Chitosan Film as a Replacement for Conventional Sulphur Dioxide Treatment of White Wines: A 1H NMR Metabolomic Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Chitosan–Genipin (Ch-Ge) Films
2.2. Wine Samples and Preservation Treatments
2.3. Organoleptic Tests
2.4. NMR Spectroscopy
2.5. Statistical Analysis
3. Results
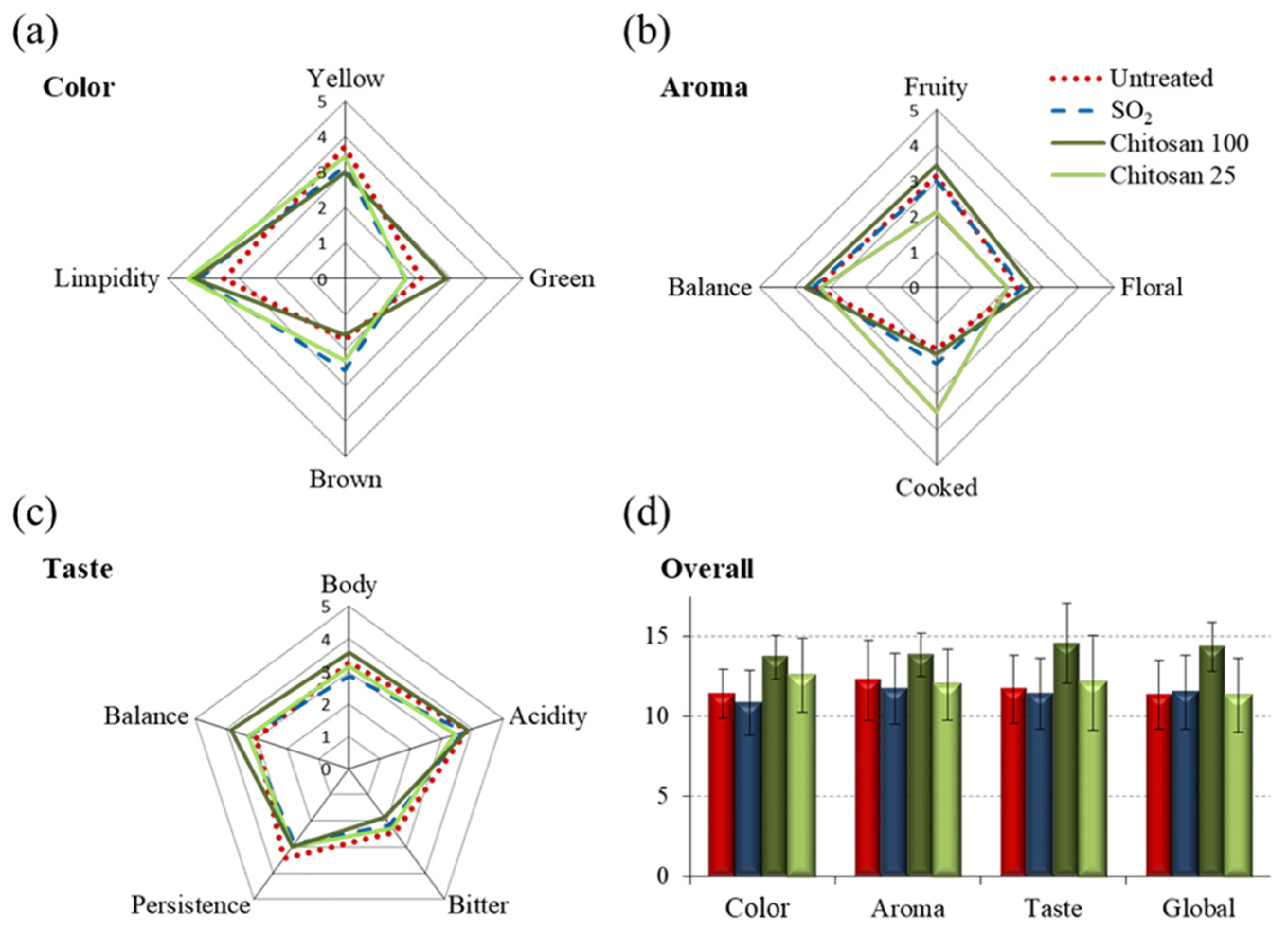
3.1. Organoleptic Characteristics of White Wines
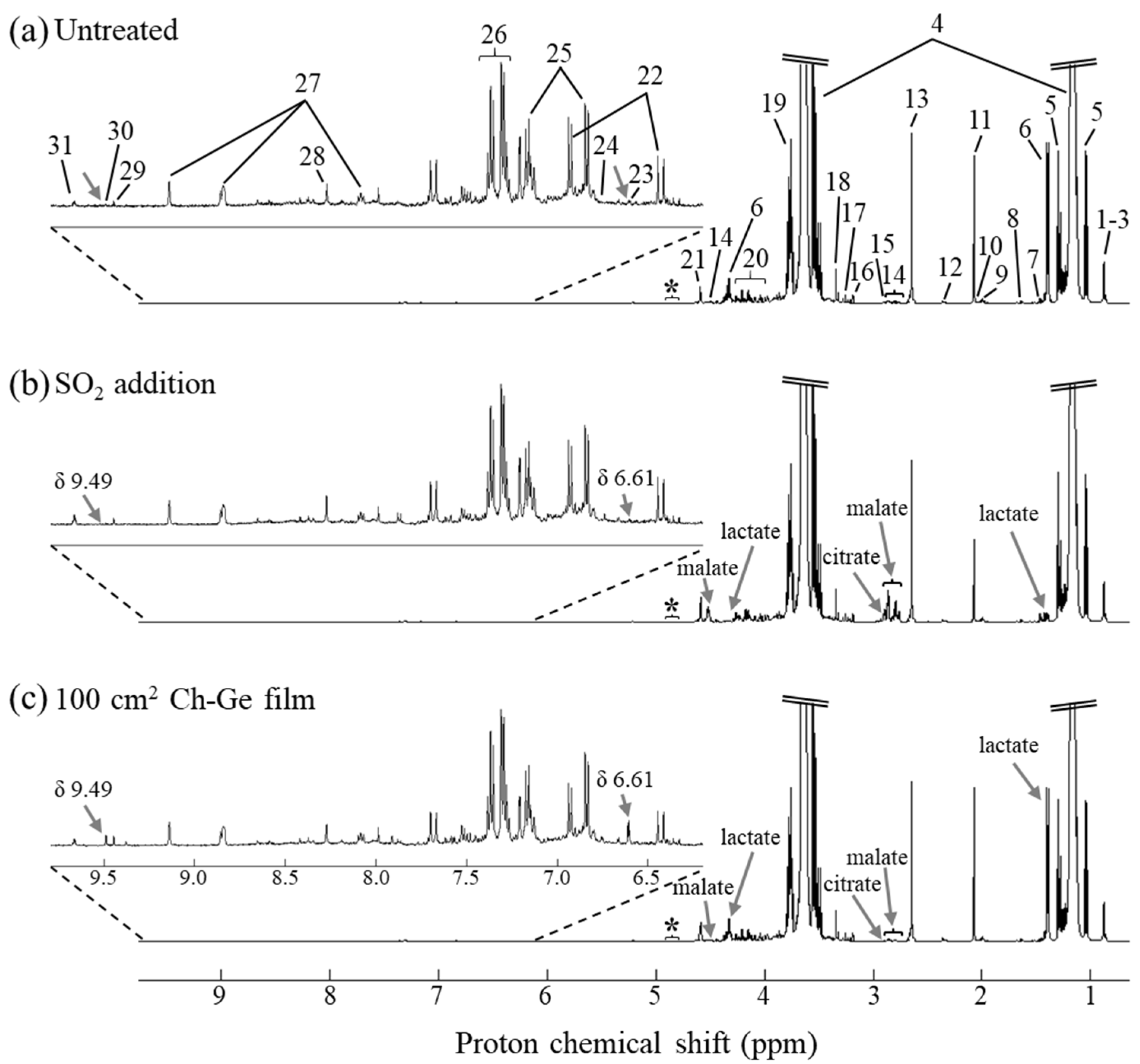
3.2. NMR Characterization of White Wines
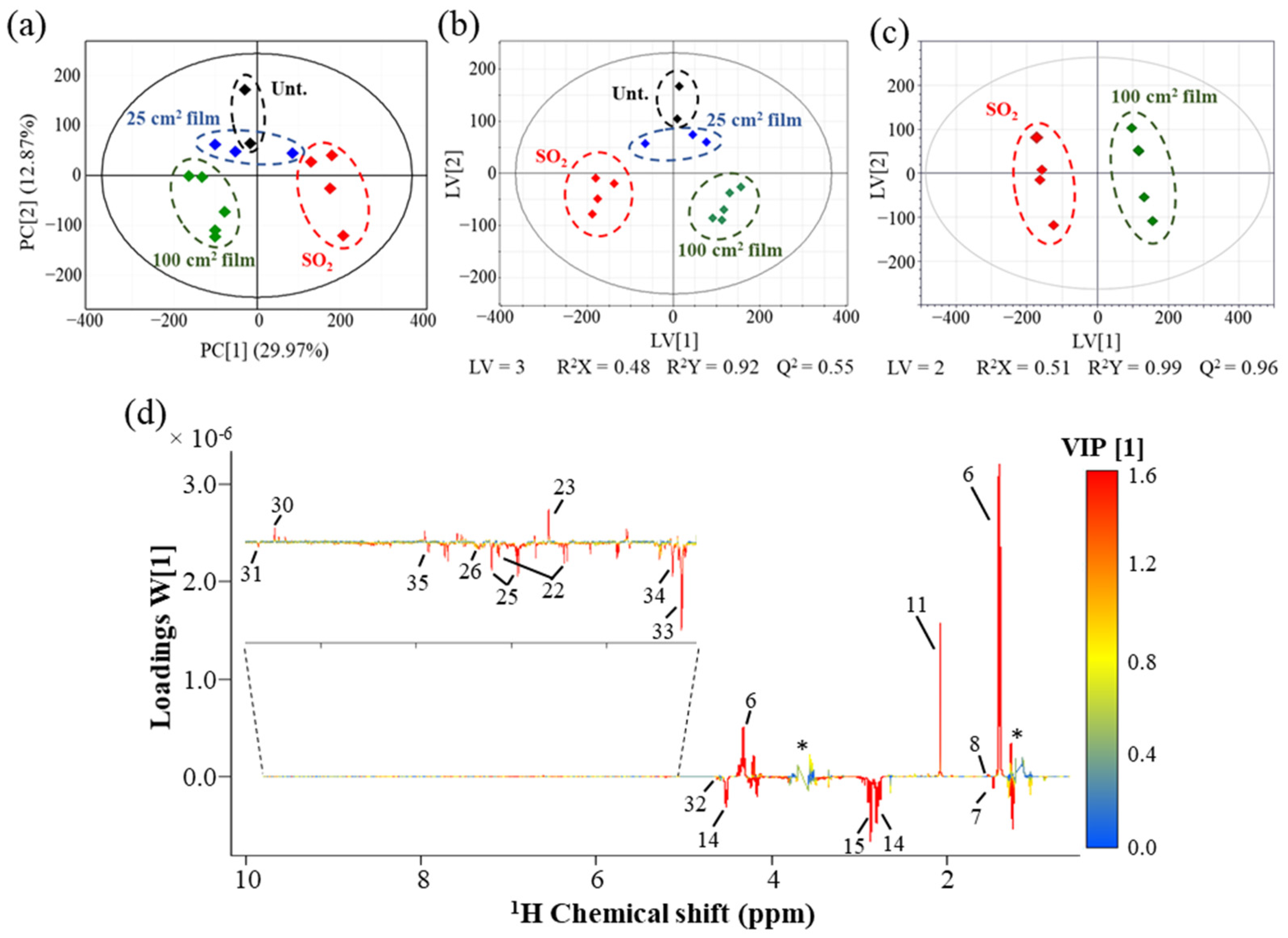
3.3. Multivariate Analysis of White Wines NMR Spectra
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vally, H.; Misso, N.L. Adverse Reactions to the Sulphite Additives. Gastroenterol. Hepatol. Bed. Bench 2012, 5, 16–23. [Google Scholar] [PubMed]
- Ferrer-Gallego, R.; Puxeu, M.; Martín, L.; Nart, E.; Hidalgo, H.; Andorrà, I. Microbiological, Physical, and Chemical Procedures to Elaborate High-Quality SO2-Free Wines. In Grapes and Wines-Advances in Production, Processing, Analysis and Valorization; Jordão, A.M., Cosme, F., Eds.; Intechopen: London, UK, 2017. [Google Scholar]
- Lisanti, M.T.; Blaiotta, G.; Nioi, C.; Moio, L. Alternative Methods to SO2 for Microbiological Stabilization of Wine. Compr. Rev. Food Sci. Food Saf. 2019, 18, 455–479. [Google Scholar] [CrossRef] [PubMed]
- Harkin, C.; Mehlmer, N.; Woortman, D.V.; Brück, T.B.; Brück, W.M. Nutritional and Additive Uses of Chitin and Chitosan in the Food Industry. In Sustainable Agriculture Reviews 36: Chitin and Chitosan: Applications in Food, Agriculture, Pharmacy, Medicine and Wastewater Treatment; Crini, G., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–43. [Google Scholar]
