Study of Water Distribution, Textural and Colour Properties of Cold Formulated and Air-Dried Apple Snacks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Vacuum Impregnation
2.3. Air-Drying
2.4. Analytical Determinations
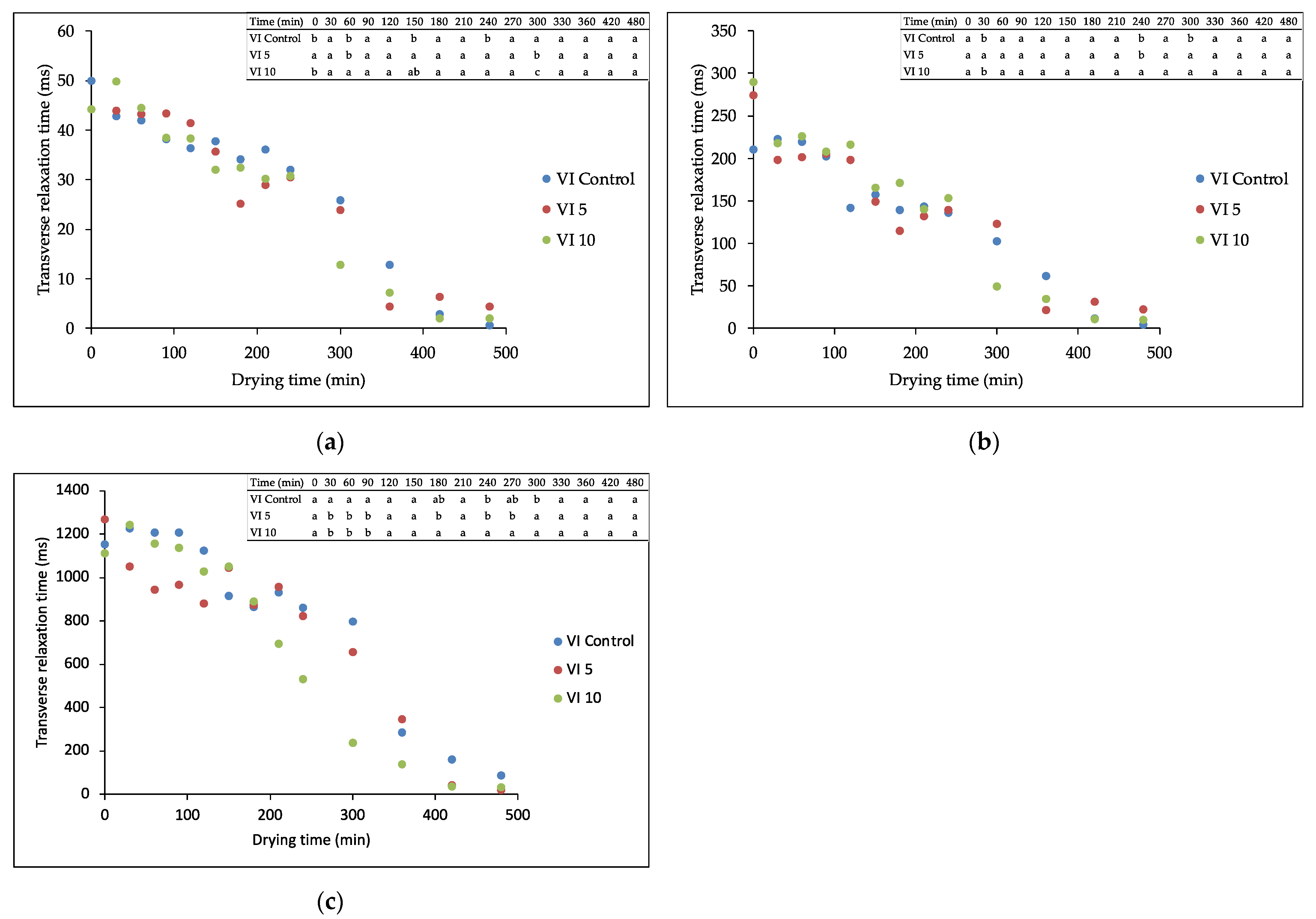
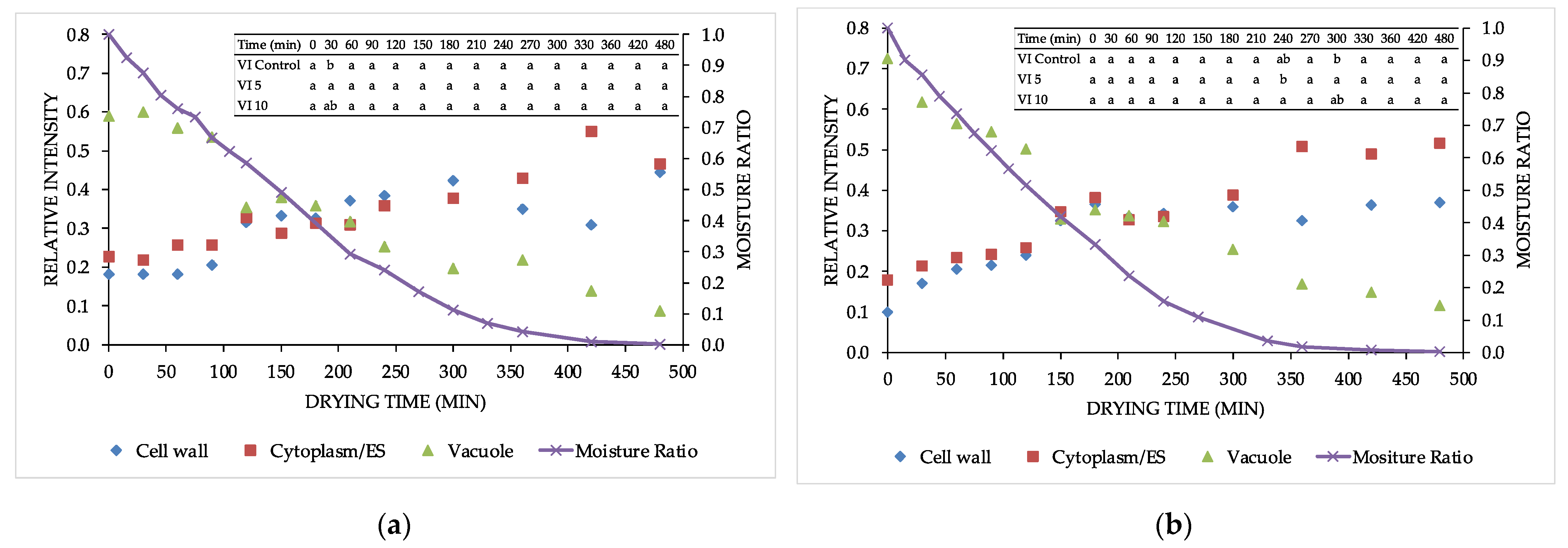
2.4.1. TD-NMR
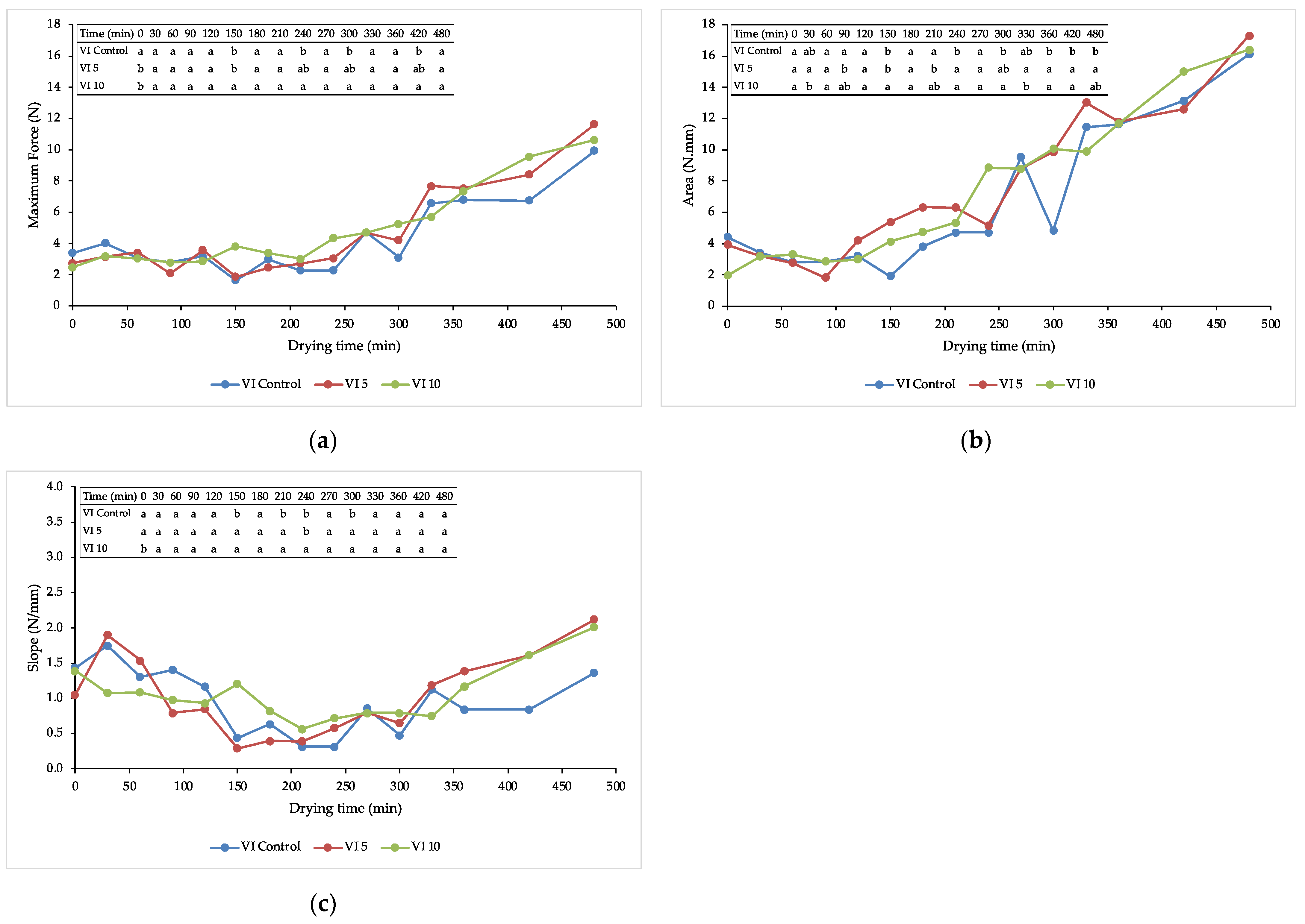
2.4.2. Texture Analysis
2.4.3. Computer Vision System (CVS)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Water State
3.2. Texture during Drying
3.3. Colour Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arbit, N.; Ruby, M.; Rozin, P. Development and Validation of the Meaning of Food in Life Questionnaire (MFLQ): Evidence for a New Construct to Explain Eating Behavior. Food Qual. Prefer. 2017, 59, 35–45. [Google Scholar] [CrossRef]
- Kokkoris, M.D.; Stavrova, O. Meaning of Food and Consumer Eating Behaviors. Food Qual. Prefer. 2021, 94, 104343. [Google Scholar] [CrossRef]
- Caso, D.; Guidetti, M.; Capasso, M.; Cavazza, N. Finally, the Chance to Eat Healthily: Longitudinal Study about Food Consumption during and after the First COVID-19 Lockdown in Italy. Food Qual. Prefer. 2022, 95, 104275. [Google Scholar] [CrossRef] [PubMed]
- Castagnini, J.M.; Betoret, N.; Betoret, E.; Fito, P. Vacuum Impregnation and Air Drying Temperature Effect on Individual Anthocyanins and Antiradical Capacity of Blueberry Juice Included into an Apple Matrix. LWT Food Sci. Technol. 2015, 64, 1289–1296. [Google Scholar] [CrossRef]
- Betoret, E.; Sentandreu, E.; Betoret, N.; Codoñer-Franch, P.; Valls-Bellés, V.; Fito, P. Technological Development and Functional Properties of an Apple Snack Rich in Flavonoid from Mandarin Juice. Innov. Food Sci. Emerg. Technol. 2012, 16, 298–304. [Google Scholar] [CrossRef]
