Abstract
Microbial transformation is an alternative method for structural modification. The current study aimed at application of microbial transformation for discovering new derivatives and investigating the structure-activity relationship of isobavachalcone (1), 4-hydroxyderricin (2), and xanthoangelol (3) isolated from the herb Angelica keiskei. In the initial screening process, 1–3 were incubated with microbes using a two-stage fermentation method and analyzed through TLC monitoring. The screening results showed that Rhizopus oryzae and Mucor hiemalis were able to transform 1 and 2, respectively. Additionally, M. hiemalis and Mortierella ramanniana var. angulispora were able to transform 3. Following scale-up fermentation, four new (4, 5, 7, and 10) and five known (6, 8, 9, 11, and 12) metabolites were produced. Cytotoxicity of all the compounds (1–12) was investigated using three human cancer cell lines including A375P, HT-29, and MCF-7 by MTT method. Meanwhile, the tyrosinase inhibitory activity of 1–12 was evaluated using l-tyrosine as a substrate. Overall, 1 and 3 displayed the highest cytotoxicity, and 5 and 7 exhibited the most potent tyrosinase inhibitory activity with relatively low cytotoxicity. This allowed us to postulate that the introduction of 4′-O-glucopyranosyl group led to the reduction in cytotoxicity and improvement in tyrosinase inhibitory activity.
1. Introduction
Angelica keiskei (Umbelliferae) is a perennial leafy herb, mainly distributed in Asian countries, including Korea and Japan. The herb is named as ‘Myeong-il yeob’ in Korean and ‘Ashitaba’ in Japanese, both literally meaning tomorrow’s leaves. Another prevalent name in Korean for A. keiskei is ‘Sinsuncho’ meaning the herb of eternal youth [1]. In daily life, it is drunk as a tea and cooked as a vegetable. Traditionally, it is consumed as medicinal herb with tonic, mild cathartic, diuretic, and galactagogue effects [2]. Preceding phytochemical investigations on A. keiskei revealed the presence of abundant prenylated chalcones in its leaves, stems, and roots [3]. These chalcones are proposed as effective agents for diverse health-beneficial properties such as anti-tumor [4], anti-inflammatory [5], anti-bacterial [6], anti-diabetic [7], anti-melanogenic [8], and anti-obesity [9] effects. Particularly, 4-hydroxyderricin (HD) and xanthoangelol (XT) are the predominant prenylchalcones in this herb, with relative abundances of 1.97% and 5.05%, respectively [10]. HD and XT have been proposed as potent cytotoxic agents that promote apoptosis and suppress tumor-induced angiogenesis in cancer cells [11]. After surgically removing implanted tumors, the administration of HD or XT inhibited metastasis and increased overall survival in mice. They were considered as promising therapeutic agents for the treatment of melanoma [12,13]. Isobavachalcone (IBC) is another bioactive prenylchalcone isolated from A. keiskei [14]. Accumulative studies have demonstrated that IBC suppressed the proliferation and induced cell apoptosis in diverse cancer cell lines including colorectal cancer, liver cancer, breast cancer, leukemia, tongue squamous cell carcinoma [15]. Meanwhile, IBC demonstrated reduced cytotoxicity toward normal cells in comparison with cancer cells. After oral administration in mice, IBC inhibited the growth of subcutaneous HL-60 xenograft tumor without obvious toxicity [16].
Microbial transformation has been used as an effective means for structural modification of natural products at non-activated positions. It has advantages over conventional chemical synthesis largely due to its simpler operation, higher selectivity, and milder conditions [17]. In some cases, biological activities may have been enhanced for the transformed metabolites [18,19]. Previous studies on the microbial transformation of natural bioactive chalcones identified diverse metabolites with promising activities [20,21,22]. The aim of the study was to apply microbial transformation of three major bioactive prenylchalcones (1–3) in order to identify new derivatives and evaluate their biological activities to understand their structure-activity relationship.
2. Materials and Methods
2.1. Materials and Reagents
The herbal material Angelica keiskei was obtained in September 2020 and identified by the herb company Damaon (Yeongcheon, Gyeongsangbuk-do, Korea). Voucher specimen (AK2009) has been deposited at the Herbarium of the College of Pharmacy, Chonnam National University (Gwangju, Korea).
The microbial media including malt extract, peptone, d-glucose, and potato dextrose were obtained from Becton, Dickinson and Co. (Sparks, MD, USA). Sucrose was obtained from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle medium (DMEM), penicillin, and streptomycin were purchased from Gibco (Invitrogen, Carlsbad, CA, USA). Fetal bovine serum (FBS) was purchased from Welgene Inc. (Gyeongsan, Gyeongsangbuk-do, Korea). Phosphate-buffered saline (PBS) tablets were obtained from Takara Korea Biomedical Inc. (Seoul, Korea). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Thermo Fisher Scientific (Waltham, MA, USA). Demethylzeylasteral (DZ) used as a reference standard in the bioassay was purchased from Biopurify Phytochemicals, Ltd. (Chengdu, China). Tyrosinase from mushroom and l-tyrosine were purchased from Sigma-Aldrich (St. Louis, MO, USA) and Daejung Chemicals and Metals Co., Ltd. (Siheung, Gyeonggi-do, Korea), respectively. Kojic acid used as a reference standard in the bioassay was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan).
The microbial strains including Cunninghamella elegans var. elegans KCTC 6992, Mortierella ramanniana var. angulispora KCTC 6137, Mucor hiemalis KCTC 26779, Mucor plumbeus KCCM 60265, Rhizopus oryzae KCCM 60556 were purchased from the Korean Collection for Type Cultures (KCTC, Daejeon, Korea) or Korean Culture Center of Microorganisms (KCCM, Seoul, Korea). The human cancer cell lines A375P, HT-29, and MCF-7 were obtained from the Korean Cell Line Bank (Seoul, Korea).
2.2. Isolation and Identification of Chalcones 1–3 from the Aerial Parts of A. keiskei
The air-dried herbal material (2.0 kg) was powdered and extracted by soaking in 95% ethanol (10 L × 3). After concentration of the filtrate, the residue (432 g) was suspended in water and sequentially partitioned with dichloromethane (CH2Cl2) and ethyl acetate (EtOAc). The crude CH2Cl2 residue (59.0 g) was subsequently separated by silica gel column chromatography using a gradient of n-hexane/EtOAc (30:1–1:1) and CH2Cl2/methanol (10:1–0:1) to yield eleven fractions (Fr. A–K). Fr. D (3.76 g) was further subjected to a Sephadex LH-20 column chromatography using methanol to yield five subfractions D1–D5. Subfr. D5 was separated using high performance liquid chromatography (HPLC) to provide substrates 1 (50 mg), 2 (95 mg), and 3 (110 mg). The structures of 1–3 were established as isobavachalcone (1) [23], 4-hydroxyderricin (2) [24] and xanthoangelol (3) [25] by 1H- and 13C-nuclear magnetic resonance (NMR) spectral analysis (see Supplementary Materials Figures S2–S7).