- Rhim, J.W.; Ng, P.K. Natural Biopolymer-Based Nanocomposite Films for Packaging Applications. Crit. Rev. Food Sci. Nutr. 2007, 47, 411–433. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Maricato, É.; Cunha, Â.; Nunes, A.; da Silva, J.A.L.; Coimbra, M.A. Chitosan–Caffeic Acid–Genipin Films Presenting Enhanced Antioxidant Activity and Stability in Acidic Media. Carbohydr. Polym. 2013, 91, 236–243. [Google Scholar] [CrossRef]
- Rocha, M.A.M.; Coimbra, M.A.; Rocha, S.M.; Nunes, C. Impact of Chitosan-Genipin Films on Volatile Profile of Wine Along Storage. Appl. Sci. 2021, 11, 6294. [Google Scholar] [CrossRef]
- OIV. Resolution 336a-2009—Codex Oenologique International; Organisation Internationale de la Vigne et du Vin: Paris, France, 2009. [Google Scholar]
- Nunes, C.; Maricato, É.; Cunha, Â.; Rocha, M.A.M.; Santos, S.; Ferreira, P.; Silva, M.A.; Rodrigues, A.; Amado, O.; Coimbra, J.; et al. Chitosan–Genipin Film, a Sustainable Methodology for Wine Preservation. Green Chem. 2016, 18, 5331–5341. [Google Scholar] [CrossRef]
- Cubero-Leon, E.; Peñalver, R.; Maquet, A. Review on Metabolomics for Food Authentication. Food Res. Int. 2014, 60, 95–107. [Google Scholar] [CrossRef]
- Hatzakis, E. Nuclear Magnetic Resonance (Nmr) Spectroscopy in Food Science: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 189–220. [Google Scholar] [CrossRef]
- Consonni, R.; Cagliani, L.R. The Potentiality of Nmr-Based Metabolomics in Food Science and Food Authentication Assessment. Magn. Reson. Chem. 2019, 57, 558–578. [Google Scholar] [CrossRef]
- Rodrigues, J.A.; Barros, A.S.; Carvalho, B.; Brandão, T.; Gil, A.M. Probing Beer Aging Chemistry by Nuclear Magnetic Resonance and Multivariate Analysis. Anal. Chim. Acta 2011, 702, 178–187. [Google Scholar] [CrossRef]
