Abstract
Rice bran is a rich source of health-promoting nutrition and bioactive compounds; nevertheless, the properties of rice brans depend on cultivars, ages, and preparation methods, drawing the potential of raw materials for health benefits. Therefore, this research aimed to investigate the health-promoting properties of fermented rice bran extracts from cultivar black rice (H7F) and germinated brown rice (G13F), focusing on their prebiotic, antipathogenic bacteria activity and safety demonstrated in vitro and in vivo study models, respectively. Here, the screening of metabolites’ change after rice bran fermentation by ATR-FTIR spectra revealed specific peaks corresponding to the composited components of protein, carbohydrate, and lipid. Then, in the in vitro study, the prebiotic capability of H7F and G13F extracts was demonstrated by a growth-promoting effect on Lactobacillus delbrueckii subsp. lactis under specific acidic conditions. Furthermore, antipathogenic bacterial activity against Escherichia coli and Staphylococcus aureus was presented at 25 mg/mL of MIC values and 50 mg/mL of MBC of both fermented rice bran extracts, eliminating the bacteria by interfering with the biofilm formation. For safety, an acute and chronic toxicity study using Wistar rats was conducted, in which changes in the body and organ weights, histopathology of organs, blood chemistry, and hematological parameters were observed after H7F and G13F treatment. Desirably, they showed no toxicity, with a significant reduction in blood cholesterol levels in the chronic treatment of H7F and G13F. Conclusively, the overall results evidenced the health benefits of H7F and G13F related to their prebiotic and antipathogenic bacteria properties and hypocholesterolemia potential with a high level of safety. Therefore, the fermented rice bran extracts were demonstrated as potential materials for the further development of functional ingredients and health products.
1. Introduction
Rice is one of the most important crops cultivated worldwide, especially in the Asian region [1,2]. Thailand is one of the top 10 rice-producing countries and a banner exporter of processed rice products [3]. Rice bran, a rice milling by-product, is a nourishing source of phytochemical compounds, including oryzanol, γ-amino butyric acid (GABA), phenolics, flavonoids, arabinoxylan, phytosterols, phytic acid, and vitamins, etc. [4,5,6,7]. According to several previous studies, rice bran extracts from various kinds of colored rice (pink, red, purple, brown, and black) have been proven to contain health-promoting benefits, e.g., antioxidation, anti-inflammation, and anticancer effects, as well as the improvement of lipid profiles [6,8,9].
Prebiotics and probiotics are functional health-promoting food ingredients on demand in the market with highly safe and naturally health-balancing features. Prebiotics are nondigestible foods that can enhance the propagation and functions of health-beneficial gut microbiomes—probiotics such as Bifidobacterium and Lactobacillus spp. [10], the two common genera of probiotic bacteria, with some isolated species: L. plantarum, L. casei, L. acidophilus, B. animalis, and B. bifidum. Probiotics have antipathogenic activity associated with producing bioactive compounds, short-chain fatty acids (SCFA), peptides, and bacteriocins. Prebiotics possess health-promoting effects by modulating the immune system, lowering allergy risk, reducing the production of harmful protein metabolites, and suppressing pathogenic bacterial growth in the gastrointestinal tract [11,12]. Prebiotics such as dietary fiber are food components that enhance the growth of probiotic bacteria; for instance, the fructooligosaccharides found in onion, asparagus, and wheat are the source of Bifidobacterium probiotics [12]. In addition, combining prebiotics and probiotics, known as synbiotics, offers intensive synergistic benefits on multiple health issues: nutrition, preventing infant diseases, reducing irritable bowel syndrome-relating symptoms and compromising bacterial vaginosis and urinary tract infection, and lowering cholesterol blood level [12,13,14,15].
Interestingly, the prebiotic and synbiotic properties of rice bran extracts have been evidenced. Dietary rice bran enhanced the growth and colonization of Lactobacillus rhamnosus GG and Escherichia coli Nissle 1917 [16], which was effective against human rotavirus (HRV) diarrhea, promoted maintenance of gut epithelial health during infection, and improved innate immune response by increasing intestinal IFN-γ and total IgA level in gnotobiotic pigs [17]. Moreover, fermented rice bran by Lactobacillus plantarum B2 featured a synbiotic product in promoting fecal lactic acid bacteria growth and inhibiting pathogens (Escherichia coli and Salmonella spp.) in Wistar rat intestine [18]. Interestingly, synergistic effects from combinational treatment of Thai black rice bran extract and yeast beta-glucan showed an increase in antioxidant capacity and reduced inflammatory cytokines in colitis-induced rats [6,19]. Moreover, fermentation is a biochemical process to improve rice and rice bran’s nutritional quality and biological activities [20,21]. Fermentation increased crude protein contents, phytochemicals, and gamma-butyric acid in fermented rice bran [21,22].
Although many prior studies have reported various health benefits of rice bran extracts, there is very little information regarding fermented rice bran from KKU ULR0381 black rice cultivar (H7F) and germinated brown rice (G13F). Thus, this study aimed to investigate the biochemical components of fermented rice bran extracts by attenuated total reflection–Fourier transform infrared spectroscopy (ATR-FTIR), the prebiotic property promoting Lactobacillus dellbrueckii (L. dellbrueckii) subsp. lactis growth, antipathogenic bacteria activity, and a safety and toxicity test in both in vitro and in vivo models. The obtained information will deliver a guideline to support health product development from H7F and G13F.
2. Results
2.1. Comparison of Biochemical Components between Nonfermented and Fermented Rice Bran by Derivative FT-IR Spectra
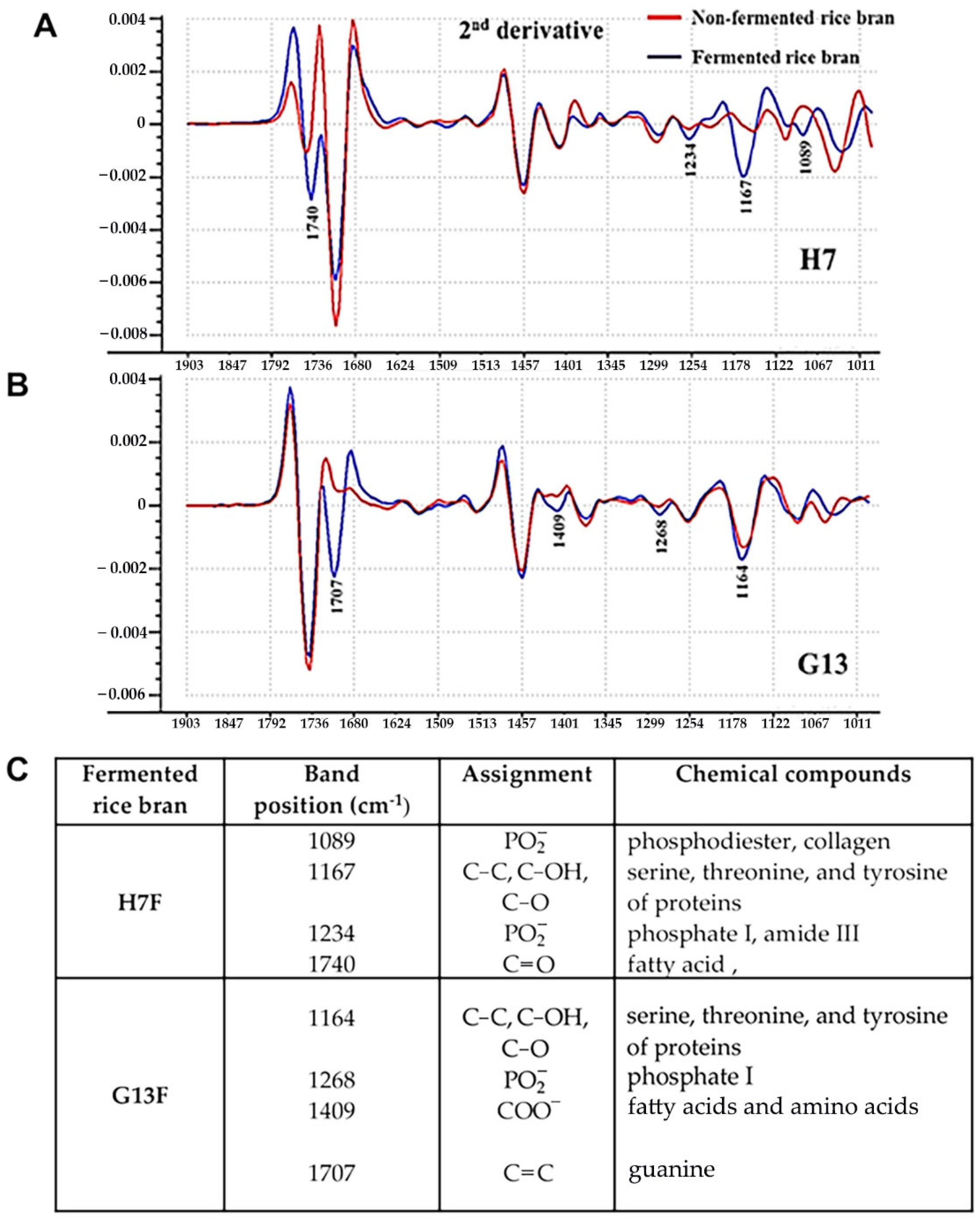
In this study, ATR-FTIR was used to monitor two different fermentative rice brans compared with nonfermentative forms of those rice brans. The second derivative ATR-FTIR spectra of H7F and G13F showed different compositions of biochemical ingredients from nonfermented rice bran (H7NF and G13NF) (Figure 1). Both fermented rice bran extracts exhibited feature peaks corresponding to protein, carbohydrate, and lipid composition. For the H7F sample, the specific peaks included phosphate and collagen at wavelength number position 1089 cm−1, serine, threonine, and tyrosine of proteins at 1167 cm−1, phosphate I and amide III at 1234 cm−1, and fatty acid at 1740 cm−1 of the H7F spectrum (Figure 1A). On the other hand, at the G13F spectra, the major peaks were found at 1164 cm−1 (serine, threonine, and tyrosine of proteins), 1268 cm−1 (phosphate I), 1409 cm−1 (fatty acid and amino acid), and 1707 cm−1 (guanine). The detailed information on biochemical compositions’ change after fermentation is shown in Figure 1C.