2.3. Preparation and Analysis of Screening Samples for Microbial Transformation
Fermentation experiments were carried out in two kinds of media. The malt medium consisting of peptone (1 g), d-glucose (20 g), and malt extract (20 g) was prepared in 1 L of distilled water for the incubation of M. hiemalis. The potato sucrose medium consisting of sucrose (20 g) and potato dextrose (24 g) was prepared in 1 L of distilled water for the culture of C. elegans var. elegans, M. ramanniana var. angulispora, M. plumbeus, or R. oryzae.
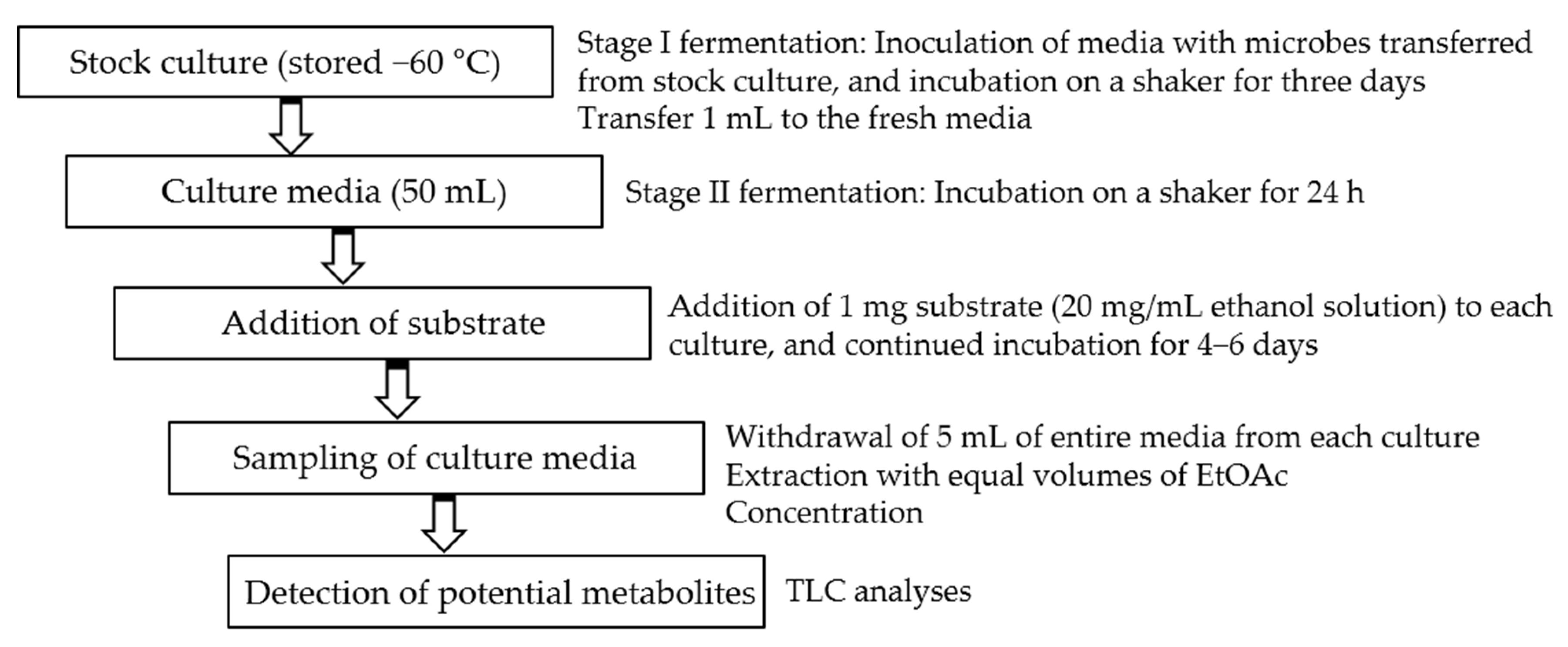
Initial fermentations were performed in 50 mL media. A two-stage fermentation method was employed in all experiments [26]. After inoculation and continued incubation for 24 h, the solution of each substrate (1–3) in ethanol (20 mg/mL) was distributed to each flask. The concentration of each substrate in the media was 0.02 mg/mL. Incubation was further continued for 4–6 days. The two parallel controls were conducted under the same conditions, i.e., substrate controls (substrate in microorganism-free culture media) and culture controls (microorganisms in substrate-free culture media). Substrate-containing microbial media were sampled by withdrawing 5 mL of entire media at every two days after addition of substrate. They were extracted with equal volumes of EtOAc. The organic layers were concentrated and spotted on thin layer chromatography (TLC) plates, and developed with chloroform/methanol (6:1). After spraying anisaldehyde-sulfuric acid reagent, the TLC plates were heated over 100 °C for detection of potential metabolites (Scheme 1).
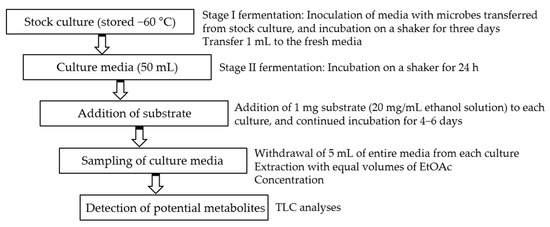
Scheme 1.
Two-stage fermentation method for screening.
On the basis of TLC analyses, the microbes M. plumbeus KCCM 60556 and M. hiemalis KCTC 26779 were selected for scale-up fermentations of substrates 1 and 2, respectively, as they showed higher transformational efficiency, while the microbes M. hiemalis KCTC 26779 and Mortierella ramanniana var. angulispora KCTC 6137 were selected for scale-up fermentations of 3, as they showed potential capability of transforming 3 (See Supplementary Materials Figure S1).
2.4. Preparation and Isolation of Scale-Up Samples to Obtain Metabolites 4–11
Scale-up fermentation of each substrate (1–3) was conducted following the aforementioned procedures in 150 mL of pre-cultured media. The scale-up fermentation culture of each substrate (1–3) was extracted thrice with equal volume of EtOAc. The combined EtOAc extract was evaporated in vacuo to give a residue.