- Son, H.S.; Kim, K.M.; van den Berg, F.; Hwang, G.S.; Park, W.M.; Lee, C.H.; Hong, Y.S. 1H Nuclear Magnetic Resonance-Based Metabolomic Characterization of Wines by Grape Varieties and Production Areas. J. Agric. Food Chem. 2008, 56, 8007–8016. [Google Scholar] [CrossRef] [PubMed]
- López-Rituerto, E.; Savorani, F.; Avenoza, A.; Busto, J.H.; Peregrina, J.M.; Engelsen, S.B. Investigations of La Rioja Terroir for Wine Production Using 1H Nmr Metabolomics. J. Agric. Food Chem. 2012, 60, 3452–3461. [Google Scholar] [CrossRef] [PubMed]
- Godelmann, R.; Fang, F.; Humpfer, E.; Schütz, B.; Bansbach, M.; Schäfer, H.; Spraul, M. Targeted and Nontargeted Wine Analysis by 1H Nmr Spectroscopy Combined with Multivariate Statistical Analysis. Differentiation of Important Parameters: Grape Variety, Geographical Origin, Year of Vintage. J. Agric. Food Chem. 2013, 61, 5610–5619. [Google Scholar] [CrossRef] [PubMed]
- Alves Filho, E.G.; Silva, L.M.A.; Ribeiro, P.R.V.; de Brito, E.S.; Zocolo, G.J.; Souza-Leão, P.C.; Marques, A.T.B.; Quintela, A.L.; Larsen, F.H.; Canuto, K.M. 1H Nmr and Lc-Ms-Based Metabolomic Approach for Evaluation of the Seasonality and Viticultural Practices in Wines from São Francisco River Valley, a Brazilian Semi-Arid Region. Food Chem. 2019, 289, 558–567. [Google Scholar] [CrossRef]
- Alves Filho, E.G.; Silva, L.M.A.; Lima, T.O.; Ribeiro, P.R.V.; Vidal, C.S.; Carvalho, E.S.S.; Druzian, J.I.; Marques, A.T.B.; Canuto, K.M. 1H Nmr and Uplc-Hrms-Based Metabolomic Approach for Evaluation of the Grape Maturity and Maceration Time of Touriga Nacional Wines and Their Correlation with the Chemical Stability. Food Chem. 2022, 382, 132359. [Google Scholar] [CrossRef]
- Mascellani, A.; Hoca, G.; Babisz, M.; Krska, P.; Kloucek, P.; Havlik, J. 1H Nmr Chemometric Models for Classification of Czech Wine Type and Variety. Food Chem. 2021, 339, 127852. [Google Scholar] [CrossRef]