- Betoret, N.; Puente, L.; Díaz, M.; Pagán, M.; García, M.; Gras, M.L.; Martínez-Monzó, J.; Fito, P.; Pag, M.J.; Mart, J.; et al. Development of Probiotic-Enriched Dried Fruits by Vacuum Impregnation. J. Food Eng. 2003, 56, 273–277. [Google Scholar] [CrossRef]
- Nawirska-Olszańska, A.; Pasławska, M.; Stępień, B.; Oziembłowski, M.; Sala, K.; Smorowska, A. Effect of Vacuum Impregnation with Apple-Pear Juice on Content of Bioactive Compounds and Antioxidant Activity of Dried Chokeberry Fruit. Foods 2020, 9, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tylewicz, U.; Mannozzi, C.; Romani, S.; Castagnini, J.M.; Samborska, K.; Rocculi, P.; Dalla Rosa, M. Chemical and Physicochemical Properties of Semi-Dried Organic Strawberries Enriched with Bilberry Juice-Based Solution. LWT 2019, 114, 108377. [Google Scholar] [CrossRef]
- Tappi, S.; Tylewicz, U.; Romani, S.; Dalla Rosa, M.; Rizzi, F.; Rocculi, P. Study on the Quality and Stability of Minimally Processed Apples Impregnated with Green Tea Polyphenols during Storage. Innov. Food Sci. Emerg. Technol. 2017, 39, 148–155. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, Y. Use of Vacuum Impregnation to Develop High Quality and Nutritionally Fortified Frozen Strawberries. J. Food Process. Preserv. 2004, 28, 117–132. [Google Scholar] [CrossRef]
- Alzamora, S.M.; Salvatori, D.; Tapia, M.S.; López-Malo, A.; Welti-Chanes, J.; Fito, P. Novel Functional Foods from Vegetable Matrices Impregnated with Biologically Active Compounds. J. Food Eng. 2005, 67, 205–214. [Google Scholar] [CrossRef]
- Castagnini, J.M.; Tappi, S.; Tylewicz, U.; Romani, S.; Rocculi, P.; Dalla Rosa, M. Sustainable Development of Apple Snack Formulated with Blueberry Juice and Trehalose. Sustainability 2021, 13, 9204. [Google Scholar] [CrossRef]
- Kręcisz, M.; Stępień, B.; Pasławska, M.; Popłoński, J.; Dulak, K. Physicochemical and Quality Properties of Dried Courgette Slices: Impact of Vacuum Impregnation and Drying Methods. Molecules 2021, 26, 4597. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, M.; Yang, P. Combination of LF-NMR and BP-ANN to Monitor Water States of Typical Fruits and Vegetables during Microwave Vacuum Drying. LWT 2019, 116, 108548. [Google Scholar] [CrossRef]