The organic residue of 1 cultured with R. oryzae was separated on HPLC eluted with 53% to 84% methanol to yield metabolites 4 (tR = 30.4 min, 2.8 mg) and 6 (tR = 34.9 min, 6.5 mg) together with fractions RO1 (tR = 34.1 min) and RO2 (tR = 36.8 min) at 2.0 mL/min. Metabolite 5 (tR = 32.0 min, 3.7 mg) was obtained by a further purification of fr. RO1 on HPLC with 55% to 72% methanol. Metabolite 7 (tR = 36.8 min, 3.2 mg) was obtained by a further purification of fr. RO2 on HPLC with a gradient of 53% to 85% methanol.
The organic residue of 2 cultured with M. hiemalis was separated on HPLC with 58% to 85% methanol to furnish 8 (tR = 16.6 min, 1.8 mg,) and 9 (tR = 26.4 min, 2.2 mg) at 2.0 mL/min.
The organic residue of 3 cultured with M. hiemalis was separated on HPLC with 58% to 97% methanol to yield metabolite 10 (3.5 mg, tR = 32.8 min) at 2.0 mL/min.
The organic residue of 3 cultured with Mortierella ramanniana var. angulispora was separated on HPLC with 58% to 78% methanol to provide fraction MR1 (tR = 32.8 min). Metabolites 11 (2.4 mg, tR = 14.8 min) and 12 (2.2 mg, tR = 17.8 min) were yielded by a further purification of fr. MR1 on HPLC with n-hexane: anhydrous EtOH (85: 15, v/v) at 1.0 mL/min.
2.5. Structural Characterization of New Metabolites 4, 5, 7, and 10
2.5.1. 3′-(3-Hydroxy-3-methylbutyl)-4′-O-β-d-glucopyranosyl-4,2′-dihydroxychalcone (4)
Yellow solid; –25.2 (c 0.10, MeOH); UV (MeOH) λmax: 371 nm; IR νmax: 3333, 2855, 1632, 1233, 990 cm−1; HRESIMS m/z: 527.1892 [M+Na]+ (calcd. for C26H32O10Na, 527.1893); 1H- and 13C-NMR spectral data: see Table 1.

Table 1.
1H- and 13C-NMR data for 4, 5, 7, and 10.
2.5.2. 3′-(3-O-Methyl-3-methylbutyl)-4′-O-β-d-glucopyranosyl-4,2′-dihydroxychalcone (5)
Yellow solid; –26.9 (c 0.10, MeOH); UV (MeOH) λmax: 365 nm; IR νmax: 3330, 2927, 1780, 1632, 1284, 1105, 1074 cm−1; HRESIMS m/z: 541.2049 [M+Na]+ (calcd. for C27H34O10Na, 541.2050); 1H- and 13C-NMR spectral data: see Table 1.
2.5.3. 3′-(3-O-Ethyl-3-methylbutyl)-4′-O-β-d-glucopyranosyl-4,2′-dihydroxychalcone (7)
Yellow solid; –46.5 (c 0.10, MeOH); UV (MeOH) λmax: 363 nm; IR νmax: 3319, 2919, 1604, 1285, 1105, 835 cm−1; HRESIMS m/z: 555.2208 [M+Na]+ (calcd. for C28H36O10Na, 555.2206); 1H- and 13C-NMR spectral data: see Table 1.
2.5.4. 4′-O-β-d-Glucopyranosyl xanthoangelol (10)
Yellow solid; –28.0 (c 0.10, MeOH); UV (MeOH) λmax: 365 nm; IR νmax: 3342, 2926, 1713, 1631, 1233, 1076 cm−1; HRESIMS m/z: 577.2414 [M+Na]+ (calcd. for C31H38O9Na, 577.2414); 1H- and 13C-NMR spectral data: see Table 1.
2.6. Acid Hydrolysis of 4, 5, 7, and 10
Each solution of compounds 4, 5, 7, and 10 (each 0.8 mg) in 2 mL 2N HCl was heated at 85 °C for 3 h. After cooling and neutralization, the reaction mixture was extracted with EtOAc. The water layer was concentrated and confirmed by TLC in comparison with authentic d-glucose as a standard. The organic layer containing the aglycone of 10 was determined as the corresponding substrate 3 by comparing their retention time on HPLC.
2.7. Cytotoxic Activity Evaluation
The cytotoxicity assays were conducted according to the MTT method as previously described [27] using human melanoma A375P, human colorectal HT-29, and human breast cancer MCF-7 cell lines. Briefly, cells were incubated in 96-well plates at an approximate density of 5.0 × 103 cells/well with 100 μL DMEM medium supplemented with 5% FBS, penicillin (100 U/mL) and streptomycin (100 μg/mL) at 37 °C with 5% CO2 for 24 h. Then, cells were fed with different concentrations of the test compounds (1–12) for another 48 h. After gently removing the culture media, 100 μL of MTT solution (0.5 mg/mL) was applied for staining the cells. Finally, absorbance of each plate was observed at 490 nm by the microplate reader after removal of MTT solution and addition of 100 μL dimethyl sulfoxide.
2.8. Tyrosinase Inhibitory Activity
l-Tyrosine was used as a substrate to evaluate the tyrosinase inhibition of 1–12 following the previously developed method [28,29] with some modifications. The tyrosinase inhibition assay was performed using 0.1 M phosphate buffer (pH 6.5), 2 mM l-tyrosine, and test compound solution with different concentrations ranging from 10 μM to 100 μM. The mixture was prepared 5 min before treatment with the enzyme solution (150 U/mL). In the experiments, the reaction mixture without tyrosinase was used as a blank, and the reaction mixture without sample solution was used as a negative control. Kojic acid was used as a positive control. After incubation for 30 min at 37 °C, absorbance of the reaction mixture was recorded at 490 nm to evaluate the amount of dopachrome produced in the process. The extent of tyrosinase inhibition by compounds 1–12 is displayed as the half maximal inhibitory concentration (IC50) in Table 2.

Table 2.
Cytotoxic and tyrosinase inhibitory activities of 1–12 a.