- Ali, K.; Maltese, F.; Toepfer, R.; Choi, Y.H.; Verpoorte, R. Metabolic Characterization of Palatinate German White Wines According to Sensory Attributes, Varieties, and Vintages Using Nmr Spectroscopy and Multivariate Data Analyses. J. Biomol. NMR 2011, 49, 255–266. [Google Scholar] [CrossRef]
- Nilsson, M.; Duarte, I.F.; Almeida, C.; Delgadillo, I.; Goodfellow, B.J.; Gil, A.M.; Morris, G.A. High-Resolution Nmr and Diffusion-Ordered Spectroscopy of Port Wine. J. Agric. Food Chem. 2004, 52, 3736–3743. [Google Scholar] [CrossRef]
- Peng, C.; Viana, T.; Petersen, M.A.; Larsen, F.H.; Arneborg, N. Metabolic Footprint Analysis of Metabolites That Discriminate Single and Mixed Yeast Cultures at Two Key Time-Points During Mixed Culture Alcoholic Fermentations. Metabolomics 2018, 14, 93. [Google Scholar] [CrossRef]
- Gougeon, L.; da Costa, G.; le Mao, I.; Ma, W.; Teissedre, P.; Guyon, F.; Richard, T. Wine Analysis and Authenticity Using 1H-Nmr Metabolomics Data: Application to Chinese Wines. Food Anal. Methods 2018, 11, 3425–3434. [Google Scholar] [CrossRef]
- Anastasiadi, M.; Zira, A.; Magiatis, P.; Haroutounian, S.A.; Skaltsounis, A.L.; Mikros, E. 1H Nmr-Based Metabonomics for the Classification of Greek Wines According to Variety, Region, and Vintage. Comparison with Hplc Data. J. Agric. Food Chem. 2009, 57, 11067–11074. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. Hmdb 4.0: The Human Metabolome Database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Veselkov, K.A.; Lindon, J.C.; Ebbels, T.M.D.; Crockford, D.; Volynkin, V.V.; Holmes, E.; Davies, D.B.; Nicholson, J.K. Recursive Segment-Wise Peak Alignment of Biological 1H Nmr Spectra for Improved Metabolic Biomarker Recovery. Anal. Chem. 2009, 81, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I.T.; Springer-Verlag. Principal Component Analysis; Springer: New York, USA, 2002. [Google Scholar]