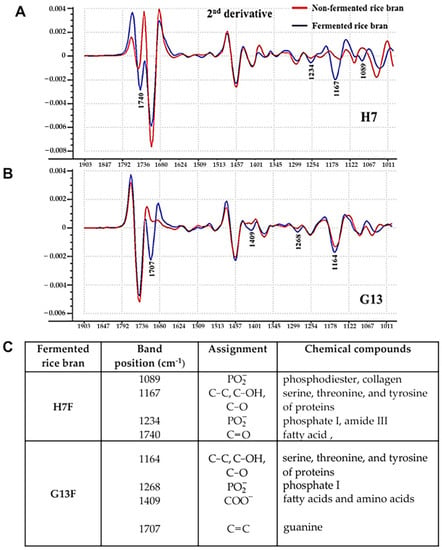
Figure 1.
Comparison of the second derivative spectra between H7NF vs. H7F (A) and G13NF vs. G13F (B) by ATRFTIR. The prediction of biochemical compositions’ change by using the band positions at specific wavelength number (cm−1) of IR spectra of H7F and G13F compared to H7NF and G13NF, respectively (C).
2.2. Lactic Acid Bacteria Growth Promoting of Fermented Rice Bran Extracts
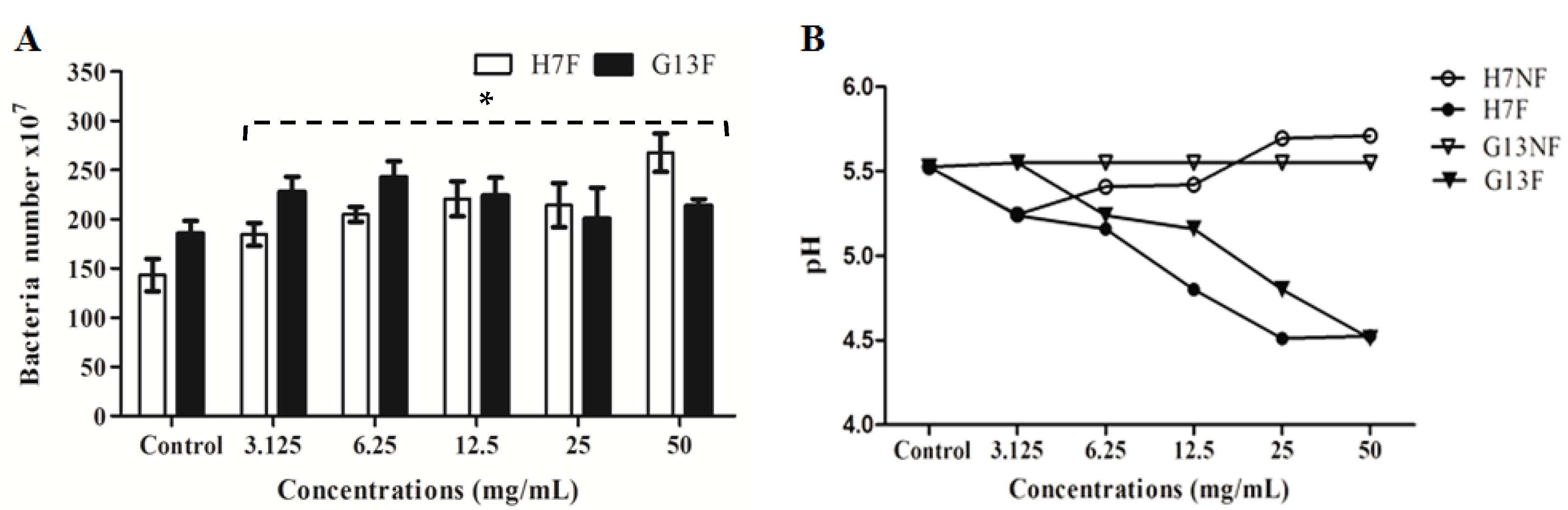
According to the metabolites’ change of rice bran under the fermentation, the fermented rice bran extracts (H7F and G13F) were used to prove the prebiotic property for a probiotic bacterium—L. delbrueckii subsp. lactis. To determine whether H7F and G13F extracts can promote the growth probiotic bacteria in in vitro study, the numbers of L. delbrueckii subsp. lactis were determined after culture with the extracts. The probiotic was significantly increased in coculture condition of all concentrations (3.125, 6.25, 12.5, 25, and 50 mg/mL) of both H7F and G13F extracts (Figure 2A), and it can observe the plateau in the numbers of lactic acid bacteria at concentrations of 12.5 mg/mL of the G13F extract. Moreover, the pH of nonfermented rice bran extracts (H7NF and G13NF) and fermented rice bran extracts (H7F and G13F) were evaluated to indicate optimal acid production to promote the lactic acid bacteria growth. The reduction in pH values, the higher level of lactic acid production (Figure 2B) with a dose-dependent trend (decreasing pH) depending on the extract concentrations. The results supported that the fermented rice bran extracts (H7F and G13F) could promote L. delbrueckii subsp. lactis in the apparent optimal growth at acidic pH; optimal pH range between 4.5–5.2.
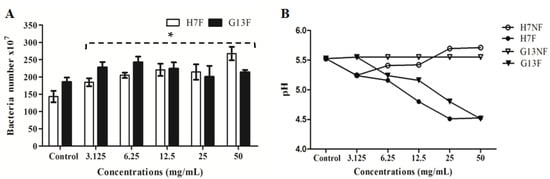
Figure 2.
The fermented rice bran extracts (H7F and G13F) promoting L. delbrueckii subsp. lactis growth determined by flow cytometry compared to the control (A) and a pH gradient during various concentrations of nonfermented rice bran extracts (H7NF and G13NF) and fermented rice bran extracts (H7F and G13F) (B); * significant difference when compared to the control (p-value < 0.05).
