Occurrence of Aflatoxins and Ochratoxin A during Merkén Pepper Powder Production in Chile
Abstract
:1. Introduction
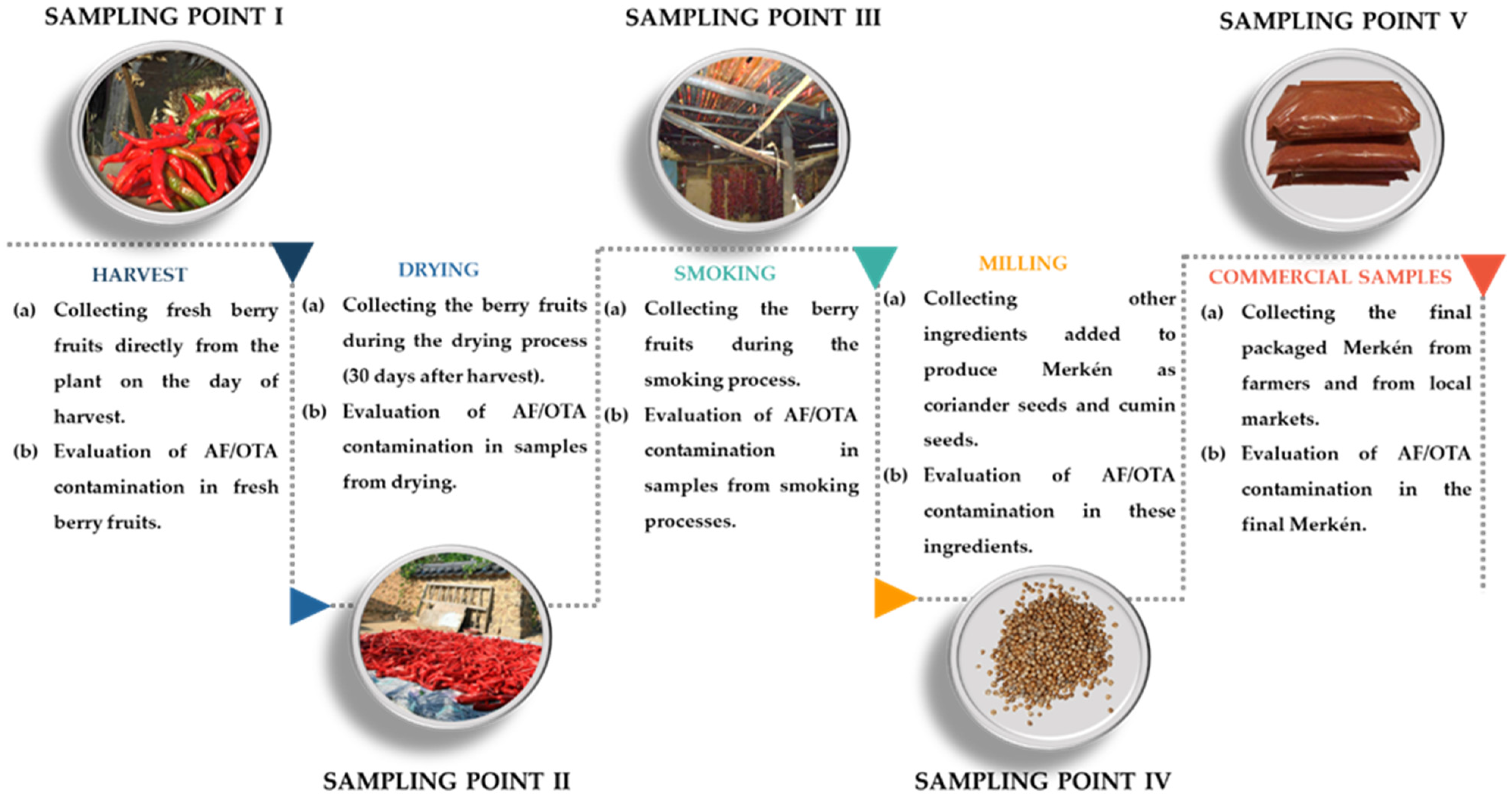
2. Materials and Methods
2.1. Sampling
2.2. Mycotoxins Extraction from Substrates
2.3. Mycological Analyses
2.3.1. Genomic DNA Extraction
2.3.2. PCR Amplification
2.4. Toxigenic Capacity of Fungal Isolates
2.5. Mycotoxin Detection
3. Results and Discussion
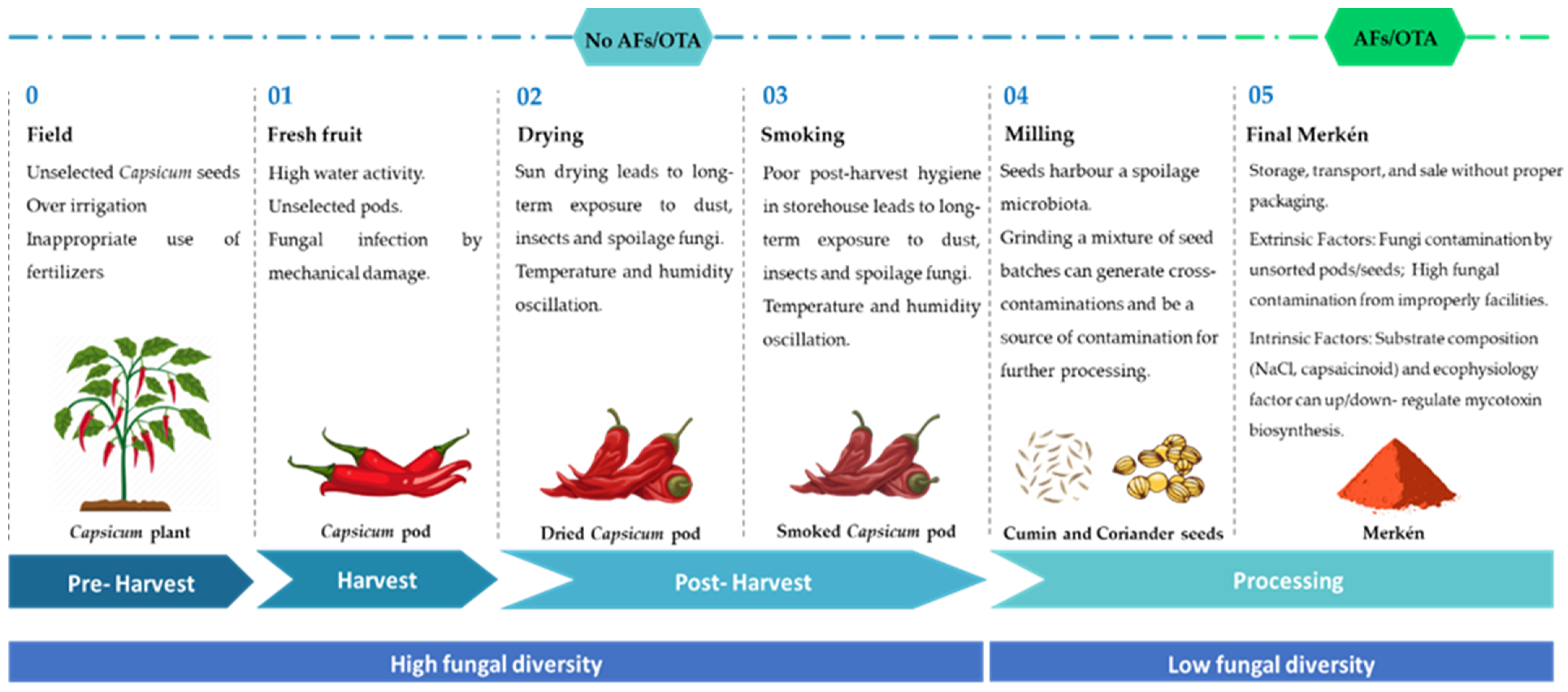
3.1. Fungal Contamination
3.2. Occurrence of AFs and OTA
3.3. Recommendations for Merkén Production Chain Food Safety
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, J.; Lima, N.; Santos, C. Chilean pepper: Spoilage fungi and mycotoxins contamination risk in Capsicum products. In Conhecimento, Conservação e uso de Fungos, 1st ed.; Oliveira, L.A., Jesus, M.A., Jackisch-Matsuura, A.B., Gasparotto, L., Oliveira, L.G.S., Lima-Neto, R.G., Rocha, L.C., Eds.; Editora INPA: Manaus, Brasil, 2019; pp. 29–39. [Google Scholar]
- Muñoz-concha, D.; Quiñones, X.; Pablo, J. Chili Pepper Landrace Survival and Family Farmers in Central Chile. Agronomy 2020, 10, 1541. [Google Scholar] [CrossRef]
- Oyarzún, M.T.; Riveros, H.; Vandecandelaere, E. Cómo Promover La Calidad Vinculada al Origen Para Contribuir al Desarrollo en América Latina: Enseñanzas de Cuatro Casos Piloto; FAO: Rome, Italy, 2013; pp. 44–59. [Google Scholar]