- Janowicz, M.; Ciurzyńska, A.; Lenart, A. Effect of Osmotic Pretreatment Combined with Vacuum Impregnation or High Pressure on the Water Diffusion Coefficients of Convection Drying: Case Study on Apples. Foods 2021, 10, 2605. [Google Scholar] [CrossRef] [PubMed]
- Marcone, M.F.; Wang, S.; Albabish, W.; Nie, S.; Somnarain, D.; Hill, A. Diverse Food-Based Applications of Nuclear Magnetic Resonance (NMR) Technology. Food Res. Int. 2013, 51, 729–747. [Google Scholar] [CrossRef]
- Tylewicz, U.; Aganovic, K.; Vannini, M.; Toepfl, S.; Bortolotti, V.; Dalla Rosa, M.; Oey, I.; Heinz, V. Effect of Pulsed Electric Field Treatment on Water Distribution of Freeze-Dried Apple Tissue Evaluated with DSC and TD-NMR Techniques. Innov. Food Sci. Emerg. Technol. 2016, 37, 352–358. [Google Scholar] [CrossRef]
- Hills, B.P.; Duce, S.L. The Influence of Chemical and Diffusive Exchange on Water Proton Transverse Relaxation in Plant Tissues. Magn. Reson. Imaging 1990, 8, 321–331. [Google Scholar] [CrossRef]
- Nowacka, M.; Tylewicz, U.; Laghi, L.; Dalla Rosa, M.; Witrowa-Rajchert, D. Effect of Ultrasound Treatment on the Water State in Kiwifruit during Osmotic Dehydration. Food Chem. 2014, 144, 18–25. [Google Scholar] [CrossRef]
- Mauro, M.A.; Dellarosa, N.; Tylewicz, U.; Tappi, S.; Laghi, L.; Rocculi, P.; Rosa, M.D. Calcium and Ascorbic Acid Affect Cellular Structure and Water Mobility in Apple Tissue during Osmotic Dehydration in Sucrose Solutions. Food Chem. 2016, 195, 19–28. [Google Scholar] [CrossRef]