2.3. Pathogenic Bacteria Growth Inhibition of Nonfermented and Fermented Rice Bran Extracts
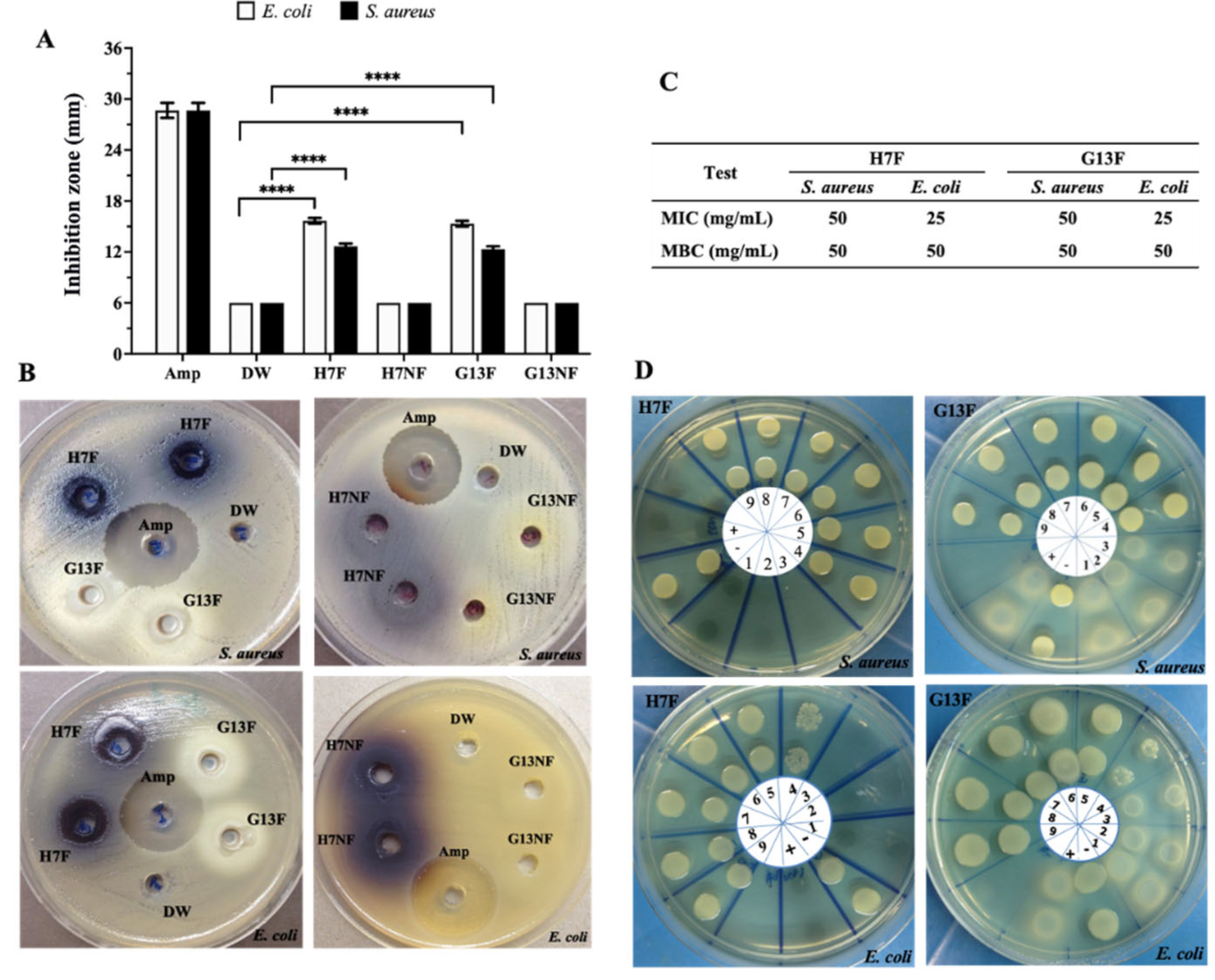
The antibacterial potential of nonfermented and fermented rice bran extract against pathogenic bacteria was evaluated by agar well diffusion assay. The 400 mg/mL concentration of either H7F or G13F extracts inhibited the growth of both S. aureus and E. coli; the averages of the inhibition zone of H7F were 12.15 and 15.96 mm, and of G13F were 12.15 and 15.50 mm, respectively. Unlike the fermented rice bran extracts, the nonfermented rice bran samples (H7NF and G13NF) could not inhibit these two pathogenic bacteria (Figure 3A,B). In addition, the results from the broth microdilution technique showed equivalent degrees of antimicrobial activities between H7F and G13F samples. The minimum inhibitory concentration (MIC) values of H7F and G13F on E. coli and S. aureus growth were identically detected at 25 and 50 mg/mL concentrations, respectively. For minimum bactericidal concentration (MBC), 50 mg/mL of both fermented rice bran extracts could inhibit the growth of S. aureus and E. coli (Figure 3C,D).
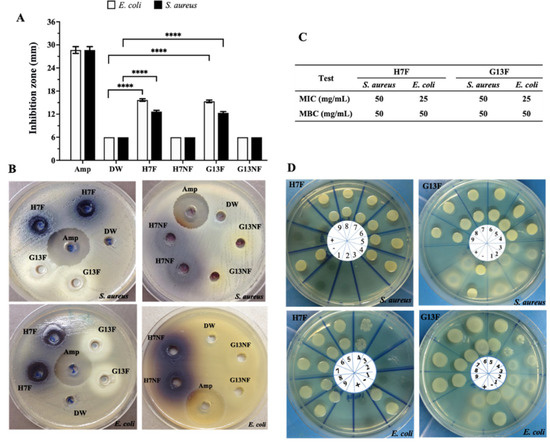
Figure 3.
Screening of antimicrobial effects of fermented rice bran H7 (H7F) and G13 extract (G13F) compared to nonfermented rice (H7NF and G13NF). According to the well diffusion assay, only H7F and G13F significantly exhibited inhibition zone against Escherichia coli and Staphylococcus aureus (A,B). A minimum inhibition concentration (MIC) and minimum bactericidal concentration (MBC) values were also evaluated by broth microdilution technique (C,D). Various concentrations of H7F for MIC and MBC were investigated, as follows: lane 1 = 200 mg/mL; 2 = 100 mg/mL; 3 = 50 mg/mL; 4 = 25 mg/mL; 5 = 12.5 mg/mL; 6 = 6.25 mg/mL; 7 = 3.12 mg/mL; 8 = 1.56 mg/mL; 9 = 0.8 mg/mL, respectively. Distilled water (DW) was used for noninhibition control (or lane +), and 5 μg/mL of Ampicillin drug (Amp) was used for inhibition control (or lane −). **** indicates statistical significance at p-value < 0.0001.
2.4. Effect of the Fermented Rice Bran on Biofilm Formation
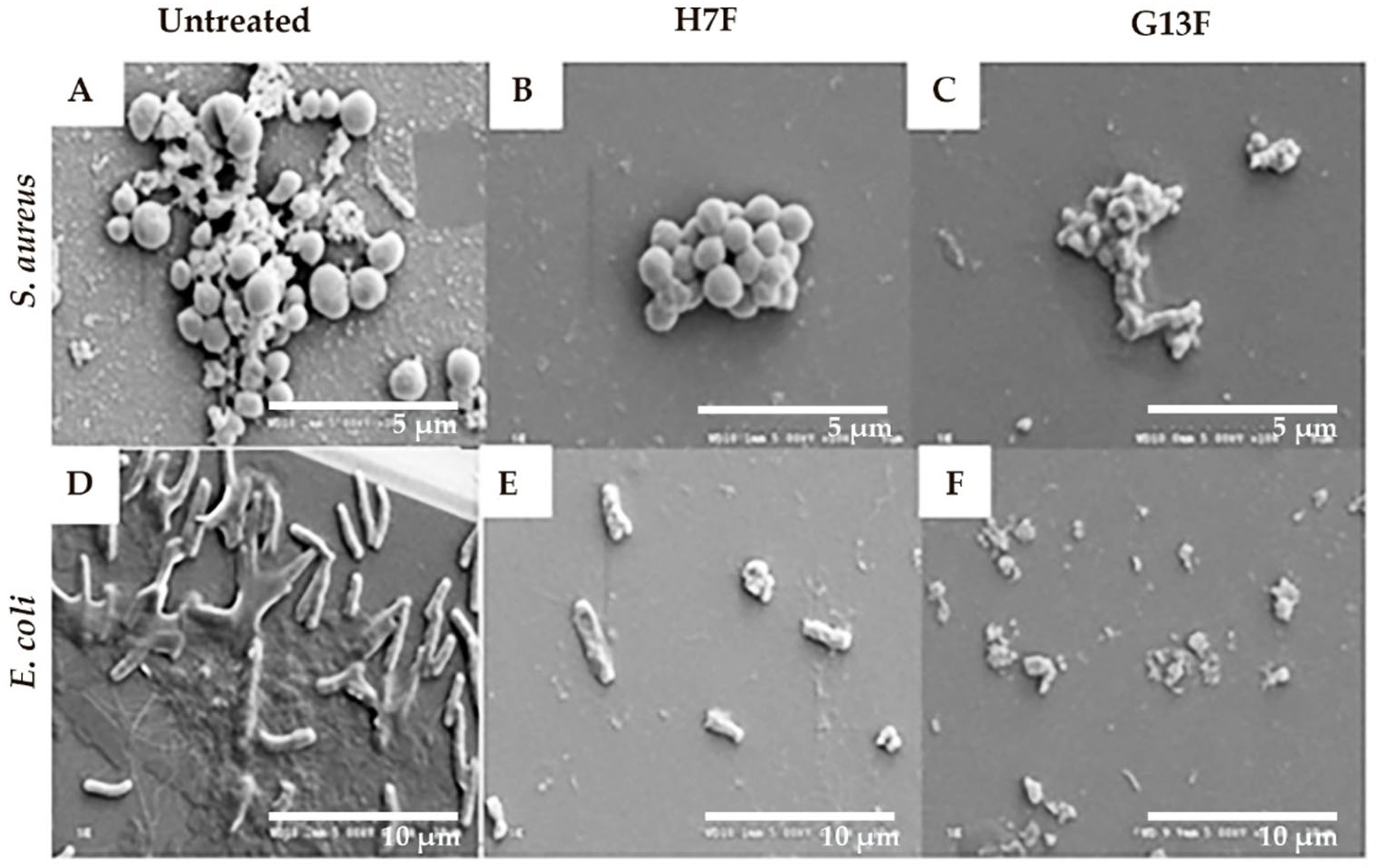
Following the demonstration of antipathogenic bacterial effects after treatment with the fermented rice bran extracts (H7F and G13F), the inhibitory mechanism against pathogenic bacteria of H7F or G13F was further investigated. Biofilm formation is an important virulence factor produced by both S. aureus and E. coli; this mechanism can prevent pathogenic bacteria destruction by toxic agents [23,24]. In our study, the H7F and G13F at half of MBC concentration (25 mg/mL) were cultured with S. aureus and E. coli; consequently, the biofilm formation and bacterial destruction were delineated by imaging the morphology of S. aureus and E. coli after the sample treatment under a scanning electron microscope (SEM). Biofilm formation reduction and bacterial cell breakage were detected in the treated groups, which differed from the control (untreated) group of each bacteria type. The control group showed colonization and biofilm formation of S. aureus; similarly, the control for E. coli presented biofilm formation and colonization with a flagella structure. Based on the results, the induction of bacterial cell membrane breakage and colony shrinkage was suggested as a mechanism of antimicrobial activity detected in H7F and G13F (Figure 4).