- ProChile. 2021. Available online: https://acceso.prochile.cl/?s=Merk%C3%A9n (accessed on 20 January 2022).
- Fundación para la Innovación Agraria (FIA). Resultados y Lecciones en Ají Merkén con Alto Valor Agregado; Fundación para la Innovación Agraria: Santiago, Chile, 2010; pp. 1–66. [Google Scholar]
- Costa, J.; Rodríguez, R.; Garcia-Cela, E.; Medina, A.; Magan, N.; Lima, N.; Battilani, P.; Santos, C. Overview of fungi and mycotoxin contamination in Capsicum pepper and in its derivatives. Toxins 2019, 11, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozturkoglu-Budak, S. A model for implementation of HACCP system for prevention and control of mycotoxins during the production of red dried chili pepper. Food Sci. Technol. 2017, 37, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Di Pillo, F.; Martínez, N. Ocratoxina A en Ají y merkén, Chile Perfil de Riesgo/ACHIPIA N.°02/2018 Ocratoxina; Agencia Chilena Para La Calidad e Inocuidad Alimentaria (ACHIPIA): Santiago, Chile, 2018. [Google Scholar]
- Casquete, R.; Rodríguez, A.; Hernández, A.; Martín, A.; Bartolomé, T.; Córdoba, J.J.; Córdoba, M.G. Occurrence of Toxigenic Fungi and Mycotoxins during Smoked Paprika Production. J. Food Prot. 2017, 80, 2068–2077. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Q.; Amjad, M.; Asi, M.R. Assessment of Hot Peppers for Aflatoxin and Mould Proliferation during Storage. J. Food Prot. 2011, 74, 830–835. [Google Scholar] [CrossRef]