3. Results
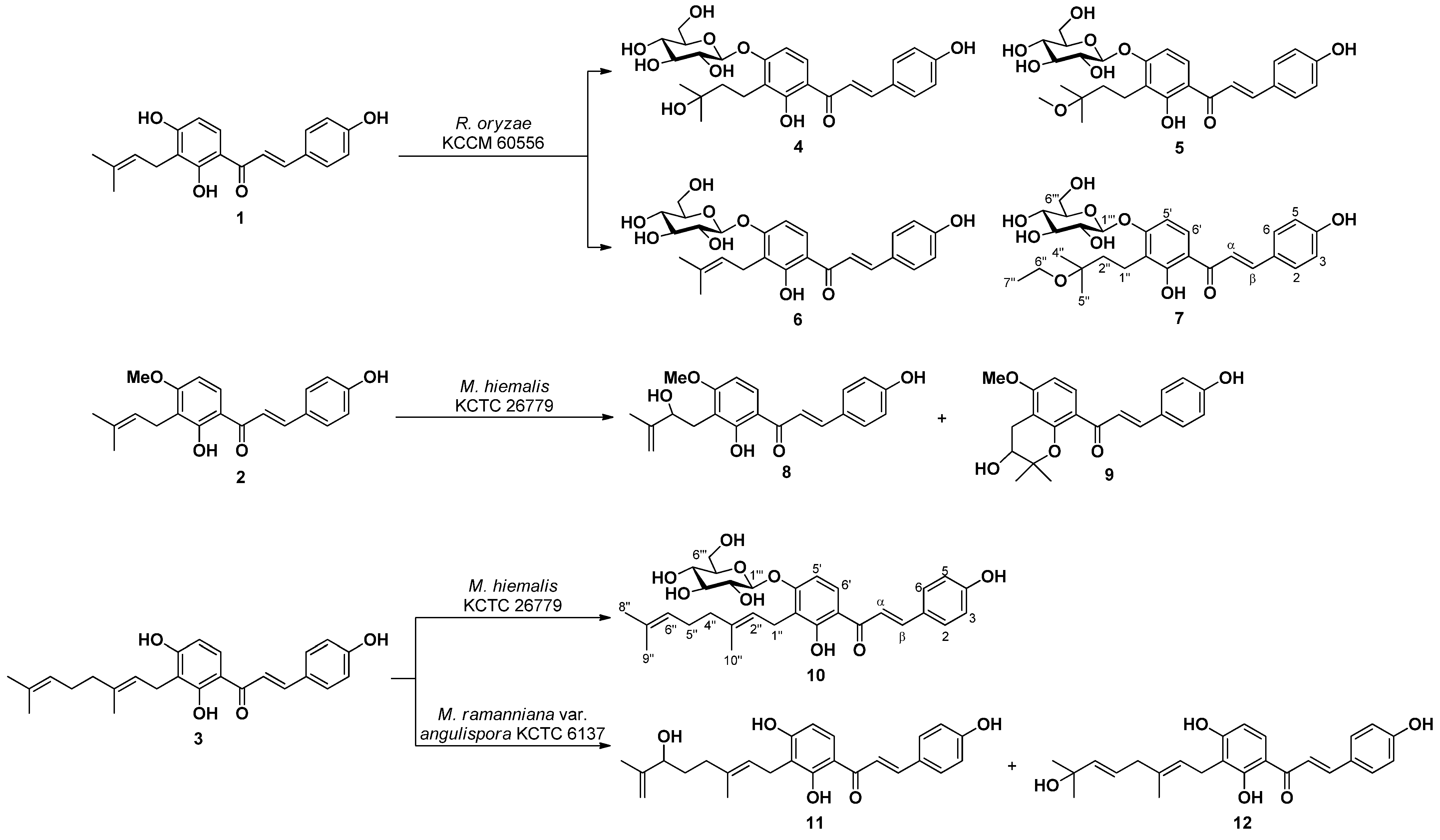
Microbial transformation of three bioactive prenylchalcones 1–3 is reported herein. Fermentation of 1 with R. oryzae KCCM 60,556 furnished three new (4, 5, and 7) and one known (6) metabolites. Fermentation of 2 with M. hiemalis KCTC 26,779 furnished two known (8 and 9) metabolites. Fermentation of 3 with M. hiemalis KCTC 26,779 furnished one new (10) metabolite, while that with M. ramanniana var. angulispora KCTC 6137 resulted in the production of two known (11 and 12) metabolites (Figure 1) (Figures S8–S41).
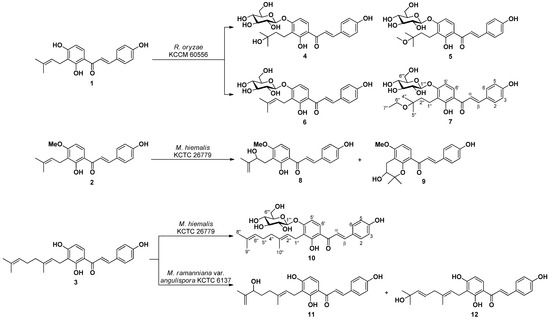
Figure 1.
Compounds 4–12 obtained by microbial transformation of 1–3.
Compound 4 was obtained as a yellow solid. High resolution electrospray ionization mass spectrometry (HRESIMS) measurements on 4 revealed a molecular formula of C26H32O10 corresponding to 11 unsaturation by the occurrence of its HRESIMS ion peak at m/z 527.1892 [M+Na]+ (calcd. for C26H32O10Na, 527.1893). The typical ultraviolet (UV) absorption at 371 nm indicated the appearance of a chalcone scaffold [4,30]. Inspection of its spectroscopic data revealed the presence of characteristic resonances for a chalcone skeleton with a 3-hydroxy-3-methylbutyl group and a sugar moiety. The 1H-NMR data presented signals assignable to two ortho-coupled aromatic protons at δH 7.98 (1H, d, J = 9.0) and 6.80 (1H, d, J = 9.0 Hz) and four aromatic protons at δH 7.64 (2H, d, J = 8.6 Hz) and 6.85 (2H, d, J = 8.6 Hz). Meanwhile, an olefin moiety was identified by the observation of signals at δH 7.82 (1H, d, J = 15.5) and 7.66 (1H, d, J = 15.5). These typical resonances suggested the existence of a 4,2′,3′,4′-tetrasubstituted chalcone. The two multiplets at δH 2.81 (2H) and 1.66 (2H) and two singlets at δH 1.28 (3H) and 1.26 (3H) were attributed to the 3-hydroxy-3-methylbutyl group, whereas the anomeric proton at δH 5.01 (1H, d, J = 7.5 Hz), together with the characteristic carbon signals at δC 102.0, 78.5, 78.2, 75.0, 71.4, and 62.6 demonstrated that the sugar moiety was a β-d-glucopyranose [31,32]. Significant heteronuclear multiple bond coherence (HMBC) correlation between the anomeric proton signal at δH 5.00 (H-1′′′) and δC 162.6 (C-4′) indicated that the β-d-glucose was attached to the C-4′ position, while the 3-hydroxy-3-methylbutyl group was located at C-3′ on the basis of the HMBC correlations between δH 2.81 (H-1′′)/1.66 (H-2′′) and δc 120.8 (C-3′). Furthermore, acid hydrolysis of 4 produced d-glucose which was confirmed through direct comparison with the standard on TLC. Thus, the structure of 4 was characterized as 3′-(3-hydroxy-3-methylbutyl)-4′-O-β-d-glucopyranosyl-4,2′-dihydroxychalcone.