- Barker, M.; Rayens, W. Partial Least Squares for Discrimination. J. Chemom. 2003, 17, 166–173. [Google Scholar] [CrossRef]
- Berben, L.; Sereika, S.M.; Engberg, S. Effect Size Estimation: Methods and Examples. Int. J. Nurs. Stud. 2012, 49, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Cloarec, O.; Dumas, M.E.; Craig, A.; Barton, R.H.; Trygg, J.; Hudson, J.; Blancher, C.; Gauguier, D.; Lindon, J.C.; Holmes, E.; et al. Statistical Total Correlation Spectroscopy: An Exploratory Approach for Latent Biomarker Identification from Metabolic 1H Nmr Data Sets. Anal. Chem. 2005, 77, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, C.; du Toit, W.J. Sauvignon Blanc Wine: Contribution of Ageing and Oxygen on Aromatic and Non-Aromatic Compounds and Sensory Composition—A Review. South Afr. J. Enol. Vitic. 2015, 36, 347–365. [Google Scholar] [CrossRef][Green Version]
- Rocha, M.A.M.; Ferreira, P.; Coimbra, M.A.; Nunes, C. Mechanism of Iron Ions Sorption by Chitosan-Genipin Films in Acidic Media. Carbohydr. Polym. 2020, 236, 116026. [Google Scholar] [CrossRef]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principles and Practices of Winemaking; Boulton, R.B., Ed.; Chapman & Hall: New York, NY, USA, 1996. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Organic Acids in Wine. In Handbook of Enology: The Chemistry of Wine Stabilization and Treatments; John Wiley & Sons, Ltd: Chichester, UK, 2006; pp. 1–49. [Google Scholar]
- Ribereau-Gayon, P.; Dubourdieu, D.; Doneche, B.; Lonvaud, A. Handbook of Enology: The Microbiology of Wine and Vinifications; John Wiley & Sons: Chichester, UK, 2006. [Google Scholar]