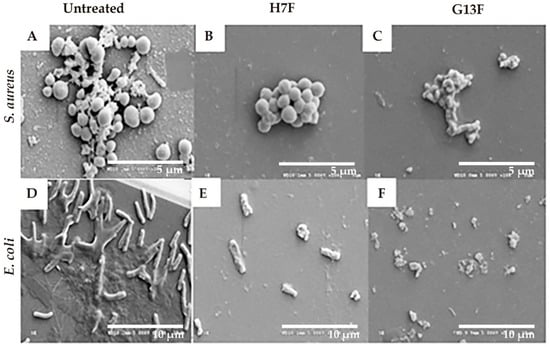
Figure 4.
Fermented rice bran H7 (H7F) and G13 extract (G13F) inhibited biofilm formation. However, biofilm and colonization of Staphylococcus aureus were still observed in the untreated condition (A), while they were decreased in the treated groups with either H7F (B) or G13F extracts (C). In addition, while the control (untreated) group of Escherichia coli still presented biofilm formation, flagella structure, and colonization (D), they were decreased in the treated groups with either H7F (E) and G13F extracts (F). Interestingly, cell membrane breakage of E. coli (a Gram-negative bacillus) was displayed in the treatment of either H7F or G13F (E,F).
2.5. Toxicity of the Fermented Rice Bran Extracts In Vitro Study
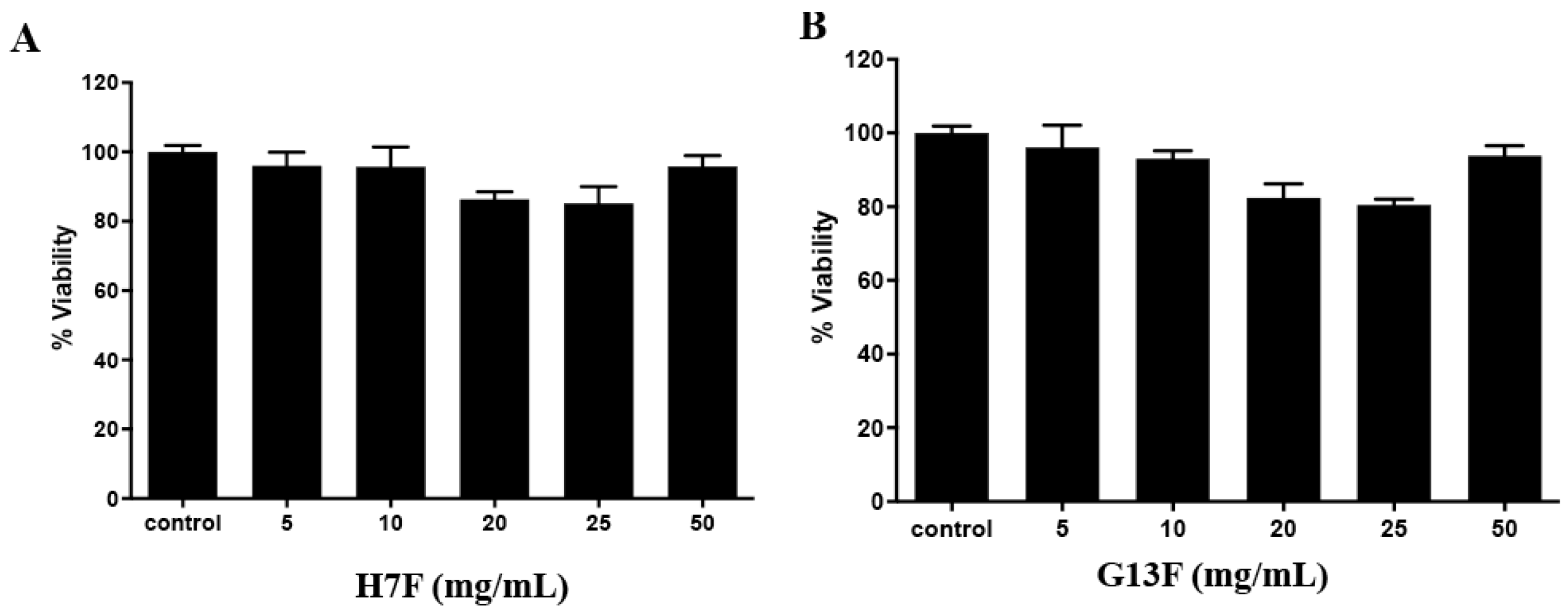
After fermentation of the rice brans, we demonstrated the metabolites’ change and acidic increase; thus, before using them as a supplement or functional food/beverage, toxicity to human and animal models should be investigated. The toxicity to human peripheral mononuclear cells (PBMCs) was demonstrated in in vitro study. For the result, all the concentrations of either H7F or G13F extract showed no toxicity to human PBMCs with more than 80% cell viability compared to the control (untreated group) (Figure 5A,B). The %survival of the cells of both fermented rice bran extracts’ treatment might indicate that these metabolic compositions and acidic conditions after the rice bran fermentative process were nontoxic to human PBMCs [25].
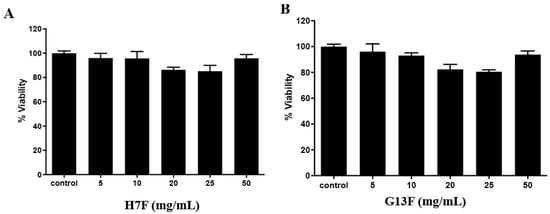
Figure 5.
Toxicity test of fermented rice bran H7 (H7F) and G13 extract (G13F) to human PBMCs by MTT assay. Both H7F (A) and G13F (B) extracts showed no toxicity to human PBMCs, expressing more than 80% of cell viability at all tested concentrations.
2.6. Toxicity in Acute or Chronic Manner of the Fermented Rice Bran Extracts In Vivo Study
To confirm the safety and bioavailability of product use, the study in the animal models should be demonstrated. In our study, Wistar rats were used for toxicity study in the acute or chronic manner. The growth and behavior of all the animals after receiving a single dose of G13F and H7F extracts at each concentration of 1000, 2500, and 5000 mg/kg/day were normal over a 14-day period without detection of mortality, change of hematological and biochemical values, or any pathological lesion of organs.
For chronic toxicity, both the male and female animals that received fermented rice bran H7F or G13F showed healthiness and no signs of toxicity when compared to the control group. In addition, there were no significant differences in body and organ weights among the treated groups that received different doses of the extracts. However, there were changes in WBC, platelet, and cholesterol levels after treatment with specific doses of H7F or G13F. For hematological values, the increase in WBC levels in all treated groups of H7F and G13F of both genders was demonstrated; however, significant differences were detected at high doses; 2000 mg/kg of H7F and 500 mg/kg of G13F in female rats as well as of 1000 and 2000 mg/kg of G13F in male rats, when compared to the vehicle group (Table 1). Furthermore, compared to the untreated group, higher platelet count levels of male rats receiving 500, 1000, and 2000 mg/kg of G13F extract were significantly revealed (Table 1). Additionally, the reduction in cholesterol levels in 500 and 2000 mg/kg of H7F and 1000 mg/kg of G13F-treated groups of male rats was significant compared to both the vehicle and untreated groups. In contrast, others presented no statistical significance (Table 2).

Table 1.
Effects on hematological values in male (A) and female Wistar rats (B) of fermented rice bran H7 (H7F) and G13 extracts (G13F) in chronic toxicity study.

Table 2.
Effects on biochemical parameters in male (A) and female Wistar rats (B) of fermented rice bran H7 (H7F) and G13 extracts (G13F) in chronic toxicity study.
3. Discussion
Rice bran—the outer layer fractions of rice grains produced from rice milling—is a rich source of fiber, vitamins, amino acids, and phenolic compounds. The high bioactivity and antioxidative properties of rice are potentially fortified through germination and fermentation [20,21,26,27,28,29]. Therefore, evidence of the genuine benefits of germinated rice and fermented rice and their extracts is pivotal in supporting the rationale for health-promoting applications. The present study has aimed to examine the properties of fermented rice bran of selected rice cultivars, focusing on the screening of biochemical composition by ATR-FTIR, prebiotic properties, antipathogenic effects, safety, and toxicity in both acute and chronic manners. This is to obtain scientific evidence that will serve as guidelines for further developing new functional food supplementary products from these ingredients.