Compound 5 was assigned the composition C27H34O10 from its HRESIMS at m/z 541.2049 [M+Na]+ (calcd. for C27H34O10Na, 541.2050). The 1H- and 13C-NMR spectra of 5 were quite similar to those of 4, except for the resonances of a methoxy group (δH 3.31/δC 49.8) in 5. The attachment of the methoxy group was determined to be at C-3′′ according to the HMBC correlations from the methoxy protons H-6′′ (δH 3.31) to C-3′′ (δC 76.9) and C-2′′ (δC 39.2). Thus, the structure of 5 was determined as 3′-(3-O-methyl-3-methylbutyl)-4′-O-β-d-glucopyranosyl-4,2′-dihydroxychalcone.
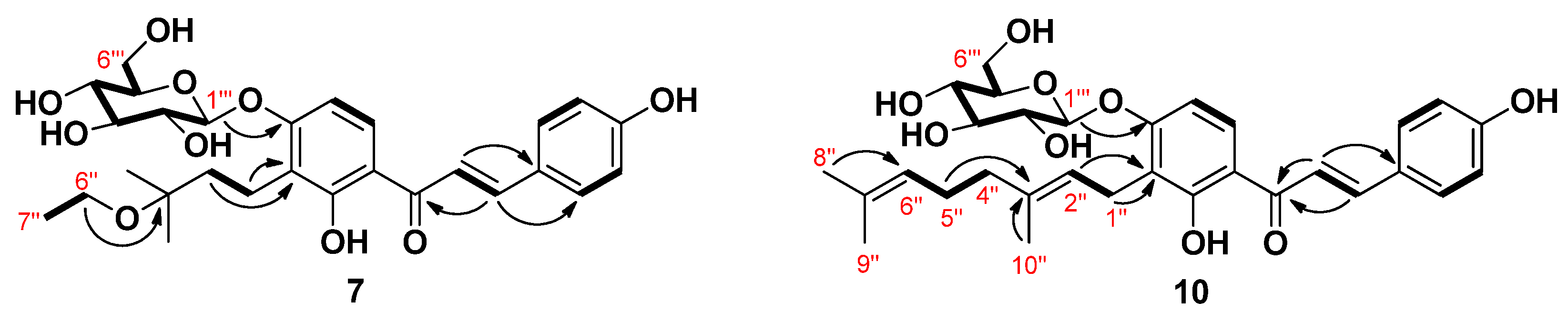
The molecular formula of compound 7 was determined as C28H36O10 using HRESIMS on the basis of a molecular ion at m/z 555.2208 [M+Na]+ (calcd. for C28H36O10Na,555.2206), which corresponded to 11 degrees of unsaturation. The NMR data of 7 were almost identical to those observed for 4 except for the appearance of one O-methylene (δH 3.55, 2H, q, J = 7.0 Hz/δC 58.0) and one methyl (δH 1.21, 3H, t, J = 7.0 Hz/δC 16.5) signals corresponding to H-6′’ and H-7′’, readily attributable to an ethyloxy group. Further evidence was obtained from the obvious correlation between proton signals H-6′′ and H-7′′ (Figure 2) in the 1H-1H homonuclear correlation spectroscopy (COSY) experiment. HMBC correlation between H-6′’ and C-3′’ confirmed the location of the ethyloxy group at C-3′’. Accordingly, the structure of 7 was assigned 3′-(3-O-ethyl-3-methylbutyl)-4′-O-β-d-glucopyranosyl-4,2′-dihydroxychalcone.
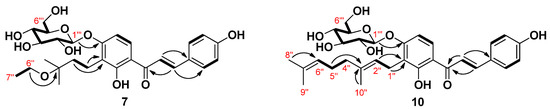
Figure 2.
Key COSY (1H-1H) and HMBC (1H→13C) correlations of compounds 7 and 10.
Compound 10 was confirmed to have C31H38O9 as the elemental composition according to the analysis of its HRESIMS data. The 1H-NMR spectrum of compound 10 displayed two sets of aromatic proton resonances at δH 8.21 (1H, d, J = 9.2) and 6.74 (1H, d, J = 9.2), 7.79 (2H, d, J = 8.5), and 6.85 (2H, d, J = 8.5) together with two olefinic proton resonances at δH 7.84 (1H, d, J = 15.3) and 7.80 (1H, d, J = 15.3) assignable to the 4,2′,3′,4′-tetrasubstituted chalcone. In addition, proton resonance signals for a geranyl group and a sugar moiety were identified in the up-field region. Analysis of the COSY spectrum together with the heteronuclear single quantum coherence (HSQC) and HMBC spectra allowed the establishment of the structure as 4,2′,4′-trihydroxychalcone substituted with a geranyl group and a sugar moiety in 10 (Figure 2). The 13C-NMR data showed six signals at δC 100.1, 77.3, 76.8, 73.4, 69.8, and 60.7, which are characteristic of a glucose moiety [31]. The anomeric proton signal at δH 5.00 (1H, d, J = 7.3 Hz) indicated a β-configuration of the glucosidic bond. The connectivity of the glucose moiety in 10 was confirmed to be at C-4′ through an ether linkage by the HMBC correlation from the anomeric proton signal H-1′’’ to C-4′. Presence of the glucose moiety and its aglycone after acid hydrolysis was further confirmed by comparison of their TLC and HPLC identification pattern with those of the authentic samples. Linkage of the geranyl group was deduced to be at C-3′ position by the observation of HMBC correlations from H-1′′/H-2′′ to C-3′. Hence, 10 was assigned 4′-O-β-d-glucopyranosyl xanthoangelol.
Structures of the five known metabolites were identified as 3′-(3-methyl-2-butenyl)-4′-O-β-d-glucopyranosyl-4,2′-dihydroxychalcone (6) [33], xanthoangelols H (8) [34,35] and D (9) [36], xanthokeismin A (11) [5], and xanthoangelol B (12) [25] by comparing 1H- and 13C-NMR data with those reported in the literature.
In this study, cytotoxicity of compounds 1–12 was determined by MTT assays against A375P, HT-29, and MCF-7 human cancer cells. As shown in Table 2, compounds 1 and 3 showed most potent cytotoxicity against A375P, HT-29, and MCF-7. Compound 2 exhibited moderate cytotoxic activity toward human cancer cell lines tested. However, all the metabolites 4–12 exhibited lower cytotoxicity compared with their corresponding substrates 1, 2, and 3. It appears that the C-prenyl or C-geranyl side chain is essential for cytotoxic activity. Meanwhile, glucosylation or methylation at 4′-OH decreased the cytotoxicity.