- Nowacka, M.; Laghi, L.; Rybak, K.; Dalla Rosa, M.; Witrowa-Rajchert, D.; Tylewicz, U. Water State and Sugars in Cranberry Fruits Subjected to Combined Treatments: Cutting, Blanching and Sonication. Food Chem. 2019, 299, 125122. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Fan, L.; Li, Y.; Dong, S.; Li, K.; Bai, Y. A Review on Recent Advances in Plasma-Activated Water for Food Safety: Current Applications and Future Trends. In Critical Reviews in Food Science and Nutrition; Bellwether Publishing, Ltd.: Columbia, MD, USA, 2020. [Google Scholar] [CrossRef]
- Thalerngnawachart, S.; Duangmal, K. Influence of Humectants on the Drying Kinetics, Water Mobility, and Moisture Sorption Isotherm of Osmosed Air-Dried Papaya. Dry. Technol. 2016, 34, 574–583. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Bhandari, B.; Zhou, L. LF-NMR Online Detection of Water Dynamics in Apple Cubes during Microwave Vacuum Drying. Dry. Technol. 2018, 36, 2006–2015. [Google Scholar] [CrossRef]
- Lv, W.; Zhang, M.; Wang, Y.; Adhikari, B. Online Measurement of Moisture Content, Moisture Distribution, and State of Water in Corn Kernels during Microwave Vacuum Drying Using Novel Smart NMR/MRI Detection System. Dry. Technol. 2018, 36, 1592–1602. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, M.; Mujumdar, A.S.; Yang, P. Combined LF-NMR and Artificial Intelligence for Continuous Real-Time Monitoring of Carrot in Microwave Vacuum Drying. Food Bioprocess Technol. 2019, 12, 551–562. [Google Scholar] [CrossRef]
- Castagnini, J.M.; Zapata, L.M.; Quinteros, C.F.; Noceti, A. Multiple Response Optimization of Blueberry Juice Depectinization. Ciência Rural 2017, 47, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Betoret, N.; Andrés, A.; Segui, L.; Fito, P. Application of Safes (Systematic Approach to Food Engineering Systems) Methodology to Dehydration of Apple by Combined Methods. J. Food Eng. 2007, 83, 186–192. [Google Scholar] [CrossRef]