- Albers, E.; Larsson, C.; Lidén, G.; Niklasson, C.; Gustafsson, L. Influence of the Nitrogen Source on Saccharomyces Cerevisiae Anaerobic Growth and Product Formation. Appl. Environ. Microbiol. 1996, 62, 3187–3195. [Google Scholar] [CrossRef]
- Hu, B.; Gao, J.; Xu, S.; Zhu, J.; Fan, X.; Zhou, X. Quality Evaluation of Different Varieties of Dry Red Wine Based on Nuclear Magnetic Resonance Metabolomics. Appl. Biol. Chem. 2020, 63, 24. [Google Scholar] [CrossRef]
- Li, H.; Guo, A.; Wang, H. Mechanisms of Oxidative Browning of Wine. Food Chem. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Sochacka, E.; Smuga, D. Uracil Ring Opening in the Reaction of 5-Formyl-2′-Deoxyuridine with Primary Alkyl Amines. Tetrahedron Lett. 2007, 48, 1363–1367. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Pretorius, I.S. Yeast Modulation of Wine Flavor. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2005; pp. 131–175. [Google Scholar]
- Kleerebezem, M.; Boekhorst, J.; van Kranenburg, R.; Molenaar, D.; Kuipers, O.P.; Leer, R.; Tarchini, R.; Peters, S.A.; Sandbrink, H.M.; Fiers, M.W.E.J.; et al. Complete Genome Sequence of Lactobacillus Plantarum Wcfs1. Proc. Natl. Acad. Sci. USA 2003, 100, 1990–1995. [Google Scholar] [CrossRef] [PubMed]
- Devi, A.; Anu-Appaiah, K.A.; Lin, T. Timing of Inoculation of Oenococcus Oeni and Lactobacillus Plantarum in Mixed Malo-Lactic Culture Along with Compatible Native Yeast Influences the Polyphenolic, Volatile and Sensory Profile of the Shiraz Wines. LWT 2022, 158, 113130. [Google Scholar] [CrossRef]
- Osborne, J.P.; Morneau, A.D.; de Orduña, R.M. Degradation of Free and Sulfur-Dioxide-Bound Acetaldehyde by Malolactic Lactic Acid Bacteria in White Wine. J. Appl. Microbiol. 2006, 101, 474–479. [Google Scholar] [CrossRef] [PubMed]