The capability of 1–12 to inhibit tyrosinase was determined using l-tyrosine as a substrate. The most potent compounds were 5 and 7, which displayed respectively 2.5- and 2-fold stronger inhibition compared with the positive control. Compound 4 showed moderate activity comparable to kojic acid. It was suggested that methyl or ethyl moiety introduced at the 3-O position of 3-hydroxy-3-methylbutyl group could improve the inhibitory activity against mushroom tyrosinase. The anti-tyrosinase potencies were enhanced in chalcone 4′-O-glucosides (6 and 10) compared with their corresponding aglycones (1 and 3). This allowed us to postulate that the introduction of 4′-O-glucopyranosyl group led to the improvement in the tyrosinase inhibitory capacity.
4. Discussion
In this work, three bioactive prenylchalcones, isobavachalcone (IBC, 1), 4-hydroxyderricin (HD, 2), and xanthoangelol (XT, 3), were isolated from A. keiskei and subjected to microbial transformation. The reaction types consisted of hydroxylation, methylation, ethylation, rearrangement, and glucosylation. These highly specific reactions are difficult to achieve by synthetic methods, especially under mild conditions. Therefore, microbial transformation is a useful way in structural diversification of chalcones to discover valuable derivatives. In addition, microbial transformation permits the possibility of understanding the metabolic pathway [37]. It is particularly noteworthy that microbial transformation of HD (2) with the microbe M. hiemalis produced two metabolites xanthoangelols H (8) and D (9), while microbial transformation of XT (3) with the microbe Mortierella ramanniana var. angulispora resulted in the production of two metabolites xanthokeismin A (11) and xanthoangelol B (12). All the four known metabolites (8, 9, 11, and 12) were previously identified from the herb A. keiskei. The information could be beneficial for understanding the metabolic pathways of these compounds in this plant.
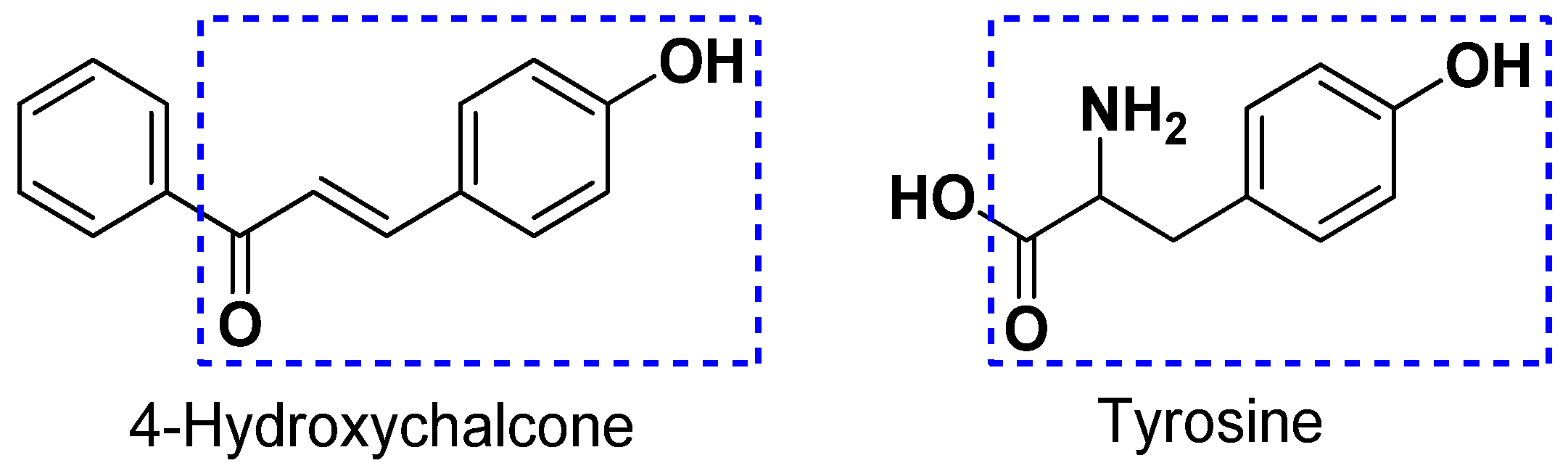
Tyrosinase is a metalloenzyme containing two copper atoms and acts as a rate-limiting oxidase involved in the production of melanin [38]. During melanogenesis, tyrosinase is able to act as a hydroxylase to catalyze the conversion of l-tyrosine to l-3,4-dihydroxyphenylalanine (l-DOPA) and subsequently is able to act as an oxidase to catalyze the conversion of l-DOPA to l-dopaquinone, which results in the accumulation of melanin and hyperpigmentation [39]. In recent years, it has been demonstrated that various dermatological disorders, such as age spots, melasma freckle, and sites of actinic damage, derive from the accumulation of an exaggerated level of epidermal pigmentation. Tyrosinase has been recognized as a significant target for the treatment of skin disorders related to irregular pigmentation [40]. Thus, the identification of new and potent leads with tyrosinase inhibitory activity has attracted considerable interest in medication and cosmetics. Chalcones represent a diverse class of compounds generally presented in higher plants. Structurally, compounds with the scaffold of 4-hydroxychalcone are considered as potent tyrosinase inhibitors owing to the structural similarity between 4-hydroxychalcone and tyrosine (Figure 3) [41]. In this study, the structure -activity relationship of twelve compounds (1–12) with the scaffold of 4-hydroxychalcone was investigated for its tyrosinase inhibitory activity. The results revealed that the glucosylation of the OH group at C-4′ position is important in the enhancement of the inhibition. Furthermore, the insertion of a methyl (compound 5) or an ethyl group (compound 7) into the 3-hydroxy-3-methylbutyl group at the 3-O position dramatically improved the inhibition. Considering that 5 and 7 showed the most potent tyrosinase inhibition with relatively low cytotoxicity, as shown in Table 2, they can act as potential leads for the development of effective and safe anti-browning and skin-whitening agents.
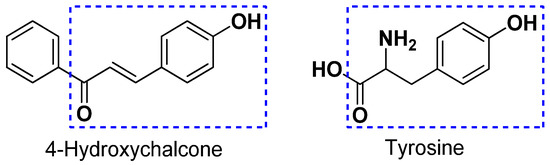
Figure 3.
The structural similarity between 4-hydroxychalcone and tyrosine.