- Costa, J.; Rodríguez, R.; Santos, C.; Soares, C.; Lima, N.; Santos, C. Mycobiota in Chilean chilli Capsicum annuum L. used for production of Merkén. Int. J. Food Microbiol. 2020, 334, 108833. [Google Scholar] [CrossRef] [PubMed]
- Frimpong, G.K.; Adekunle, A.A.; Ogundipe, O.T.; Solanki, M.K.; Sadhasivam, S. Identification and Toxigenic Potential of Fungi Isolated from Capsicum Peppers. Microorganisms 2019, 7, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, L.; Marín, S.; Mateo, E.M.; Gil-Serna, J.; Valle-Algarra, F.M.; Patiño, B.; Ramos, A.J. Mycobiota and co-occurrence of mycotoxins in Capsicum powder. Int. J. Food Microbiol. 2011, 151, 270–276. [Google Scholar] [CrossRef]
- Gambacorta, L.; Magistà, D.; Perrone, G.; Murgolo, S.; Logrieco, A.F.; Solfrizzo, M. Co-occurrence of toxigenic moulds, aflatoxins, ochratoxin A, Fusarium and Alternaria mycotoxins in fresh sweet peppers (Capsicum annuum) and their processed products. World Mycotoxin J. 2018, 11, 159–173. [Google Scholar] [CrossRef]
- Rapid Alert System for Food and Feed Hazards (RASFF)—European Commission. Available online: https://webgate.ec.europa.eu/rasff-window/portal/ (accessed on 20 October 2021).