- Dellarosa, N.; Ragni, L.; Laghi, L.; Tylewicz, U.; Rocculi, P.; Dalla Rosa, M. Time Domain Nuclear Magnetic Resonance to Monitor Mass Transfer Mechanisms in Apple Tissue Promoted by Osmotic Dehydration Combined with Pulsed Electric Fields. Innov. Food Sci. Emerg. Technol. 2016, 37, 345–351. [Google Scholar] [CrossRef]
- Borgia, G.C.; Brown, R.J.S.; Fantazzini, P. Uniform-Penalty Inversion of Multiexponential Decay Data. J. Magn. Reson. 1998, 132, 65–77. [Google Scholar] [CrossRef] [Green Version]
- Panarese, V.; Laghi, L.; Pisi, A.; Tylewicz, U.; Rosa, M.D.; Rocculi, P. Effect of Osmotic Dehydration on Actinidia Deliciosa Kiwifruit: A Combined NMR and Ultrastructural Study. Food Chem. 2012, 132, 1706–1712. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, M.; Adhikari, B. Effect of Trehalose and Ultrasound-Assisted Osmotic Dehydration on the State of Water and Glass Transition Temperature of Broccoli (Brassica Oleracea L. Var. Botrytis L.). J. Food Eng. 2013, 119, 640–647. [Google Scholar] [CrossRef]
- Prothon, F.; Ahrné, L.M.; Funebo, T.; Kidman, S.; Langton, M.; Sjöholm, I. Effects of Combined Osmotic and Microwave Dehydration of Apple on Texture, Microstructure and Rehydration Characteristics. LWT Food Sci. Technol. 2001, 34, 95–101. [Google Scholar] [CrossRef]
- Vargas, A.; Perez, J.; Zoffoli, J.P.; Perez, A. Evolucion de La Textura de Bayas de Uva Del Cv. Thompson Seedless. Cienc. Investig. Agrar. 2000, 27, 117–126. [Google Scholar] [CrossRef]
- Martynenko, A.; Janaszek, M.A. Texture Changes During Drying of Apple Slices. Dry. Technol. 2014, 32, 567–577. [Google Scholar] [CrossRef]
- Önal, B.; Adiletta, G.; Crescitelli, A.; Di Matteo, M.; Russo, P. Optimization of Hot Air Drying Temperature Combined with Pre-Treatment to Improve Physico-Chemical and Nutritional Quality of ‘Annurca’ Apple. Food Bioprod. Process. 2019, 115, 87–99. [Google Scholar] [CrossRef]
- Katsoufi, S.; Lazou, A.E.; Giannakourou, M.C.; Krokida, M.K. Air Drying Kinetics and Quality Characteristics of Osmodehydrated-Candied Pumpkins Using Alternative Sweeteners. Dry. Technol. 2021, 39, 2194–2205. [Google Scholar] [CrossRef]