5. Conclusions
Microbial transformation of three bioactive prenylchalcones 1–3 isolated from A. keiskei afforded four new (4, 5, 7, and 10) and five known (6, 8, 9, 11, and 12) metabolites. The five known metabolites were all previously reported from natural sources. In the evaluation of all compounds for their cytotoxic and tyrosinase inhibitory activities, metabolites 5 and 7 exhibited the most potent tyrosinase inhibition with relatively low cytotoxicity. The two metabolites 5 and 7 can be considered as new leads for the further design of tyrosinase inhibitors. The present results indicate that microbial transformation can be an efficient and useful tool for the identification of natural product-like compounds as bioactive leads.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods11040543/s1, Figure S1: TLC analyses for microbial transformation of 1–3 by selected microbes, Figures S2–S41: 1D, 2D NMR, and HRESIMS spectra of 1–12.
Author Contributions
Conceptualization, I.-S.L.; methodology, Y.X. and I.-S.L.; validation, Y.X.; formal analysis, I.-S.L.; investigation, Y.X. and I.-S.L.; resources, Y.X.; data curation, Y.X.; writing—original draft preparation, Y.X.; writing—review and editing, I.-S.L.; visualization, Y.X.; supervision, I.-S.L.; project administration, I.-S.L.; funding acquisition, Y.X. and I.-S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2019R1I1A3A01043084 and NRF-2021R1I1A1A01056116).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge the Center for Research Facilities, Chonnam National University, and the Korea Basic Science Institute (KBSI) for their support to operate the NMR, IR, and HRESIMS experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kil, Y.-S.; Pham, S.T.; Seo, E.K.; Jafari, M. Angelica keiskei, an emerging medicinal herb with various bioactive constituents and biological activities. Arch. Pharm. Res. 2017, 40, 655–675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Pang, X.; Hua, P.; Gao, X.; Li, Q.; Li, Z. Simultaneous optimization of ultrasound-assisted extraction for flavonoids and antioxidant activity of Angelica keiskei using response surface methodology (RSM). Molecules 2019, 24, 3461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caesar, L.K.; Cech, N.B. A review of the medicinal uses and pharmacology of ashitaba. Planta Med. 2016, 82, 1236–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akihisa, T.; Tokuda, H.; Hasegawa, D.; Ukiya, M.; Kimura, Y.; Enjo, F.; Suzuki, T.; Nishino, H. Chalcones and other compounds from the exudates of Angelica keiskei and their cancer chemopreventive effects. J. Nat. Prod. 2006, 69, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Aoki, N.; Muko, M.; Ohta, E.; Ohta, S. C-geranylated chalcones from the stems of Angelica keiskei with superoxide-scavenging activity. J. Nat. Prod. 2008, 71, 1308–1310. [Google Scholar] [CrossRef] [PubMed]
- Inamori, Y.; BABA, K.; Tsujibo, H.; Taniguchi, M.; Nakata, K.; KOZAWA, M. Antibacterial activity of two chalcones, xanthoangelol and 4-hydroxyderricin, isolated from the root of Angelica keiskei KOIDZUMI. Chem. Pharm. Bull. 1991, 39, 1604–1605. [Google Scholar] [CrossRef] [Green Version]
- Enoki, T.; Ohnogi, H.; Nagamine, K.; Kudo, Y.; Sugiyama, K.; Tanabe, M.; Kobayashi, E.; Sagawa, H.; Kato, I. Antidiabetic activities of chalcones isolated from a Japanese herb, Angelica keiskei. J. Agric. Food Chem. 2007, 55, 6013–6017. [Google Scholar] [CrossRef]
- Arung, E.T.; Furuta, S.; Sugamoto, K.; Shimizu, K.; Ishikawa, H.; Matsushita, Y.-I.; Kondo, R. The inhibitory effects of representative chalcones contained in Angelica keiskei on melanin biosynthesis in B16 melanoma cells. Nat. Prod. Commun. 2012, 7, 1007–1010. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wu, W.; Li, X.; Xin, X.; Liu, D. Daily supplementation with fresh Angelica keiskei juice alleviates high-fat diet-induced obesity in mice by modulating gut microbiota composition. Mol. Nutr. Food Res. 2019, 63, 1900248. [Google Scholar] [CrossRef]
- Kim, D.W.; Curtis-Long, M.J.; Yuk, H.J.; Wang, Y.; Song, Y.H.; Jeong, S.H.; Park, K.H. Quantitative analysis of phenolic metabolites from different parts of Angelica keiskei by HPLC–ESI MS/MS and their xanthine oxidase inhibition. Food Chem. 2014, 153, 20–27. [Google Scholar] [CrossRef]
- Sumiyoshi, M.; Taniguchi, M.; Baba, K.; Kimura, Y. Antitumor and antimetastatic actions of xanthoangelol and 4-hydroxyderricin isolated from Angelica keiskei roots through the inhibited activation and differentiation of M2 macrophages. Phytomedicine 2015, 22, 759–767. [Google Scholar] [CrossRef]
- Kimura, Y.; Taniguchi, M.; Baba, K. Antitumor and antimetastatic activities of 4-hydroxyderricin isolated from Angelica keiskei roots. Planta Med. 2004, 70, 211–219. [Google Scholar]
- Zhang, T.; Wang, Q.; Fredimoses, M.; Gao, G.; Wang, K.; Chen, H.; Wang, T.; Oi, N.; Zykova, T.A.; Reddy, K. The ashitaba (Angelica keiskei) chalcones 4-hydroxyderricin and xanthoangelol suppress melanomagenesis by targeting BRAF and PI3K. Cancer Prev. Res. 2018, 11, 607–620. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Li, H.; Kweon, M.; Choi, Y.; Kim, M.J.; Ryu, J.-H. Isobavachalcone from Angelica keiskei inhibits adipogenesis and prevents lipid accumulation. Int. J. Mol. Sci. 2018, 19, 1693. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Lin, L.; Lu, J.-J.; Chen, X. Pharmacological review of isobavachalcone, a naturally occurring chalcone. Pharmacol. Res. Commun. 2021, 165, 105483. [Google Scholar] [CrossRef]