- Chilean Ministry of Health. Available online: https://www.portaltransparencia.cl/PortalPdT/ingreso-sai-v2?idOrg=undefined (accessed on 20 October 2021).
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens—The IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Benkerroum, N. Chronic and Acute Toxicities of Aflatoxins: Mechanisms of Action. Int. J. Environ. Res. Public Health 2020, 1, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, J.; Lima, N.; Santos, C. An overview on possible links between aflatoxin B1 exposure and gallbladder cancer. Mycotoxin Res. 2021, 37, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Imaoka, T.; Yang, J.; Wang, L.; Mcdonald, M.G.; Afsharinejad, Z.; Bammler, T.K.; van Ness, K.; Yeung, C.K.; Rettie, A.E.; Himmelfarb, J.; et al. Microphysiological system modeling of ochratoxin A-associated nephrotoxicity. Toxicology 2020, 444, 152582. [Google Scholar] [CrossRef] [PubMed]
- Chilean Ministry of Health. Decreto 22. 2013. Available online: https://www.bcn.cl/leychile/navegar?idNorma=1054913 (accessed on 21 October 2021).
- Ham, H.; Kim, S.; Kim, M.H.; Lee, S.; Hong, S.K.; Ryu, J.G.; Lee, T. Mycobiota of ground red pepper and their aflatoxigenic potential. J. Microbiol. 2016, 54, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, M.; Chalyy, Z.; Sedova, I.; Aksenov, I. Stability of Mycotoxins in Individual Stock and Multi-Analyte Standard Solutions. Toxins 2020, 12, 94. [Google Scholar] [CrossRef] [Green Version]
- Samson, R.A.; Hocking, A.D.; Pitt, J.I.; King, A.D. Modern methods in food mycology. In Developments in Food Science; Elsevier: Baarn, The Netherlands, 1992. [Google Scholar]
- International Seed Testing Association-ISTA. Available online: https://www.seedtest.org/en/home.html (accessed on 27 August 2022).
- Nelson, P.E.; Toussoun, T.A.; Marasas, W.F.O. Fusarium Species: An Illustrated Manual for Identification; The Pennsylvania State University Press, University Park: London, UK, 1983. [Google Scholar]
- Klich, M.A. Identification of Common Aspergillus Species; Amer Society for Microbiology: Utrecht, The Netherlands, 2002. [Google Scholar]
- Samson, R.A.; Hockstra, E.S.; Frisvad, J.C.; Filtenborg, O. Introduction to Food and Airborne Fungi; Centaalbureau Voorschimmelculturs-Utrecht Ponson and Looyen Press: Wageningen, The Netherlands, 2000. [Google Scholar]
- Rodrigues, P.; Venâncio, A.; Kozakiewicz, Z.; Lima, N. A polyphasic approach to the identification of aflatoxigenic and non-aflatoxigenic strains of Aspergillus section Flavi isolated from Portuguese almonds. Int. J. Food Microbiol. 2009, 129, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [Green Version]
