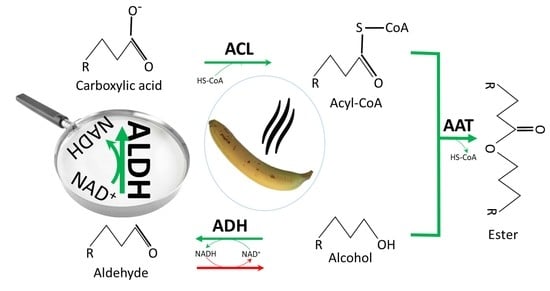
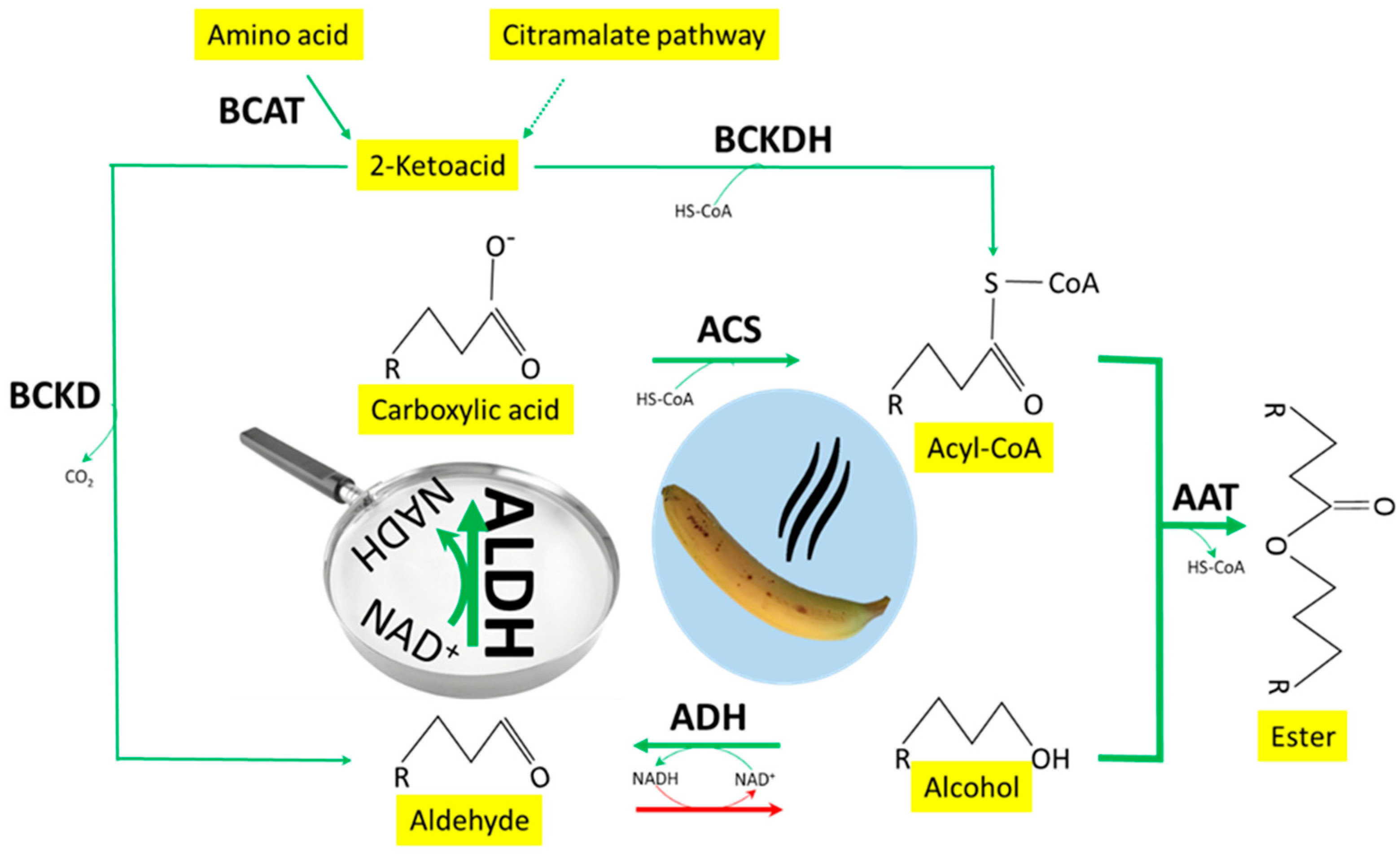
Functional Characteristics of Aldehyde Dehydrogenase and Its Involvement in Aromatic Volatile Biosynthesis in Postharvest Banana Ripening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials
2.3. Substrate Feeding Experiments
2.3.1. Feeding of Alcohols and 2-Keto-4-Methylpentanoic Acid
2.3.2. Feeding of Aldehydes and Trapping of Carboxylic Acids
2.3.3. Setting and Monitoring of O2 and CO2 in Incubation
2.4. Crude ALDH Extraction from Banana Pulps, and Functional Characteristics of the Enzyme
2.4.1. Crude Protein Extraction
2.4.2. Functional Characteristics of ALDH
2.4.3. Activity of ADH in Crude Protein Extractions and Inhibition by 4-Methyl Pyrazole
2.5. Statistical Analysis
3. Results
3.1. Feeding Alcohols to Banana Pulps to Produce Esters
3.2. Feeding Aldehydes to Banana Pulps to Produce the Corresponding Acids
3.3. Extraction and Functional Characteristics of ALDH
3.4. Feeding 2-Keto-4-Methylpentanoic Acid to Banana Pulps to Produce Branched Alcohol, Aldehyde and Ester
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brocker, C.; Vasiliou, M.; Carpenter, S.; Carpenter, C.; Zhang, Y.; Wang, X.; Kotsoni, S.O.; Wood, A.J.; Kirch, H.-H.; Kopečný, D.; et al. Aldehyde dehydrogenase (ALDH) superfamily in plants: Gene nomenclature and comparative genomics. Planta 2013, 237, 189–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Wyllie, S.G.; Fellman, J.K. Formation of volatile branched chain esters in bananas (Musa sapientum L.). J. Agric. Food Chem. 2000, 48, 3493–3496. [Google Scholar] [CrossRef] [PubMed]
- Tressl, R.; Drawert, F. Biogenesis of banana volatiles. J. Agric. Food Chem. 1973, 21, 560–565. [Google Scholar] [CrossRef]