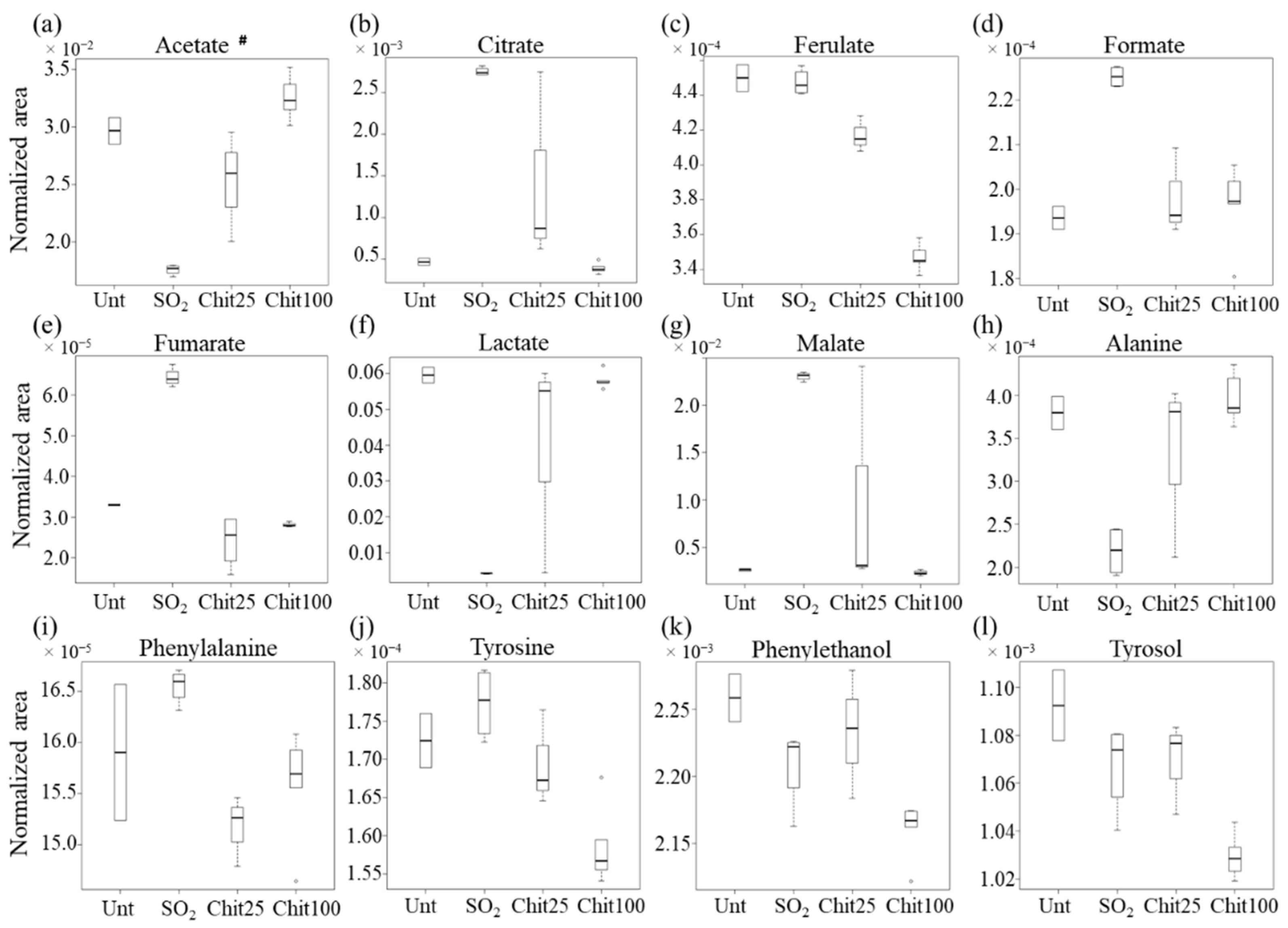
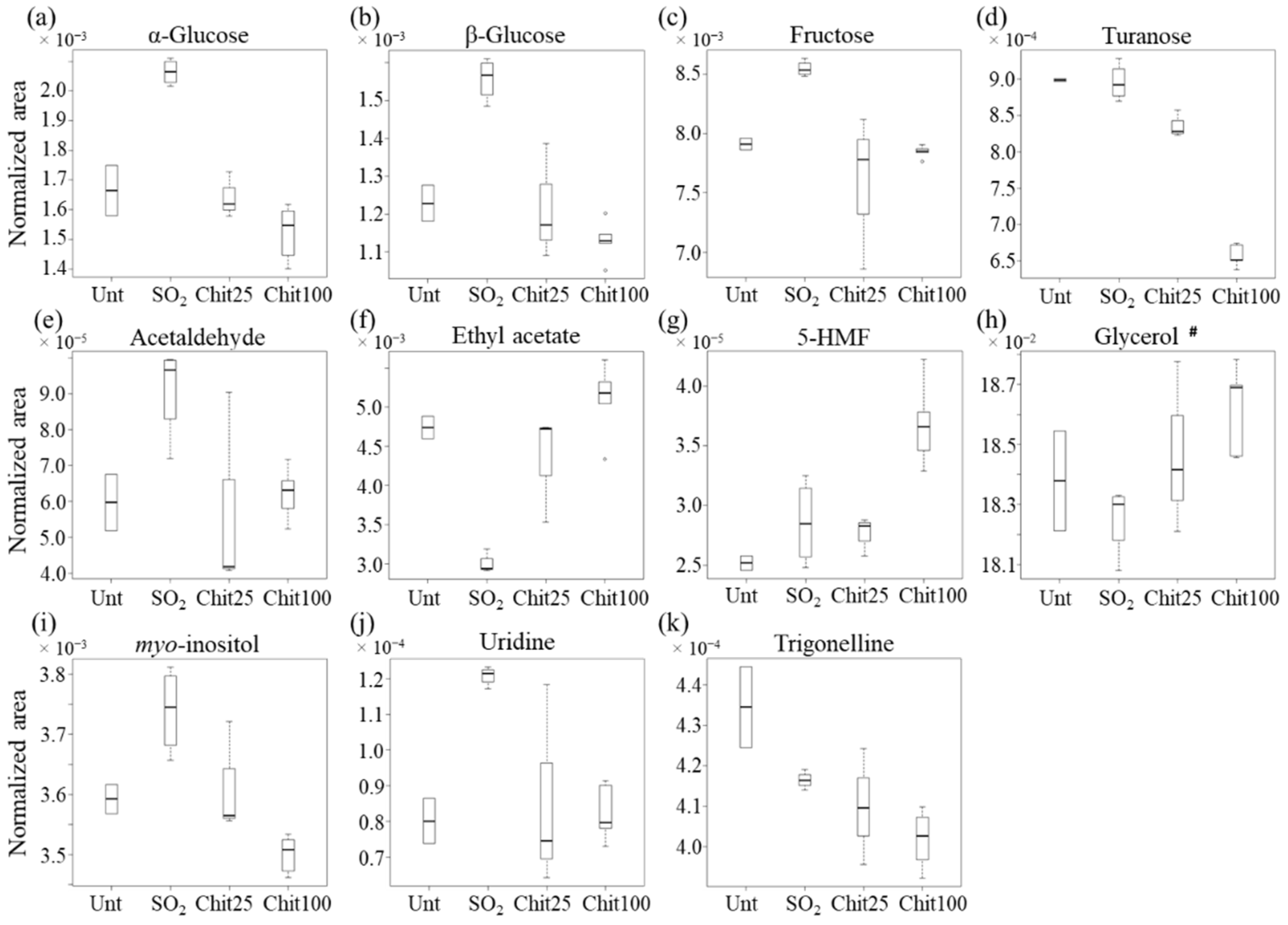
| Family of Compounds | Metabolites | Chemical Shift/ppm (Multiplicity) a | 100 cm2 Ch-Ge Films vs. SO2 | |
|---|---|---|---|---|
| Effect Size b | p-Value c | |||
| Organic acids | Acetate # | 2.07 (s) | 8.8 ± 4.2 | 3.5 × 10−5 |
| Citrate | 2.93 (d) | −35.7 ± 16.5 | 7.8 × 10−11 | |
| Ferulate | 6.94 (d) | −11.4 ± 5.4 | 4.0 × 10−7 | |
| Formate | 8.27 (s) | −3.5 ± 2.0 | 1.7 × 10−3 | |
| Fumarate | 6.74 (s) | −17.4 ± 8.1 | 1.5 × 10−6 | |
| Lactate | 1.41 (d) | 25.9 ± 12.0 | 9.3 × 10−7 | |
| Malate | 2.80 (dd) | −54.4 ± 25.1 | 2.1 × 10−8 | |
| Amino acids and corresponding aromatic alcohols | Alanine | 1.51 (d) | 5.3 ± 2.7 | 5.8 × 10−5 |
| Phenylalanine | 7.40 (m) | −2.0 ± 1.5 | 1.5 × 10−2 | |
| Tyrosine | 6.86 (d) | −3.3 ± 1.9 | 8.9 × 10−4 | |
| Phenylethanol | 7.37 (m) | −1.7 ± 1.4 | 4.1 × 10−2 | |
| Tyrosol | 6.84 (d) | −2.3 ± 1.6 | 2.0 × 10−2 | |
| Sugars | Fructose | 3.86 (m) | −10.8 ± 5.1 | 3.3 × 10−6 |
| β-glucose | 4.65 (d) | −7.0 ± 3.4 | 1.4 × 10−5 | |
| α-glucose | 5.22 (d) | −6.3 ± 3.1 | 3.3 × 10−5 | |
| Turanose d | 5.30 (d) | −10.5 ± 5.0 | 2.0 × 10−5 | |
| Other metabolites | Acetaldehyde | 9.66 (q) | −2.5 ± 1.7 | 1.3 × 10−2 |
| Ethyl acetate | 2.06 (s) | 5.1 ± 2.6 | 2.9 × 10−4 | |
| 5-HMF | 9.45 (s) | 2.1 ± 1.5 | 1.1 × 10−2 | |
| Glycerol # | 3.56 (dd) | 2.4 ± 1.6 | 4.6 × 10−3 | |
| myo-inositol | 3.25 (t) | −4.1 ± 2.2 | 3.4 × 10−3 | |
| Uridine | 7.87 (d) | −5.4 ± 2.8 | 1.6 × 10−4 | |
| Trigonelline | 8.83 (m) | −2.3 ± 1.6 | 8.4 × 10−3 | |
| Unassigned resonances | U1 g | 1.46 (d) | −12.5 ± 5.9 | 1.3 × 10−7 |