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.-B.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samson, R.A. Identification and nomenclature of the genus Penicillium. Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef] [Green Version]
- Samson, R.A.; Visagie, C.M.; Houbraken, J.; Hong, S.-B.; Hubka, V.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Susca, A.; Tanney, J.B.; et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014, 7, 141–173. [Google Scholar] [CrossRef] [Green Version]
- White, T.J.; Burns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications, 1st ed.; Gelgard, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Glass, L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Bragulat, M.R.; Abarca, M.L.; Cabanes, F.J. An easy screening method for fungi producing ochratoxin A in pure culture. Int. J. Food Microbiol. 2001, 71, 139–144. [Google Scholar] [CrossRef]
- Chuaysrinule, C.; Maneeboon, T.; Roopkham, C.; Mahakarnchanakul, W. Occurrence of aflatoxin- and ochratoxin A-producing Aspergillus species in Thai dried chilli. J. Agric. Res. 2020, 2, 100054. [Google Scholar] [CrossRef]
- Araújo, C.A.S.; Ferreira, P.C.; Pupin, B.; Dias, L.P.; Avalos, J.; Edwards, J.; Hallsworth, J.E.; Rangel, D.E.N. Osmotolerance as a determinant of microbial ecology: A study of phylogenetically diverse fungi. Fungal Biol. 2020, 124, 273–288. [Google Scholar] [CrossRef]
- Butinar, L.; Zalar, P.; Frisvad, J.C.; Gunde-Cimerman, N. The genus Eurotium members of indigenous fungal community in hypersaline waters of salterns. FEMS Microbiol. Ecol. 2005, 51, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Yogendrarajah, P.; Jacxsens, L.; Saeger, S.; Meulenaer, B. Co-occurrence of multiple mycotoxins in dry chilli (Capsicum annum L.) samples from the markets of Sri Lanka and Belgium. Food Control 2014, 46, 26–34. [Google Scholar] [CrossRef]
- Ozbey, F.; Kabak, B. Natural co-occurrence of aflatoxins and ochratoxin A in spices. Food Control 2012, 28, 354–361. [Google Scholar] [CrossRef]
- Tosun, A.; Ozden, S. Ochratoxin A in red pepper flakes commercialised in Turkey. Food Addit. Contam. B Surveill. 2015, 9, 46–50. [Google Scholar] [CrossRef]
- Ikoma, T.; Tsuchiya, Y.; Asai, T.; Okano, K.; Ito, N.; Endoh, K.; Yamamoto, M.; Nakamura, K. Ochratoxin A Contamination of Red Chili Peppers from Chile, Bolivia and Peru, Countries with a High Incidence of Gallbladder Cancer. Asian Pac. J. Cancer Prev. 2015, 16, 5987–5991. [Google Scholar] [CrossRef] [Green Version]