- Wu, D.; Wang, W.; Chen, W.; Lian, F.; Lang, L.; Huang, Y.; Xu, Y.; Zhang, N.; Chen, Y.; Liu, M. Pharmacological inhibition of dihydroorotate dehydrogenase induces apoptosis and differentiation in acute myeloid leukemia cells. Haematologica 2018, 103, 1472. [Google Scholar] [CrossRef]
- Cao, H.; Chen, X.; Jassbi, A.R.; Xiao, J. Microbial biotransformation of bioactive flavonoids. Biotechnol. Adv. 2015, 33, 214–223. [Google Scholar] [CrossRef]
- Chen, G.; Yang, M.; Nong, S.; Yang, X.; Ling, Y.; Wang, D.; Wang, X.; Zhang, W. Microbial transformation of 20 (S)-protopanaxadiol by Absidia corymbifera. Cytotoxic activity of the metabolites against human prostate cancer cells. Fitoterapia 2013, 84, 6–10. [Google Scholar] [CrossRef]
- Farooq, R.; Hussain, N.; Yousuf, S.; Ahmad, M.S.; Choudhary, M.I. Microbial transformation of mestanolone by Macrophomina phaseolina and Cunninghamella blakesleeana and anticancer activities of the transformed products. RSC Adv. 2018, 8, 21985–21992. [Google Scholar] [CrossRef] [Green Version]
- Akihisa, T.; Motoi, T.; Seki, A.; Kikuchi, T.; Fukatsu, M.; Tokuda, H.; Suzuki, N.; Kimura, Y. Cytotoxic activities and anti-tumor-promoting effects of microbial transformation products of prenylated chalcones from Angelica keiskei. Chem. Biodivers. 2012, 9, 318–330. [Google Scholar] [CrossRef]
- Kozłowska, J.; Potaniec, B.; Żarowska, B.; Anioł, M. Microbial transformations of 4′-methylchalcones as an efficient method of obtaining novel alcohol and dihydrochalcone derivatives with antimicrobial activity. RSC Adv. 2018, 8, 30379–30386. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Yim, S.-H.; Han, F.; Kang, B.Y.; Choi, H.J.; Jung, D.-W.; Williams, D.R.; Gustafson, K.R.; Kennelly, E.J.; Lee, I.-S. Biotransformed metabolites of the hop prenylflavanone isoxanthohumol. Molecules 2019, 24, 394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, Y.; Wu, J.; Liu, X.; Zhao, B.; Wang, Z. Study on structural and spectral properties of isobavachalcone and 4-hydroxyderricin by computational method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 126, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Son, Y.K.; Kim, G.H.; Hwang, K.H. Xanthoangelol and 4-hydroxyderricin are the major active principles of the inhibitory activities against monoamine oxidases on Angelica keiskei K. Biomol. Ther. 2013, 21, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.E.; Choi, E.J.; Jin, Q.; Jin, H.-G.; Woo, E.-R. Chalcones isolated from Angelica keiskei and their inhibition of IL-6 production in TNF-α-stimulated MG-63 cell. Arch. Pharm. Res. 2011, 34, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.M.; McChesney, J.D.; Hufford, C.D. The use of microorganisms for the study of drug metabolism. Med. Res. Rev. 1985, 5, 231–253. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Vincent, J.; Hearing, J. Mammalian monophenol monooxygenase (tyrosinase): Purification, properties, and reactions catalyzed. Methods Enzymol. 1987, 142, 154–165. [Google Scholar]
- Deering, R.W.; Chen, J.; Sun, J.; Ma, H.; Dubert, J.; Barja, J.L.; Seeram, N.P.; Wang, H.; Rowley, D.C. N-acyl dehydrotyrosines, tyrosinase inhibitors from the marine bacterium Thalassotalea sp. PP2-459. J. Nat. Prod. 2016, 79, 447–450. [Google Scholar] [CrossRef] [Green Version]
- Kaennakam, S.; Sukandar, E.R.; Rassamee, K.; Siripong, P.; Tip-pyang, S. Cytotoxic chalcones and isoflavones from the stems of Dalbergia velutina. Phytochem. Lett. 2019, 31, 187–191. [Google Scholar] [CrossRef]
- Roslund, M.U.; Tähtinen, P.; Niemitz, M.; Sjöholm, R. Complete assignments of the 1H and 13C chemical shifts and JH,H coupling constants in NMR spectra of d-glucopyranose and all d-glucopyranosyl-d-glucopyranosides. Carbohydr. Res. 2008, 343, 101–112. [Google Scholar] [CrossRef]
- Ninomiya, M.; Efdi, M.; Inuzuka, T.; Koketsu, M. Chalcone glycosides from aerial parts of Brassica rapa L. ‘hidabeni’, turnip. Phytochem. Lett. 2010, 3, 96–99. [Google Scholar] [CrossRef]
- Cioffi, G.; Escobar, L.M.; Braca, A.; De Tommasi, N. Antioxidant chalcone glycosides and flavanones from Maclura (Chlorophora) tinctoria. J. Nat. Prod. 2003, 66, 1061–1064. [Google Scholar] [CrossRef]
- Kumazawa, S.; Suzuki, S.; Ahn, M.-R.; Kamihira, M.; Udagawa, Y.; Bang, K.-S.; Nakayama, T. A new chalcone from propolis collected on Jeju island, Korea. Food Sci. Technol. 2006, 12, 67–69. [Google Scholar] [CrossRef]
- Sugamoto, K.; Matsusita, Y.-i.; Matsui, K.; Kurogi, C.; Matsui, T. Synthesis and antibacterial activity of chalcones bearing prenyl or geranyl groups from Angelica keiskei. Tetrahedron 2011, 67, 5346–5359. [Google Scholar] [CrossRef]
- Baba, K.; Nakata, K.; Taniguchi, M.; Kido, T.; Kozawa, M. Chalcones from Angelica keiskei. Phytochemistry 1990, 29, 3907–3910. [Google Scholar] [CrossRef]
- Shanu-Wilson, J.; Evans, L.; Wrigley, S.; Steele, J.; Atherton, J.; Boer, J. Biotransformation: Impact and application of metabolism in drug discovery. ACS Med. Chem. Lett. 2020, 11, 2087–2107. [Google Scholar] [CrossRef]
- Decker, H.; Schweikardt, T.; Tuczek, F. The first crystal structure of tyrosinase: All questions answered? Angew. Chem. Int. Ed. 2006, 45, 4546–4550. [Google Scholar] [CrossRef]
- Burestedt, E.; Narvaez, A.; Ruzgas, T.; Gorton, L.; Emnéus, J.; Domínguez, E.; Marko-Varga, G. Rate-limiting steps of tyrosinase-modified electrodes for the detection of catechol. Anal. Chem. 1996, 68, 1605–1611. [Google Scholar] [CrossRef]
- Chang, T.-S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [Green Version]
- Nerya, O.; Musa, R.; Khatib, S.; Tamir, S.; Vaya, J. Chalcones as potent tyrosinase inhibitors: The effect of hydroxyl positions and numbers. Phytochemistry 2004, 65, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).