- Sugimoto, N.; Forsline, P.; Beaudry, R. Volatile profiles of members of the USDA Geneva Malus Core Collection: Utility in evaluation of a hypothesized biosynthetic pathway for esters derived from 2-methylbutanoate and 2-methylbutan-1-ol. J. Agric. Food Chem. 2015, 63, 2106–2116. [Google Scholar] [CrossRef]
- Sugimoto, N.; Jones, A.D.; Beaudry, R. Changes in free amino acid content in ‘Jonagold’apple fruit as related to branched-chain ester production, ripening, and senescence. J. Am. Soc. Hortic. Sci. 2011, 136, 429–440. [Google Scholar] [CrossRef] [Green Version]
- Tavaria, F.K.; Dahl, S.; Carballo, F.J.; Malcata, F.X. Amino acid catabolism and generation of volatiles by lactic acid bacteria. J. Dairy Sci. 2002, 85, 2462–2470. [Google Scholar] [CrossRef]
- Beck, H.C.; Hansen, A.M.; Lauritsen, F.R. Metabolite production and kinetics of branched-chain aldehyde oxidation in Staphylococcus xylosus. Enzym. Microb. Technol. 2002, 31, 94–101. [Google Scholar] [CrossRef]
- Dickinson, J.R.; Harrison, S.J.; Dickinson, J.A.; Hewlins, M.J. An investigation of the metabolism of isoleucine to active amyl alcohol in Saccharomyces cerevisiae. J. Biol. Chem. 2000, 275, 10937–10942. [Google Scholar] [CrossRef] [Green Version]
- Bai, J.; Baldwin, E.A.; Imahori, Y.; Kostenyuk, I.; Burns, J.; Brecht, J.K. Chilling and heating may regulate C6 volatile aroma production by different mechanisms in tomato (Solanum lycopersicum) fruit. Postharvest Biol. Technol. 2011, 60, 111–120. [Google Scholar] [CrossRef]
- Yang, X.; Song, J.; Fillmore, S.; Pang, X.; Zhang, Z. Effect of high temperature on color, chlorophyll fluorescence and volatile biosynthesis in green-ripe banana fruit. Postharvest Biol. Technol. 2011, 62, 246–257. [Google Scholar] [CrossRef]