- Foerster, C.; Muñoz, K.; Delgado-rivera, L.; Rivera, A.; Cortés, S.; Müller, A.; Arriagada, G.; Ferreccio, C.; Rios, G. Occurrence of relevant mycotoxins in food commodities consumed in Chile. Mycotoxin Res. 2019, 36, 63–72. [Google Scholar] [CrossRef]
- Foerster, C.; Gisela, R.; Patricia, G.; Muñoz, K.; Cort, S. Assessment of Mycotoxin Exposure in a Rural County of Chile by Urinary Biomarker Determination. Toxin 2021, 13, 439. [Google Scholar] [CrossRef]
- Asai, T.; Tsuchiya, Y.; Okano, K.; Piscoya, A.; Nishi, C.Y.; Ikoma, T.; Oyama, T.; Ikegami, K.; Yamamoto, M. Aflatoxin Contamination of Red Chili Pepper from Bolivia and Peru, Countries with High Gallbladder Cancer Incidence Rates. Asian Pac. J. Cancer Prev. 2012, 13, 5167–5170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogueira, L.; Foerster, C.; Groopman, J.; Egner, P.; Koshiol, J.; Ferreccio, C. Association of Aflatoxin with Gallbladder Cancer in Chile. JAMA 2015, 313, 2075–2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra, I.; Yamamoto, M.; Calvo, A.; Cavada, G.; Báez, S.; Endoh, K.; Watanabe, H.; Tajima, K. Association of chili pepper consumption, low socioeconomic status and longstanding gallstones with gallbladder cancer in a Chilean population. Int. J. Cancer 2012, 102, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Terao, M.; Okano, K.; Nakamura, K.; Oyama, M.; Ikegami, K.; Yamamoto, M. Mutagenicity and Mutagens of the Red Chili Pepper as Gallbladder Cancer Risk Factor in Chilean Women. Asian Pac. J. Cancer Prev. 2011, 12, 471–476. [Google Scholar]
- Cabral, L.C.; Terminiello, L.; Fernández Pinto, V.; Fog Nielsen, K.; Patriarca, A. Natural occurrence of mycotoxins and toxigenic capacity of Alternaria strains from mouldy peppers. Int. J. Food Microbiol. 2016, 236, 155–160. [Google Scholar] [CrossRef]
- Santos, L.; Marín, S.; Sanchis, V.; Ramos, A.J. Co-occurrence of aflatoxins, ochratoxin A and zearalenone in Capsicum powder samples available on the Spanish market. Food Chem. 2010, 122, 826–830. [Google Scholar] [CrossRef]
- European Commission Regulation (EC). No 594/2012 of, 5 July 2012 amending Regulation (EC) 1881/2006 as regard the maximum levels of the contaminants ochratoxin A, non-dioxin-like PCBs and melamine in foodstuffs. Off. J. Eur. Union 2012, 176, 43–45. [Google Scholar]
- European Commission Regulation (EU). No 2015/1137, of 13 July 2015 amending Regulation (EC) No 1881/2006 as regards the maximum level of Ochratoxin A in Capsicum spp. spices. Off. J. Eur. Union 2015, 185, 11–12. [Google Scholar]
- Food and Agriculture Organization of the United Nations-FAO. Available online: http://www.fao.org/fao-whocodexalimentarius (accessed on 20 October 2021).