| U7 | 3.01 (s) | −9.3 ± 4.5 | 7.0 × 10−5 | |
| U12 | 4.18 (s) | −42.2 ± 19.5 | 2.3 × 10−6 | |
| U13 e | 4.20 (d) | 28.3 ± 13.1 | 1.0 × 10−9 | |
| U14 | 4.27 (q) | −16.8 ± 7.9 | 6.5 × 10−8 | |
| U15 e | 4.37 (d) | 26.1 ± 12.1 | 9.4 × 10−7 | |
| U19 e | 5.78 (d) | 5.5 ± 2.8 | 2.9 × 10−5 | |
| U20 | 5.87 (s) | −4.2 ± 2.3 | 6.5 × 10−4 | |
| U22 | 6.17 (s) | −5.0 ± 2.6 | 3.5 × 10−3 | |
| U23 f | 6.61 (d) | 21.0 ± 9.8 | 1.3 × 10−8 | |
| U24 | 7.21 (d) | −8.9 ± 4.3 | 2.0 × 10−5 | |
| U27 f | 7.91 (s) | 11.1 ± 5.3 | 2.6 × 10−7 | |
| U33 f | 9.49 (s) | 16.4 ± 7.7 | 1.3 × 10−7 | |
| U41 g | 3.20 (dd) | −6.3 ± 3.1 | 4.2 × 10−5 | |
| U43 g | 3.37 (d) | −8.9 ± 3.4 | 8.4 × 10−6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, J.A.; Nunes, C.; Coimbra, M.A.; Goodfellow, B.J.; Gil, A.M. Chitosan Film as a Replacement for Conventional Sulphur Dioxide Treatment of White Wines: A 1H NMR Metabolomic Study. Foods 2022, 11, 3428. https://doi.org/10.3390/foods11213428
Rodrigues JA, Nunes C, Coimbra MA, Goodfellow BJ, Gil AM. Chitosan Film as a Replacement for Conventional Sulphur Dioxide Treatment of White Wines: A 1H NMR Metabolomic Study. Foods. 2022; 11(21):3428. https://doi.org/10.3390/foods11213428
Chicago/Turabian StyleRodrigues, Joao A., Cláudia Nunes, Manuel A. Coimbra, Brian J. Goodfellow, and Ana M. Gil. 2022. "Chitosan Film as a Replacement for Conventional Sulphur Dioxide Treatment of White Wines: A 1H NMR Metabolomic Study" Foods 11, no. 21: 3428. https://doi.org/10.3390/foods11213428
APA StyleRodrigues, J. A., Nunes, C., Coimbra, M. A., Goodfellow, B. J., & Gil, A. M. (2022). Chitosan Film as a Replacement for Conventional Sulphur Dioxide Treatment of White Wines: A 1H NMR Metabolomic Study. Foods, 11(21), 3428. https://doi.org/10.3390/foods11213428