- Wendakoon, S.K.; Ueda, Y.; Imaahori, Y.; Tomimasu, Y. Presence of acetyl-CoA synthetase in ripened bananas. Food Preserv. Sci. 2005, 31, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Ueda, Y.; Tsuda, A.; Bai, J.-H.; Fujishita, N.; Chachin, K. Characteristic pattern of aroma ester formation from banana, melon and strawberry with reference to the substrate specificity of ester synthetase and alcohol contents in pulp. J. Jpn. Food Sci. Technol. 1992, 39, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Jayanty, S.; Song, J.; Rubinstein, N.M.; Chong, A.; Beaudry, R.M. Temporal relationship between ester biosynthesis and ripening events in bananas. J. Am. Soc. Hortic. Sci. 2002, 127, 998–1005. [Google Scholar] [CrossRef] [Green Version]
- Beekwilder, J.; Alvarez-Huerta, M.; Neef, E.; Verstappen, F.W.; Bouwmeester, H.J.; Aharoni, A. Functional Characterization of Enzymes Forming Volatile Esters from Strawberry and Banana. Plant Physiol. 2004, 135, 1865–1878. [Google Scholar] [CrossRef] [Green Version]
- Ueda, Y.; Fujishita, N.; Chachin, K. Presence of alcohol acetyltransferase in melons (Cucumis melo L.). Postharvest Biol. Technol. 1997, 10, 121–126. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, H.; Zhang, Y.; Hu, J.; Feng, J.; Li, C.; Li, C.; Chen, J.; Zhu, S. Comparative genomic study of ALDH gene superfamily in Gossypium: A focus on Gossypium hirsutum under salt stress. PLoS ONE 2017, 12, e0176733. [Google Scholar] [CrossRef]
- Modig, T.; Lidén, G.; Taherzadeh, M.J. Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem. J. 2002, 363, 769–776. [Google Scholar] [CrossRef]
- USDA-AMS Bananas, Market Inspection Instructions. 2004; p. 16. Available online: https://www.ams.usda.gov/sites/default/files/media/Bananas_Inspection_Instructions%5B1%5D.pdf (accessed on 11 November 2021).