- Buitimea-Cantúa, G.V.; Buitimea-Cantúa, N.E.; Rocha-Pizaña, M.D.R.; Hernández-Morales, A.; Magaña-Barajas, E.; Molina-Torres, J. Inhibitory effect of Capsicum chinense and Piper nigrum fruits, capsaicin and piperine on aflatoxins production in Aspergillus parasiticus by downregulating the expression of afl D, afl M, afl R, and afl S genes of aflatoxins biosynthetic pathway. J. Environ. Sci. Health-B 2020, 55, 835–843. [Google Scholar] [CrossRef]
- Tewksbury, J.J.; Reagan, K.M.; Machnicki, N.J.; Carlo, A.; Haak, D.C.; Peñaloza, A.; Levey, D.J. Evolutionary ecology of pungency in wild chilies. Proc. Natl. Acad. Sci. USA 2008, 105, 11808–11811. [Google Scholar] [CrossRef] [Green Version]
- Coton, M.; Auffret, A.; Poirier, E.; Debaets, S.; Coton, E. Production and migration of ochratoxin A and citrinin in Comté cheese by an isolate of Penicillium verrucosum selected among Penicillium spp. mycotoxin producers in YES medium. Food Microbiol. 2019, 82, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.; Cruz Cabral, L.; Rodríguez, M.; Rodríguez, A. Influence of ochratoxin A on adaptation of Penicillium nordicum on a NaCl-rich dry-cured ham-based medium. Int. J. Food Microbiol. 2018, 272, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, M.; Magistà, D.; Epifani, F.; Cervellieri, S.; Lippolis, V.; Gallo, A.; Perrone, G.; Susca, A. Study of gene expression and OTA production by Penicillium nordicum during a small-scale seasoning process of salami. Int. J. Food Microbiol. 2016, 227, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Medina, Á.; Córdoba, J.J.; Magan, N. The influence of salt (NaCl) on ochratoxin A biosynthetic genes, growth and ochratoxin A production by three strains of Penicillium nordicum on a dry-cured ham-based medium. Int. J. Food Microbiol. 2014, 178, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Heydt, M.; Graf, E.; Stoll, D.; Geisen, R. The biosynthesis of ochratoxin A by Penicillium as one mechanism for adaptation to NaCl rich foods. Food Microbiol. 2012, 29, 233–241. [Google Scholar] [CrossRef]
- Masih, A.; Singh, P.K.; Kathuria, S.; Agarwal, K.; Meis, J.F.; Chowdhary, A. Identification by molecular methods and matrix-assisted laser desorption ionization-time of flight mass spectrometry and antifungal susceptibility profiles of clinically significant rare Aspergillus species in a referral chest hospital in Delhi, India. J. Clin. Microbiol. 2016, 54, 2354–2364. [Google Scholar] [CrossRef] [Green Version]
- Sabz, G.; Gharaghani, M.; Mirhendi, H.; Ahmadi, B.; Gatee, M.A.; Sisakht, M.T.; Hemati, A.; Mohammadi, R.; Taghavi, J.; Nouripour-Sisakht, S. Clinical and microbial epidemiology of otomycosis in the city of Yasuj, southwest Iran, revealing Aspergillus tubingensis as the dominant causative agent. J. Med. Microbiol. 2019, 68, 585–590. [Google Scholar] [CrossRef]