The technology of ATR-FTIR was selected in this study to enable direct analysis of the chemical composition of the sample based on a total internal reflection via evanescent waves [30,31]. The ratios of prominent biochemical compounds, including amino acids (serine, threonine, and tyrosine) and polysaccharides, of fermented-rice bran derived from fermentation by L. delbrueckii subsp. lactis of both H7 and G13 were increased when compared to nonfermented rice bran samples. The results agreed with those of Russo et al. [32], who reported an increasing level of gross energy and crude protein after the fermentation of rice bran. Macek et al.’s study reported that the fermented rice can produce amino acids such as serine, threonine, and tyrosine. These amino acids are essential factors of protein biosynthesis in organism, including boosting the growth of microorganism [33]. In addition, fatty acid was increased after both rice fermentation. These results are in accordance with previous research that showed a slight increase in fatty acid after fermenting chia and sesame seeds [34]. An increase in fatty acids such as short-chain fatty acids in the fermented product cloud be attributed to microorganisms’ metabolism [35], producing SCFA (acetate, propionate, and butyrate) from carbohydrates and protein catabolism [36,37]. Through biosynthetic and metabolic versatility, fermentation by lactic acid bacteria is a value-added form of food processing to fortify the products with macronutrients, micronutrients (vitamins), or non-nutrition such as bacteriocin and phytochemical derivatives [20,32].
To answer whether H7F and G13F extracts could promote the growth and lactic acid production of L. delbrueckii subsp. lactis, a broth dilution technique was employed. The results revealed that both extracts of fermented rice bran could virtually enhance the propagation of probiotic bacteria. In addition, the acidic property of the fermented rice bran extracts, particularly at pH range 4.5–5.2, could be suitable to promote L. delbrueckii subsp. lactis, resulting in organic acid production under the growth of lactic acid bacteria. This result has implied the health impact of H7F and G13F following the principle of prebiotic-fortified probiotic functions. Probiotic bacteria in the gastrointestinal tract have various beneficial effects, e.g., maintenance of intestinal microbial balance, production of antimicrobial substances against pathogens, induction of mucin production, stimulation of a heightened immune response, and modulation of cytokine production by intestinal epithelial cells (IECs) [38,39,40]. Therefore, H7F and G13F are likely effective prebiotic agents containing bioactive compounds and nutrients for probiotic microorganism growth promotion. Moreover, the plateau of the L. delbrueckii subsp. lactis population at a G13F concentration greater than 6.25 mg/mL was demonstrated; this reflected the saturation of the L. delbrueckii subsp. lactis population in the systems affected by high concentrations of G13F extract (12.5–50 mg/mL) (Figure 2A). This has suggested the high potential and attribution of the feature biochemical composition in G13F—the combination of fatty acids, amino acids, and guanine (Figure 1).
Furthermore, the bactericidal property of H7F and G13F against pathogenic bacteria (S. aureus and E. coli)—usually causative microorganisms of intestinal infectious diseases such as acute diarrhea and inflammatory bowel disease (IBD)—was investigated [41]. This study showed that H7F and G13F had a gainful property of inhibiting the growth of these intestinal pathogens, while nonfermented extract (H7NF and G13NF) failed to demonstrate this capability. The antibacterial activities could be attributed to some non-nutrition metabolites, such as bacteriocins, coordinating with low pH conditioning from lactic acid bacteria after fermentation [12]. This presumption is supported by the previous reports. L. delbrueckii is a nonmotile Gram-positive rod bacterium that biochemically transforms sugars into lactic acids via anaerobic fermentation; therefore, many subspecies, including L. delbrueckii subsp. delbruecki, L. delbrueckii subsp. bulgaricus, and L. delbrueckii subsp. lactis, have been widely employed in the yogurt, cheese, and dairy products industry. Interestingly, the health benefits of L. delbrueckii subsp. lactis have been potentiated by several reports on the ability in bacteriocin production—namely lacticin, which has a broad spectrum of activity against Gram-positive bacteria and other microorganisms with high tolerance to intestinal antimicrobial molecules [42,43,44,45]. Moreover, this was supported by the report of Li, Lee, and Cho [24]; the effective microorganism-fermented extract (EM)-YU, which is a new version of the refreshment drink derived from fermented rice bran, seaweed, and kiwifruit, was found to exhibit antibacterial activity against E. coli O157:H7 and P. aeruginosa PAO1 under low pH (at 3.5) conditions. However, apart from fermentation extracts, nonfermented rice bran extracts of Song-Yod and jasmine rice from different extraction techniques also reported their antimicrobial activity against Salmonella spp., Shigella spp., E. coli, V. cholera, V. vulnificus, and S. aureus [41]. Consequently, a further suggestion from the present study is that the efficiency of the antibacterial activity of rice bran may depend on other factors such as rice species, the extraction method of rice bran, and the strain of bacteria.
Biofilm is an extracellular polymeric conglomeration of shading proteins, capsular polysaccharides, and slime covering the surface of their colonization. It is a naturally protective armor and contributes to the encouraging environment for bacterial growth [46,47]. In this study, biofilm formation, observed by SEM, in both S. aureus and E. coli reduced remarkedly after treatment of either H7F or G13F. Notably, both H7F and G13F could promote breakage of the cell membrane of E. coli. As supporting evidence, a biofilm inhibitory effect against E. coli O157:H7 by crystal-violet biofilm assay of diluted EM-YU was previously reported [24]. Moreover, Bermudez-Brito et al. [48] reported that lactic acid could destroy Gram-negative bacteria by inducing cell perforation and inhibiting cell membrane procreation. This was attributed to pH decreasing in bacterial cytoplasm and promoting bacteriocin production from lactic acid bacteria [48].
Safety and toxicity information on H7F and G13F is necessary to foster the development of new supplementary food products. This present research work is conducted in in vitro and in vivo test models. Human PBMCs were cocultured with fermented rice bran extracts before the percentages of live and dead cells were investigated by MTT assay. It was revealed that all concentrations of H7F and G13F showed more than 80% of cell viability, indicating no cytotoxicity to human PBMCs [25]. Relatively, their safety for acute and chronic administration was significantly agreed upon and evidenced. For acute toxicity, the fermented rice bran extracts showed no toxic effects on body weight, organ weight, blood chemical and hematological values, and histopathological analysis. These obtained results indicated the safety of these extracts in the acute phase at all dosing levels. Moreover, the chronic toxicity test showed no sign of weight loss or pathological lesions of vital organs in either male or female rats. Moreover, PBMC numbers increased significantly in many concentrations of H7F and G13F consumption. Presumably, the increase in innate immune cells (macrophages) may result from the presence of peptidoglycan, which is a part of the lactic acid bacteria cell wall via inducing the secretion of proliferative-associated cytokines [49]. Furthermore, G13F seemed to stimulate platelet production. However, the mechanism at this point remains unclear.
Interestingly, a significant reduction in cholesterol levels at 500 and 2000 mg/kg of H7F and 1000 mg/kg of G13F in male rats was revealed, compared to those in both vehicle and untreated groups. These results support health homeostasis through hepatic metabolism. Although the amount of short-chain fatty acid (SCFA) increases in prebiotic fermentation, the lipid blood level is maintained through hepatic metabolism of the SCFA as propionate and butyrate from the portal circulation to prevent systemically high SCFA concentrations [50]. Therefore, the contribution of dietary fibers and biochemical constituents from rice bran and lactic acid bacteria is presumed, which can disturb lipid digestion, absorption, and metabolism [11,12]. Dietary fibers can bind to some minor molecules, bile salt, or enzymes, resulting in reduced lipid digestibility and absorption. The binding property of fiber to bile acids, fatty acids, and cholesterol has been suggested as an underlining mechanism [51]. Besides the nutrition, phytochemicals such as anthocyanin-pigmented rice extract (black rice) could partially contribute to decreased plasma triglyceride, total cholesterol, and low-density lipoprotein cholesterol in rats [52]. Additionally, lactic acid bacteria probably disturb lipid metabolism via deconjugating bile salt and suppressing cholesterol absorption, which agrees with the findings of Pereira and Gibson [53]. Germinated brown rice improves lipid parameters and ameliorates cardiovascular disease (CVD) risk via transcriptional regulation of hepatic metabolic genes [54]. The hypocholesterolaemia effect of rice bran in high-fat diet-fed mice has been evidenced in association with the phytochemical composition of oryzanol, anthocyanins, GABA, and vitamin E [55,56]. Interestingly, the nutrition-fortified final products with GABA and phenolics from the processes of fermentation and germination have widely been evidenced [57,58,59,60,61]. Therefore, our study revealed the potential properties of both fermented rice bran extracts derived from black rice and germinated brown rice, including prebiotic properties, antipathogenic bacteria, and safety with hypocholesterolemia effect demonstrated in in vitro and in vivo models, respectively. The obtained information could emphasize that fermented black rice (H7F) and germinated brown rice (G13F) are the potential functional ingredients for the further development of health products such as probiotics, prebiotics, and synbiotics, as well as fermented foods.