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Peterson, G.L. Review of the Folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal. Biochem. 1979, 100, 201–220. [Google Scholar] [CrossRef]
- Omarov, R.; Dräger, D.; Tischner, R.; Lips, S.H. Aldehyde oxidase isoforms and subunit composition in roots of barley as affected by ammonium and nitrate. Physiol. Plant. 2003, 117, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, A.; Castelfranco, P.A. An acetaldehyde dehydrogenase from germinating seeds. Plant Physiol. 1967, 42, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: Regulation and biological consequences. Antioxid. Redox Sign. 2008, 10, 179–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mura, K.; Tanimura, W. Change of polyphenol compounds in banana pulp during ripening. Food Preserv. Sci. 2003, 29, 347–351. [Google Scholar] [CrossRef]
- Crabb, D.W.; Matsumoto, M.; Chang, D.; You, M. Overview of the role of alcohol dehydrogenase and aldehyde dehydrogenase and their variants in the genesis of alcohol-related pathology. Proc. Nutr. Soc. 2004, 63, 49–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattheis, J.; Buchanan, D.; Fellman, J.K. Volatile compounds emitted by ‘Gala ‘apples following dynamic atmosphere storage. J. Am. Soc. Hortic. Sci. 1998, 123, 426–432. [Google Scholar] [CrossRef]
- Bai, J.; Baldwin, E.A.; Mattheis, J.P. Effect of 1-MCP pretreatment, CA storage and subsequent marketing temperature on volatile profile of ‘Gala’ apple. In Proceedings of the Florida State Horticultural Society, Sheraton World Resort, Daytona Beach, FL, USA, 8–10 June 2003; Volume 116, pp. 400–404. [Google Scholar]
- Macku, C.; Jennings, W.G. Production of volatiles by ripening bananas. J. Agric. Food Chem. 1987, 35, 845–848. [Google Scholar] [CrossRef]
- Bai, J.; Hagenmaier, R.D.; Baldwin, E.A. Volatile response of four apple varieties with different coatings during marketing at room temperature. J. Agric. Food Chem. 2002, 50, 7660–7668. [Google Scholar] [CrossRef] [PubMed]
- Imahori, Y.; Yamamoto, K.; Tanaka, H.; Bai, J. Residual effects of low oxygen storage of mature green fruit on ripening processes and ester biosynthesis during ripening in bananas. Postharvest Biol. Technol. 2013, 77, 19–27. [Google Scholar] [CrossRef]
- Ueda, Y.; Bai, J.; Yoshioka, H. Effects of polyethylene packaging on flavor retention and volatile production of ‘Starking Delicious’ apple. J. Jpn. Soc. Hortic. Sci. 1993, 62, 207–213. [Google Scholar] [CrossRef]
- Ueda, Y.; Bai, J. Effect of short term exposure of elevated CO2 on flesh firmness and ester production of strawberry. J. Jpn. Soc. Hortic. Sci. 1993, 62, 457–464. [Google Scholar] [CrossRef]
- Lara, I.; Miró, R.M.; Fuentes, T.; Sayez, G.; Graell, J.; López, M.L. Biosynthesis of volatile aroma compounds in pear fruit stored under long-term controlled-atmosphere conditions. Postharvest Biol. Technol. 2003, 29, 29–39. [Google Scholar] [CrossRef]
- Bauchot, A.D.; Mottram, D.S.; Dodson, A.T.; John, P. Effect of aminocyclopropane-1-carboxylic acid oxidase antisense gene on the formation of volatile esters in cantaloupe Charentais melon (cv. Vedrandais). J. Agric. Food Chem. 1998, 46, 4787–4792. [Google Scholar] [CrossRef]
- Mir, N.A.; Perez, R.; Schwallier, P.; Beaudry, R. Relationship between ethylene response manipulation and volatile production in Jonagold variety apples. J. Agric. Food Chem. 1999, 47, 2653–2659. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda, Y.; Zhao, W.; Ihara, H.; Imahori, Y.; Tsantili, E.; Wendakoon, S.K.; Chambers, A.; Bai, J. Functional Characteristics of Aldehyde Dehydrogenase and Its Involvement in Aromatic Volatile Biosynthesis in Postharvest Banana Ripening. Foods 2022, 11, 347. https://doi.org/10.3390/foods11030347
Ueda Y, Zhao W, Ihara H, Imahori Y, Tsantili E, Wendakoon SK, Chambers A, Bai J. Functional Characteristics of Aldehyde Dehydrogenase and Its Involvement in Aromatic Volatile Biosynthesis in Postharvest Banana Ripening. Foods. 2022; 11(3):347. https://doi.org/10.3390/foods11030347
Chicago/Turabian StyleUeda, Yoshinori, Wei Zhao, Hideshi Ihara, Yoshihiro Imahori, Eleni Tsantili, Sumithra K. Wendakoon, Alan Chambers, and Jinhe Bai. 2022. "Functional Characteristics of Aldehyde Dehydrogenase and Its Involvement in Aromatic Volatile Biosynthesis in Postharvest Banana Ripening" Foods 11, no. 3: 347. https://doi.org/10.3390/foods11030347