- Santos, R.A.C.; Steenwyk, J.L.; Rivero-Menendez, O.; Mead, M.E.; Silva, L.P.; Bastos, R.W.; Alastruey-Izquierdo, A.; Goldman, G.H.; Rokas, A. Genomic and Phenotypic Heterogeneity of Clinical Isolates of the Human Pathogens Aspergillus fumigatus, Aspergillus lentulus, and Aspergillus fumigatiaffinis. Front. Genet. 2020, 11, 459. [Google Scholar] [CrossRef]
- Meteochile. Available online: http://www.meteochile.gob.cl/PortalDMC-web/index.xhtml (accessed on 23 October 2021).
- Turner, P.C.; Sylla, A.; Gong, Y.Y.; Diallo, M.S.; Sutcliffe, A.E.; Hall, A.J.; Wild, C.P. Reduction in exposure to carcinogenic aflatoxins by postharvest intervention measures in west Africa: A community-based intervention study. Lancet 2005, 365, 1950–1956. [Google Scholar] [CrossRef]
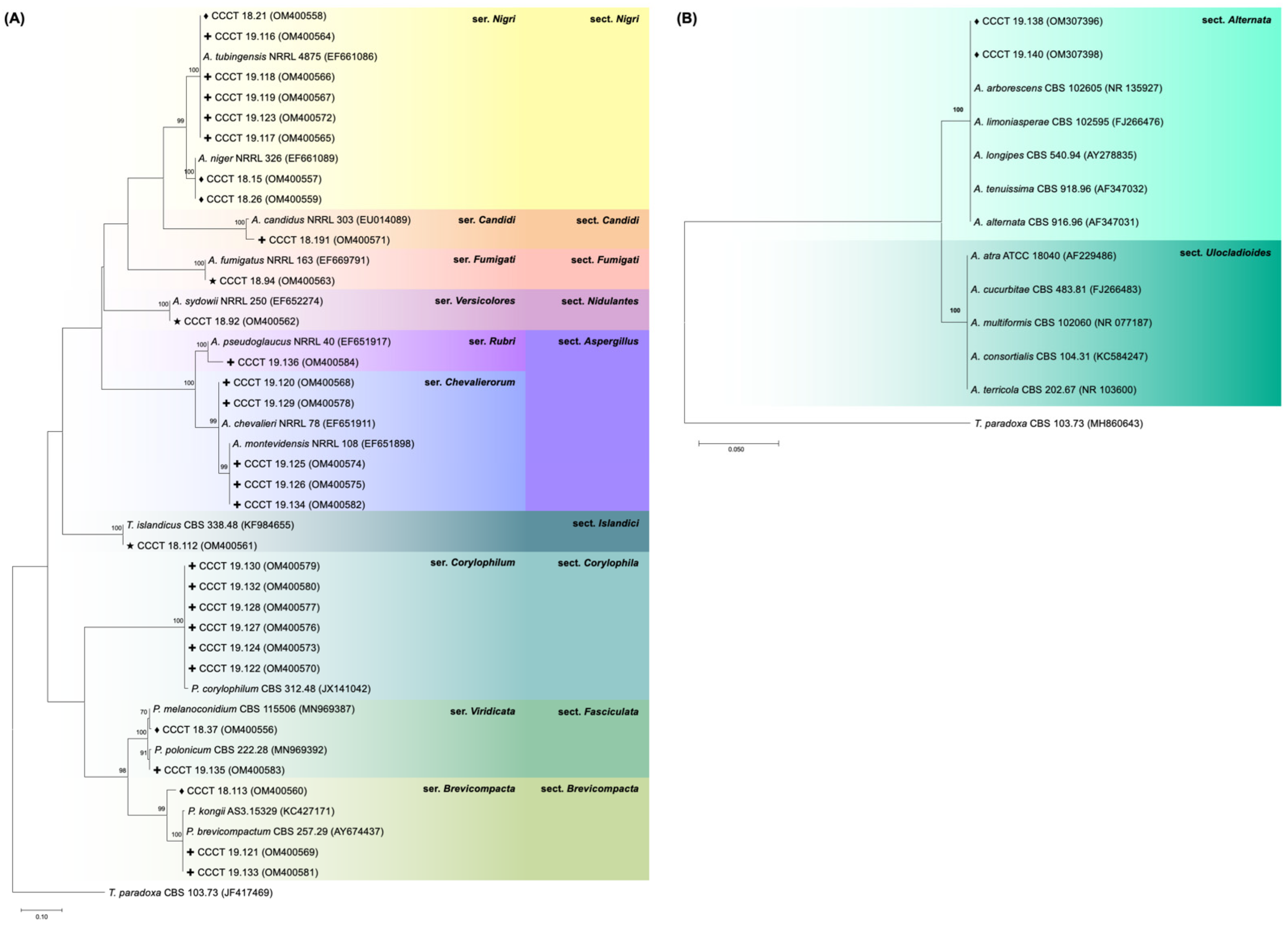
| Spices | Fungal Species | ||
|---|---|---|---|
| Aspergillus spp. | Penicillium spp. | Other Genera | |
| Merkén | A. tubingensis (5) | P. brevicompactum (2) | |
| A. candidus (1) | P. corylophilum (6) | ||
| A. chevalieri (2) | P. polonicum (1) | ||
| A. pseudoglaucus (1) | |||
| A. montevidensis (3) | |||
| Coriander | A. niger (2) | P. kongii (1) | Alternaria sect. Alternata (2) |
| A. tubingensis (1) | P. melanoconidium (1) | ||
| Cumin | A. fumigatus (1) A. sydowii (1) | T. islandicus (1) | |
| Source | Samples | OTA (µg/kg) | AFB1 (µg/kg) |
|---|---|---|---|
| Farmer | I | + | + |
| II | + | + | |
| III | + | + | |
| IV | + | + | |
| V | + | + | |
| VI | + | + | |
| VII | + | − | |
| VIII | + | − | |
| Local markets | 1 | + | − |
| 2 | + | − | |
| 3 | + | − | |
| 4 | + | + | |
| 5 | + | + | |
| 6 | + | + | |
| 7 | + | − | |
| 8 | + | + | |
| 9 | + | − | |
| 10 | + | + | |
| 11 | + | − | |
| 12 | + | + | |
| 13 | + | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, J.; Santos, C.; Soares, C.; Rodríguez, R.; Lima, N.; Santos, C. Occurrence of Aflatoxins and Ochratoxin A during Merkén Pepper Powder Production in Chile. Foods 2022, 11, 3843. https://doi.org/10.3390/foods11233843
Costa J, Santos C, Soares C, Rodríguez R, Lima N, Santos C. Occurrence of Aflatoxins and Ochratoxin A during Merkén Pepper Powder Production in Chile. Foods. 2022; 11(23):3843. https://doi.org/10.3390/foods11233843
Chicago/Turabian StyleCosta, Jéssica, Carla Santos, Célia Soares, Rodrigo Rodríguez, Nelson Lima, and Cledir Santos. 2022. "Occurrence of Aflatoxins and Ochratoxin A during Merkén Pepper Powder Production in Chile" Foods 11, no. 23: 3843. https://doi.org/10.3390/foods11233843