4. Materials and Methods
4.1. Preparation of the Rice Bran Extracts
Rice bran of Thai cultivar black rice (H7; KKU ULR0381) and germinated brown rice (G13) were purchased from local rice farmers in Khon Kaen province and Sakon Nakhon province of Thailand, respectively. The pure cultures of Aspergillus sojae (TISTR3037) and Lactobacillus delbrueckii (L. delbrueckii) subsp. lactis were purchased from Thailand Institute of Scientific and Technological Research (TISTR). The extracts of rice bran were separately prepared. Firstly, rice bran sample was pasteurized in sterile distilled water (DW) at 63 ± 1 °C for 30 min. Then, rice bran was fermented by Aspergillus sojae (TISTR3037), which was used as starter culture at 30 °C for 2–5 days and then fermented by L. delbrueckii subsp. lactis for another 2–5 days. Afterward, the fermented sap was collected by centrifugation and vacuum filtration and dried using lyophilization (Martin Christ, Osterode am Harz, Germany) under the following conditions: freezing at −80 °C, followed by a primary freeze-drying at 0.4 mbar to eliminate free water (ending at 3 °C) and by two subsequent cycles of secondary freeze-drying with a pressure variation of 1.6 mbar at 0.001 mbar to remove the bound water (ending at 10 °C). Finally, the dried powder of nonfermented rice bran (H7NF and G13NF) and fermented rice bran (H7F and G13F) were kept at −20 °C until used in experiments. For the preparation of extracts, the powder of each rice bran (nonfermentation and fermentation) was dissolved in DW for the stock of the extracts and kept in −20 °C prior to use in further experiments. To investigate probiotic growth, antibacterial activity and cytotoxicity, the extracts were prepared in the diluents, including de Man–Rogaosa–Sharpe (MRS) broth, DW, and RPMI 1640 medium, respectively.
4.2. Chemical Composition Profile by Attenuated Total Reflection–Fourier Transform Infrared Spectroscopy (ATR-FTIR)
The chemical compositions of both nonfermented and fermented rice bran extracts of H7 and G13 were investigated using ATR-FTIR (4500 Series, Agilent Technologies, Santa Clara, CA, USA). The spectra were recorded in the wave number range of 500–4000 cm−1 to manipulate the operating software of the ATR-FTIR instrument to transform the data into derivative spectra.
4.3. Prebiotic Property on L. delbrueckii Growth Using Broth Dilution Technique and the Potential of Hydrogen Ion (pH) Measurement
Various concentrations (3.125, 6.25, 12.5, 25 and 50 mg/mL) of fermented rice bran H7 (H7F) and G13 (G13F) extracts were cocultured with L. delbrueckii (2 × 104 cfu/mL) in 27.6 g/L de Man–Rogaosa–Sharpe (MRS) broth under anaerobic conditions at 37 °C for 24 h. Bacterial numbers were analyzed by flow cytometric analysis (BD FACSCantoTM II flow cytometer, BD Biosciences, San Jose, CA, USA) employing counting beads calculation (BD Biosciences, CA, USA) followed with pH value measurement, compared to nonfermented rice bran (H7NF and G13NF) extracts, by using pH meter (Starter 3100, Ohaus, Parsippany, NJ, USA).
4.4. Antimicrobial Activity Assays
4.4.1. Agar Well Diffusion and Broth Microdilution Technique
Agar well diffusion test was employed to test the inhibitory effect of fermented and nonfermented rice bran extracts against pathogenic bacteria. Mueller–Hinton (MH) agars were drilled with 6 mm diameter cork-borer and then streaked with Escherichia coli (E. coli) ATCC25922 or Staphylococcus aureus (S. aureus) ATCC29213. A total of 100 μL of 400 mg/mL (excessive concentration dose) fermented (H7F and G13F) and nonfermented rice bran (H7NF and G13NF) extracts dissolved in DW were added in holes and then incubated at 37 °C for 24 h before determining the diameter of inhibition zone. Distilled water (DW) was used for noninhibition control, whereas bacteria cocultured with 5 μg/mL of Ampicillin (Amp) were used for inhibition control.
Moreover, the solution of fermented rice bran extracts was diluted into various concentrations, ranging between 200, 100, 50, 25, 12.5, 6.25, 3.12, 1.56, and 0.8 mg/mL, and used in the experiment to define a minimum inhibition concentration (MIC) as well as a minimum bactericidal concentration (MBC) by broth microdilution technique. The fermented rice bran extract solutions were cocultured with pathogenic bacteria in microtiter plate at 37 °C for 24 h, and subsequently, 10 μL of each was dropped onto MH agar and followed with a 24 h incubation. Finally, the colonies in each unit were observed. The colony numbers of the treated group were compared to that of noninhibition control (untreated group; distilled water (DW) + microbial + medium). The MIC was indicated at the lowest concentration, resulting in colony density and size reduction as evidenced by the bacterial growth inhibitory effect. In contrast, the MBC was accounted for at the lowest concentration, resulting in no bacterial colony formation [62].
4.4.2. Biofilm Formation by Scanning Electron Microscopy (SEM)
E. coli and S. aureus were cultured in trypticase soy broth (TSB) in 6-well plates. The concentrations of fermented rice bran extract solution dissolved in TBS were adjusted according to MIC. Bacteria were incubated at 37 °C for 18 h before washing and fixation with 4% formaldehyde. Finally, samples were dehydrated with absolute ethanol and observed under a scanning electron microscope (SEM) (S-3000N Hitachi, Tokyo, Japan).
4.5. Safety and Toxicity Assays
4.5.1. Toxicity Test of Human Peripheral Blood Mononuclear Cells (PBMCs) in In Vitro Model
Human peripheral blood mononuclear cells (PBMCs) from the leftover buffy coat permitted by the Khon Kaen University Ethical Committee Review Board (HE651334) were cultured in RPMI 1640 media supplemented with 10% fetal bovine serum at 37 °C for 24 h with presenting of fermented rice bran extracts at various concentrations of 5, 10, 20, 25, and 50 mg/mL r DW for the control. Compared to the control, %viability of cell was analyzed by MTT assay.
4.5.2. Study Population in In Vivo Model
A total of 160 Wistar rats, including 80 males and 80 females, aged between 6–8 weeks, were employed in this experiment. The animals were provided by the National Laboratory Animal Center, Mahidol University, and they were later treated at the Northeast Laboratory Animal Center, Khon Kaen University, Thailand. This study was approved by the Ethical Committee of Khon Kaen University (AEKKU-NELAC 7/2557), whose acute and chronic toxicity study was performed in respect to OECD guideline 420 [63] and OECD guideline 452 [64], respectively.
4.5.3. Acute Toxicity Test
A total of 80 rats were used and divided into 8 groups with 10 rats, including 5 females and 5 males in each. Those in groups I, II, and III received an orally fed single dose of G13F for 14 days at 1000, 2500, and 5000 mg/kg/day; those in group IV, V, and VI received oral feeding of H7F in the same dose; while those in group VII and VIII were vehicle (DW) and untreated group, respectively. Each rat’s body weight was recorded daily, while the weight of organs, blood chemical values, hematological values, and histopathology of organs were examined after completion of a 14-day term of study.
4.5.4. Chronic Toxicity Test
Similar to the acute toxicity test, 80 rats were used and divided into 8 groups with 10 rats, including 5 females and 5 males, in each. For 6 months, the assigned groups I, II, and III daily received an orally fed dose of G13F at 500, 1000, and 2000 mg/kg/day; group IV, V, and VI received H7F; and VII and VIII groups were vehicle and untreated group, respectively. Each rat’s body weight was recorded weekly, while the weight of organs, blood chemical values, hematological values, and histopathology of organs were examined after 6-month term of treatment.
4.6. Statistical Analysis
The data are presented in mean ± standard deviation (SD) from at least triplicates of independent studied unit. The flow cytometry data were analyzed by the BD FACSDivasTM software (BD Biosciences, Franklin, CA, USA). The statistical analysis was performed by SPSS (version 17, SPSS Inc., San Diego, IL, USA) and GraphPad Prism software (GraphPad Software Inc., Chicago, CA, USA). The nonparametric data were analyzed by Mann–Whitney U test. Statistical significance was considered at p-value less than 0.05.
5. Conclusions
The health benefits of fermented rice bran extracts from black rice cultivar (H7F) and germinated brown rice cultivar (G13F) have been evidenced. They exhibited capacity in controlling bacterial growth with different potencies: growth-promoting for beneficial bacteria via the prebiotic property, while inhibiting pathogenic bacterial growth by diminishing biofilm formation and promoting cell membrane breakage. In addition, their high degree of safety was demonstrated. Coincidently, the reduced cholesterol levels and modulating immune cell response were manifested. Conclusively, H7F and G13F are suggested as promising functional ingredients for health-promoting products.
