.; Zhang, J.; Dai, W.; Gao, J.; Yu, H. Structural properties, antioxidant and immune activities of low molecular weight peptides from soybean dregs (Okara). Food Chem. X 2021, 12, 100175. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, M.; Ren, Y.; Cai, X.; Yin, Z.; Zhang, X.; Min, T.; Wu, H. Characterization and immunomodulatory activity of a novel peptide, ECFSTA, from wheat germ globulin. J. Agric. Food Chem. 2017, 65, 5561–5569. [Google Scholar] [CrossRef]
- Wang, K.; Han, L.; Tan, Y.; Hong, H.; Luo, Y. Generation of novel antioxidant peptides from silver carp muscle hydrolysate: Gastrointestinal digestion stability and transepithelial absorption property. Food Chem. 2023, 403, 134136. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Zhang, L.; Song, W.; Zhang, C.; Hua, Y.; Chen, Y.; Li, X. Separation, identification and molecular binding mechanism of dipeptidyl peptidase IV inhibitory peptides derived from walnut (Juglans regia L.) protein. Food Chem. 2021, 347, 129062. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Yan, R.; Jiang, Y.; Feng, S.; Sun, H.; Sun, J.; Zhao, D.; Li, H.; Wang, B.; Zhang, N. Identification of peptides in Qingke baijiu and evaluation of its angiotensin converting enzyme (ACE) inhibitory activity and stability. Food Chem. 2022, 395, 133551. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Dai, Z.; Jin, R. Purification and identification of a novel angiotensin converting enzyme inhibitory peptide from the enzymatic hydrolysate of Lepidotrigla microptera. Foods 2022, 11, 1889. [Google Scholar] [CrossRef]
- Lang, M.; Song, Y.; Li, Y.; Xiang, X.; Ni, L.; Miao, J. Purification, identification, and molecular mechanism of DPP-IV inhibitory peptides from defatted Antarctic krill powder. J. Food Biochem. 2021, 45, e13872. [Google Scholar] [CrossRef]
- He, P.; Wang, Q.; Zhan, Q.; Pan, L.; Xin, X.; Wu, H.; Zhang, M. Purification and characterization of immunomodulatory peptides from enzymatic hydrolysates of duck egg ovalbumin. Food Funct. 2021, 12, 668–681. [Google Scholar] [CrossRef]



| No. | Sequence | Length | Mass | PeptideRanker | pI | Hydrophobicity |
|---|---|---|---|---|---|---|
| 1 | PSPFPYFT | 8 | 954.44872 | 0.962878 | 5.95 | 62.50% |
| 2 | SAGFPEGF | 8 | 810.35482 | 0.898452 | 4.00 | 50.00% |
| 3 | GPQGPPGH | 8 | 745.35074 | 0.802084 | 6.74 | 37.50% |
| 4 | PGAKCYGF | 8 | 841.37926 | 0.889124 | 8.60 | 37.50% |
| 5 | PGCACLPG | 8 | 716.29857 | 0.903095 | 5.87 | 50.00% |
| 6 | GSGGCGHW | 8 | 759.27586 | 0.862882 | 6.73 | 12.50% |
| 7 | QGF | 3 | 350.15902 | 0.940972 | 5.52 | 33.33% |
| 8 | PGMR | 4 | 459.22639 | 0.900324 | 10.18 | 50.00% |
| 9 | WQR | 3 | 488.24957 | 0.840511 | 9.75 | 33.33% |
| 10 | YYSF | 4 | 578.23766 | 0.806248 | 5.52 | 25.00% |
| 11 | WCH | 3 | 444.15797 | 0.968801 | 6.73 | 33.33% |
| Sequence | MRPR (%) | Phagocytosis Rates (%) | TNF-α (ng/L) | IL-6 (ng/L) |
|---|---|---|---|---|
| PSPFPYFT | 126.27 ± 11.65% bc | 141.59 ± 2.96% bc | 70.70 ± 3.02 a | 21.63 ± 2.51 a |
| SAGFPEGF | 111.26 ± 0.77% cd | 138.99 ± 6.64% bc | 54.69 ± 1.46 b | 25.45 ± 1.63 a |
| GPQGPPGH | 150.17 ± 1.92% a | 159.41 ± 5.51% a | 56.24 ± 5.83 b | 21.94 ± 1.03 a |
| QGF | 107.67 ± 9.60% cd | 129.27 ± 9.58% c | 62.41 ± 3.01 ab | 25.98 ± 1.90 a |
| PGMR | 143.52 ± 0.76% ab | 148.12 ± 2.03% ab | 60.06 ± 7.23 b | 21.12 ± 3.69 a |
| WQR | 134.69 ± 15.23% ab | 156.59 ± 9.15% a | 56.18 ± 1.41 a | 22.22 ± 1.09 a |
| Control | 100.00 ± 0.02% d | 100.18 ± 0.07% d | 39.10 ± 2.33 c | 13.80 ± 2.00 b |
| Sequence | Affinity (kcal/mol) | |
|---|---|---|
| TLR2 (ID: 1FYW) | TLR4/MD-2 (ID: 5IJD) | |
| PSPFPYFT | −7.0 | −9.3 |
| GPQGPPGH | −7.3 | −8.7 |
| PGMR | −6.8 | −6.8 |
| WQR | −7.3 | −8.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Ren, K.; Yang, Z.; Fang, Z.; Li, Y.; Xiang, X.; Song, Y. Purification, Identification and Molecular Docking of Immunomodulatory Peptides from the Heads of Litopenaeus vannamei. Foods 2022, 11, 3309. https://doi.org/10.3390/foods11203309
Jiang W, Ren K, Yang Z, Fang Z, Li Y, Xiang X, Song Y. Purification, Identification and Molecular Docking of Immunomodulatory Peptides from the Heads of Litopenaeus vannamei. Foods. 2022; 11(20):3309. https://doi.org/10.3390/foods11203309
Chicago/Turabian StyleJiang, Weiwei, Keyu Ren, Zhiyan Yang, Zhou Fang, Yan Li, Xi Xiang, and Yishan Song. 2022. "Purification, Identification and Molecular Docking of Immunomodulatory Peptides from the Heads of Litopenaeus vannamei" Foods 11, no. 20: 3309. https://doi.org/10.3390/foods11203309