- Antal, T.; Kerekes, B.; Sikolya, L.; Tarek, M. Quality and Drying Characteristics of Apple Cubes Subjected to Combined Drying (FD Pre-Drying and HAD Finish-Drying). J. Food Process. Preserv. 2015, 39, 994–1005. [Google Scholar] [CrossRef]

| Sample | Drying Time (min) | L* | A* | B* | C* | H* |
|---|---|---|---|---|---|---|
| VI control | 0 | 65 ± 4 c | 26 ± 4 e | 17 ± 3 b | 31 ± 4 d | 33 ± 3 a |
| 480 | 64 ± 3 c | 21 ± 2 cd | 17 ± 1 b | 27 ± 2 bc | 40 ± 3 b | |
| VI 5 | 0 | 49 ± 3 a | 22 ± 3 d | 19 ± 3 b | 29 ± 4 c | 40 ± 3 b |
| 480 | 50 ± 4 a | 19 ± 1 c | 17 ± 1 ab | 25.2 ± 0.9 b | 42 ± 4 b | |
| VI 10 | 0 | 58 ± 3 b | 10.9 ± 0.8 a | 15 ± 1 a | 19 ± 1 a | 54 ± 3 c |
| 480 | 56 ± 4 b | 16 ± 3 b | 28 ± 4 c | 33 ± 3 d | 60 ± 8 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castagnini, J.M.; Tappi, S.; Tylewicz, U.; Laghi, L.; Rocculi, P. Study of Water Distribution, Textural and Colour Properties of Cold Formulated and Air-Dried Apple Snacks. Foods 2022, 11, 731. https://doi.org/10.3390/foods11050731
Castagnini JM, Tappi S, Tylewicz U, Laghi L, Rocculi P. Study of Water Distribution, Textural and Colour Properties of Cold Formulated and Air-Dried Apple Snacks. Foods. 2022; 11(5):731. https://doi.org/10.3390/foods11050731
Chicago/Turabian StyleCastagnini, Juan Manuel, Silvia Tappi, Urszula Tylewicz, Luca Laghi, and Pietro Rocculi. 2022. "Study of Water Distribution, Textural and Colour Properties of Cold Formulated and Air-Dried Apple Snacks" Foods 11, no. 5: 731. https://doi.org/10.3390/foods11050731
APA StyleCastagnini, J. M., Tappi, S., Tylewicz, U., Laghi, L., & Rocculi, P. (2022). Study of Water Distribution, Textural and Colour Properties of Cold Formulated and Air-Dried Apple Snacks. Foods, 11(5), 731. https://doi.org/10.3390/foods11050731