Author Contributions
Conceptualization, K.S.; methodology, P.T. and P.V.; software, S.S. and W.P.; formal analysis, K.S. and P.T.; investigation, K.S., P.T., S.S., W.P. and P.V.; writing—original draft preparation, K.S., P.T. and R.W.; writing—review and editing, K.S.; visualization, P.V. and P.T.; supervision, K.S.; project administration, K.S.; funding acquisition, K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded and supported by Research and Graduate Studies, Khon Kaen University: 2563-2565; the Fundamental Fund of Khon Kaen University: 2565, and the National Science, Research and Innovation Fund (NSRF): 2565.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of Khon Kaen University (protocol code AEKKU-NELAC 7/2557, approval in July 2014).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bhattacharjee, P.; Singhal, R.S.; Kulkarni, P.R. Basmati rice: A review. Int. J. Food Sci. Technol. 2002, 37, 1–12. [Google Scholar] [CrossRef]
- Roohinejad, S.; Mirhosseini, H.; Saari, N.; Shuhaimi, M.; Alias, I.; Meor Hussin, A.S.; Abdul Hamid, A.; Abd Manap, Y. Evaluation of GABA, Crude Protein and Amino Acid Composition from Different Varieties of Malaysian’s Brown Rice. Aust. J. Crop Sci. 2009, 3, 184–190. [Google Scholar]
- Atungulu, G.G.; Pan, Z. Rice industrial processing worldwide and impact on macro- and micronutrient content, stability, and retention. Ann. N. Y. Acad. Sci. 2014, 1324, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, gamma-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.J.; Ollila, C.A.; Kumar, A.; Borresen, E.C.; Raina, K.; Agarwal, R.; Ryan, E.P. Chemopreventive properties of dietary rice bran: Current status and future prospects. Adv. Nutr. 2012, 3, 643–653. [Google Scholar] [CrossRef]
- Pengkumsri, N.; Sivamaruthi, B.S.; Sirilun, S.; Suwannalert, P.; Rodboon, T.; Prasitpuriprecha, C.; Peerajan, S.; Butrungrod, W.; Chaiyasut, C. Dietary supplementation of Thai black rice bran extract and yeast beta-glucan protects the dextran sodium sulphate mediated colitis induced rat. RSC Adv. 2017, 7, 396–402. [Google Scholar] [CrossRef]
- Phetpornpaisan, P.; Tippayawat, P.; Jay, M.; Sutthanut, K. A local Thai cultivar glutinous black rice bran: A source of functional compounds in immunomodulation, cell viability and collagen synthesis, and matrix metalloproteinase-2 and -9 inhibition. J. Funct. Foods 2014, 7, 650–661. [Google Scholar] [CrossRef]
- Kannappan, R.; Yadav, V.R.; Aggarwal, B.B. gamma-Tocotrienol but not gamma-tocopherol blocks STAT3 cell signaling pathway through induction of protein-tyrosine phosphatase SHP-1 and sensitizes tumor cells to chemotherapeutic agents. J. Biol. Chem. 2010, 285, 33520–33528. [Google Scholar] [CrossRef]
- Phutthaphadoong, S.; Yamada, Y.; Hirata, A.; Tomita, H.; Hara, A.; Limtrakul, P.; Iwasaki, T.; Kobayashi, H.; Mori, H. Chemopreventive effect of fermented brown rice and rice bran (FBRA) on the inflammation-related colorectal carcinogenesis in ApcMin/+ mice. Oncol. Rep. 2010, 23, 53–59. [Google Scholar]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Carlson, J.L.; Erickson, J.M.; Lloyd, B.B.; Slavin, J.L. Health Effects and Sources of Prebiotic Dietary Fiber. Curr. Dev. Nutr. 2018, 2, nzy005. [Google Scholar] [CrossRef]
- George Kerry, R.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.-S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef]
- Khangwal, I.; Shukla, P. Prospecting prebiotics, innovative evaluation methods, and their health applications: A review. 3 Biotech 2019, 9, 187. [Google Scholar] [CrossRef]
- Nouvenne, A.; Ticinesi, A.; Tana, C.; Prati, B.; Catania, P.; Miraglia, C.; De’ Angelis, G.L.; Di Mario, F.; Meschi, T. Digestive disorders and Intestinal microbiota. Acta Biomed. 2018, 89, 47–51. [Google Scholar] [CrossRef]
- Nabavi, S.; Rafraf, M.; Somi, M.H.; Homayouni-Rad, A.; Asghari-Jafarabadi, M. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J. Dairy Sci. 2014, 97, 7386–7393. [Google Scholar] [CrossRef]
- Teng, G.; Liu, Z.; Liu, Y.; Wu, T.; Dai, Y.; Wang, H.; Wang, W. Probiotic Escherichia coli Nissle 1917 Expressing Elafin Protects Against Inflammation and Restores the Gut Microbiota. Front. Microbiol. 2022, 13, 819336. [Google Scholar] [CrossRef]
- Yang, X.; Twitchell, E.; Li, G.; Wen, K.; Weiss, M.; Kocher, J.; Lei, S.; Ramesh, A.; Ryan, E.P.; Yuan, L. High protective efficacy of rice bran against human rotavirus diarrhea via enhancing probiotic growth, gut barrier function, and innate immunity. Sci. Rep. 2015, 5, 15004. [Google Scholar] [CrossRef]
- Zubaidah, E.; Nurcholis, M.; Wulan, S.N.; Kusuma, A. Comparative Study on Synbiotic Effect of Fermented Rice Bran by Probiotic Lactic Acid Bacteria Lactobacillus casei and Newly Isolated Lactobacillus plantarum B2 in Wistar Rats. APCBEE Procedia 2012, 2, 170–177. [Google Scholar] [CrossRef]
- Wang, M.; Lei, M.; Samina, N.; Chen, L.; Liu, C.; Yin, T.; Yan, X.; Wu, C.; He, H.; Yi, C. Impact of Lactobacillus plantarum 423 fermentation on the antioxidant activity and flavor properties of rice bran and wheat bran. Food Chem. 2020, 330, 127156. [Google Scholar] [CrossRef]
- Pino, A.; Nicosia, F.D.; Agolino, G.; Timpanaro, N.; Barbagallo, I.; Ronsisvalle, S.; Caggia, C.; Randazzo, C.L. Formulation of germinated brown rice fermented products functionalized by probiotics. Innov. Food Sci. Emerg. Technol. 2022, 80, 103076. [Google Scholar] [CrossRef]
- Shuvo, A.A.S.; Rahman, M.S.; Al-Mamum, M.; Islam, K.M.S. Cholesterol reduction and feed efficiency enhancement in broiler through the inclusion of nutritionally improved fermented rice bran. J. Appl. Poult. Res. 2022, 31, 100226. [Google Scholar] [CrossRef]
- Yi, C.; Xu, L.; Luo, C.; He, H.; Ai, X.; Zhu, H. In vitro digestion, fecal fermentation, and gut bacteria regulation of brown rice gel prepared from rice slurry backfilled with rice bran. Food Hydrocoll. 2022, 133, 107986. [Google Scholar] [CrossRef]
- Jeyaraj, E.J.; Nathan, S.; Lim, Y.Y.; Choo, W.S. Antibiofilm properties of Clitoria ternatea flower anthocyanin-rich fraction towards Pseudomonas aeruginosa. Access Microbiol. 2022, 4, 000343. [Google Scholar] [CrossRef]
- Li, Z.; Lee, J.; Cho, M.H. Antioxidant, antibacterial, tyrosinase inhibitory, and biofilm inhibitory activities of fermented rice bran broth with effective microorganisms. Biotechnol. Bioprocess Eng. 2010, 15, 139–144. [Google Scholar] [CrossRef]
- Hill, H.D.; Mirkin, C.A. The bio-barcode assay for the detection of protein and nucleic acid targets using DTT-induced ligand exchange. Nat. Protoc. 2006, 1, 324–336. [Google Scholar] [CrossRef]
- Alauddin, M.; Shirakawa, H.; Koseki, T.; Kijima, N.; Ardiansyah; Budijanto, S.; Islam, J.; Goto, T.; Komai, M. Fermented rice bran supplementation mitigates metabolic syndrome in stroke-prone spontaneously hypertensive rats. BMC Complement. Altern. Med. 2016, 16, 442. [Google Scholar] [CrossRef]
- Cho, D.H.; Lim, S.T. Germinated brown rice and its bio-functional compounds. Food Chem. 2016, 196, 259–271. [Google Scholar] [CrossRef]
- Lin, Y.T.; Pao, C.C.; Wu, S.T.; Chang, C.Y. Effect of different germination conditions on antioxidative properties and bioactive compounds of germinated brown rice. BioMed Res. Int. 2015, 2015, 608761. [Google Scholar] [CrossRef]
- Kittibunchakul, S.; Yuthaworawit, N.; Whanmek, K.; Suttisansanee, U.; Santivarangkna, C. Health beneficial properties of a novel plant-based probiotic drink produced by fermentation of brown rice milk with GABA-producing Lactobacillus pentosus isolated from Thai pickled weed. J. Funct. Foods 2021, 86, 104710. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Chan, K.L. ATR-FTIR spectroscopic imaging: Recent advances and applications to biological systems. Analyst 2013, 138, 1940–1951. [Google Scholar] [CrossRef]
- Mirabella, F.M. Internal Reflection Spectroscopy. Appl. Spectrosc. Rev. 1985, 21, 45–178. [Google Scholar] [CrossRef]
- Russo, P.; Lopez, P.; Capozzi, V.; de Palencia, P.F.; Duenas, M.T.; Spano, G.; Fiocco, D. Beta-glucans improve growth, viability and colonization of probiotic microorganisms. Int. J. Mol. Sci. 2012, 13, 6026–6039. [Google Scholar] [CrossRef]
- Macek, B.; Mijakovic, I.; Olsen, J.V.; Gnad, F.; Kumar, C.; Jensen, P.R.; Mann, M. The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis. Mol. Cell. Proteom. 2007, 6, 697–707. [Google Scholar] [CrossRef]
- Calvo-Lerma, J.; Asensio-Grau, A.; Garcia-Hernandez, J.; Heredia, A.; Andres, A. Exploring the Impact of Solid-State Fermentation on Macronutrient Profile and Digestibility in Chia (Salvia hispanica) and Sesame (Sesamum Indicum) Seeds. Foods 2022, 11, 410. [Google Scholar] [CrossRef]
- Achinewhu, S.C. The effect of fermentation on carbohydrate and fatty acid composition of African oil bean seed (Pentaclethra macrophylla). Food Chem. 1986, 19, 105–116. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Mack, D.R.; Michail, S.; Wei, S.; McDougall, L.; Hollingsworth, M.A. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. 1999, 276, G941–G950. [Google Scholar] [CrossRef]
- Naidu, A.S.; Bidlack, W.R.; Clemens, R.A. Probiotic spectra of lactic acid bacteria (LAB). Crit. Rev. Food Sci. Nutr. 1999, 39, 13–126. [Google Scholar] [CrossRef]
- Wallace, T.D.; Bradley, S.; Buckley, N.D.; Green-Johnson, J.M. Interactions of lactic acid bacteria with human intestinal epithelial cells: Effects on cytokine production. J. Food Prot. 2003, 66, 466–472. [Google Scholar] [CrossRef]
- Kondo, S.; Teongtip, R.; Srichana, D.; Itharat, A. Antimicrobial activity of rice bran extracts for diarrheal disease. J. Med. Assoc. Thail. = Chotmaihet Thangphaet 2011, 94 (Suppl. S7), S117–S121. [Google Scholar]
- Hugo, A.A.; De Antoni, G.L.; Pérez, P.F. Lactobacillus delbrueckii subsp lactis (strain CIDCA 133) resists the antimicrobial activity triggered by molecules derived from enterocyte-like Caco-2 cells. Lett. Appl. Microbiol. 2010, 50, 335–340. [Google Scholar] [CrossRef]
- Toba, T.; Yoshioka, E.; Itoh, T. Lacticin, a bacteriocin produced by Lactobacillus delbrueckii subsp. lactis. Lett. Appl. Microbiol. 1991, 12, 43–45. [Google Scholar] [CrossRef]
- Morgan, S.M.; O’Connor, P.M.; Cotter, P.D.; Ross, R.P.; Hill, C. Sequential actions of the two component peptides of the lantibiotic lacticin 3147 explain its antimicrobial activity at nanomolar concentrations. Antimicrob. Agents Chemother. 2005, 49, 2606–2611. [Google Scholar] [CrossRef]
- Dischinger, J.; Basi Chipalu, S.; Bierbaum, G. Lantibiotics: Promising candidates for future applications in health care. Int. J. Med. Microbiol. 2014, 304, 51–62. [Google Scholar] [CrossRef]
- Chandki, R.; Banthia, P.; Banthia, R. Biofilms: A microbial home. J. Indian Soc. Periodontol. 2011, 15, 111–114. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Diaz, J.; Munoz-Quezada, S.; Gomez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Bersch, K.L.; DeMeester, K.E.; Zagani, R.; Chen, S.; Wodzanowski, K.A.; Liu, S.; Mashayekh, S.; Reinecker, H.-C.; Grimes, C.L. Bacterial Peptidoglycan Fragments Differentially Regulate Innate Immune Signaling. ACS Cent. Sci. 2021, 7, 688–696. [Google Scholar] [CrossRef]
- Bloemen, J.G.; Venema, K.; van de Poll, M.C.; Olde Damink, S.W.; Buurman, W.A.; Dejong, C.H. Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clin. Nutr. 2009, 28, 657–661. [Google Scholar] [CrossRef]
- Vaskonen, T.; Mervaala, E.; Krogerus, L.; Karppanen, H. Supplementation of plant sterols and minerals benefits obese Zucker rats fed an atherogenic diet. J. Nutr. 2002, 132, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Zawistowski, J.; Kopeć, A.; Kitts, D. Effects of a black rice extract (Oryza sativa L. indica) on cholesterol levels and plasma lipid parameters in Wistar Kyoto rats. J. Funct. Foods 2009, 1, 50–56. [Google Scholar]
- Pereira, D.I.; Gibson, G.R. Cholesterol assimilation by lactic acid bacteria and bifidobacteria isolated from the human gut. Appl. Environ. Microbiol. 2002, 68, 4689–4693. [Google Scholar] [CrossRef] [PubMed]
- Imam, M.U.; Ishaka, A.; Ooi, D.-J.; Zamri, N.D.M.; Sarega, N.; Ismail, M.; Esa, N.M. Germinated brown rice regulates hepatic cholesterol metabolism and cardiovascular disease risk in hypercholesterolaemic rats. J. Funct. Foods 2014, 8, 193–203. [Google Scholar] [CrossRef]
- Cicero, A.F.; Gaddi, A. Rice bran oil and gamma-oryzanol in the treatment of hyperlipoproteinaemias and other conditions. Phytother. Res. 2001, 15, 277–289. [Google Scholar] [CrossRef]
- Ho, J.N.; Son, M.E.; Lim, W.C.; Lim, S.T.; Cho, H.Y. Anti-obesity effects of germinated brown rice extract through down-regulation of lipogenic genes in high fat diet-induced obese mice. Biosci. Biotechnol. Biochem. 2012, 76, 1068–1074. [Google Scholar] [CrossRef]
- Iimure, T.; Kihara, M.; Hirota, N.; Zhou, T.; Hayashi, K.; Ito, K. A method for production of γ-amino butyric acid (GABA) using barley bran supplemented with glutamate. Food Res. Int. 2009, 42, 319–323. [Google Scholar] [CrossRef]
- Lee, S.M.; Lim, H.J.; Chang, J.W.; Hurh, B.-S.; Kim, Y.-S. Investigation on the formations of volatile compounds, fatty acids, and γ-lactones in white and brown rice during fermentation. Food Chem. 2018, 269, 347–354. [Google Scholar] [CrossRef]
- Liao, W.-C.; Wang, C.-Y.; Shyu, Y.-T.; Yu, R.-C.; Ho, K.-C. Influence of preprocessing methods and fermentation of adzuki beans on γ-aminobutyric acid (GABA) accumulation by lactic acid bacteria. J. Funct. Foods 2013, 5, 1108–1115. [Google Scholar] [CrossRef]
- Oh, S.-J.; Kim, H.S.; Lim, S.-T.; Reddy, C.K. Enhanced accumulation of gamma-aminobutyric acid in rice bran using anaerobic incubation with various additives. Food Chem. 2019, 271, 187–192. [Google Scholar] [CrossRef]
- Park, E.-J.; Garcia, C.V.; Youn, S.-J.; Park, C.-D.; Lee, S.-P. Fortification of γ-aminobutyric acid and bioactive compounds in Cucurbita moschata by novel two-step fermentation using Bacillus subtilis and Lactobacillus plantarum. LWT 2019, 102, 22–29. [Google Scholar] [CrossRef]
- Wandee, R.; Sutthanut, K.; Songsri, J.; Sonsena, S.; Krongyut, O.; Tippayawat, P.; Tukummee, W.; Rittirod, T. Tamarind Seed Coat: A Catechin-Rich Source with Anti-Oxidation, Anti-Melanogenesis, Anti-Adipogenesis and Anti-Microbial Activities. Molecules 2022, 27, 5319. [Google Scholar] [CrossRef]
- OECD. Test No. 420: Acute Oral Toxicity—Fixed Dose Procedure; OECD: Paris, France, 2002. [Google Scholar] [CrossRef]
- OECD. Test No. 452: Chronic Toxicity Studies; OECD: Paris, France, 2018. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).