Formulation Strategies for Improving the Stability and Bioavailability of Vitamin D-Fortified Beverages: A Review
Abstract
:1. Introduction
2. Examples of Vitamin D-Fortified Beverages
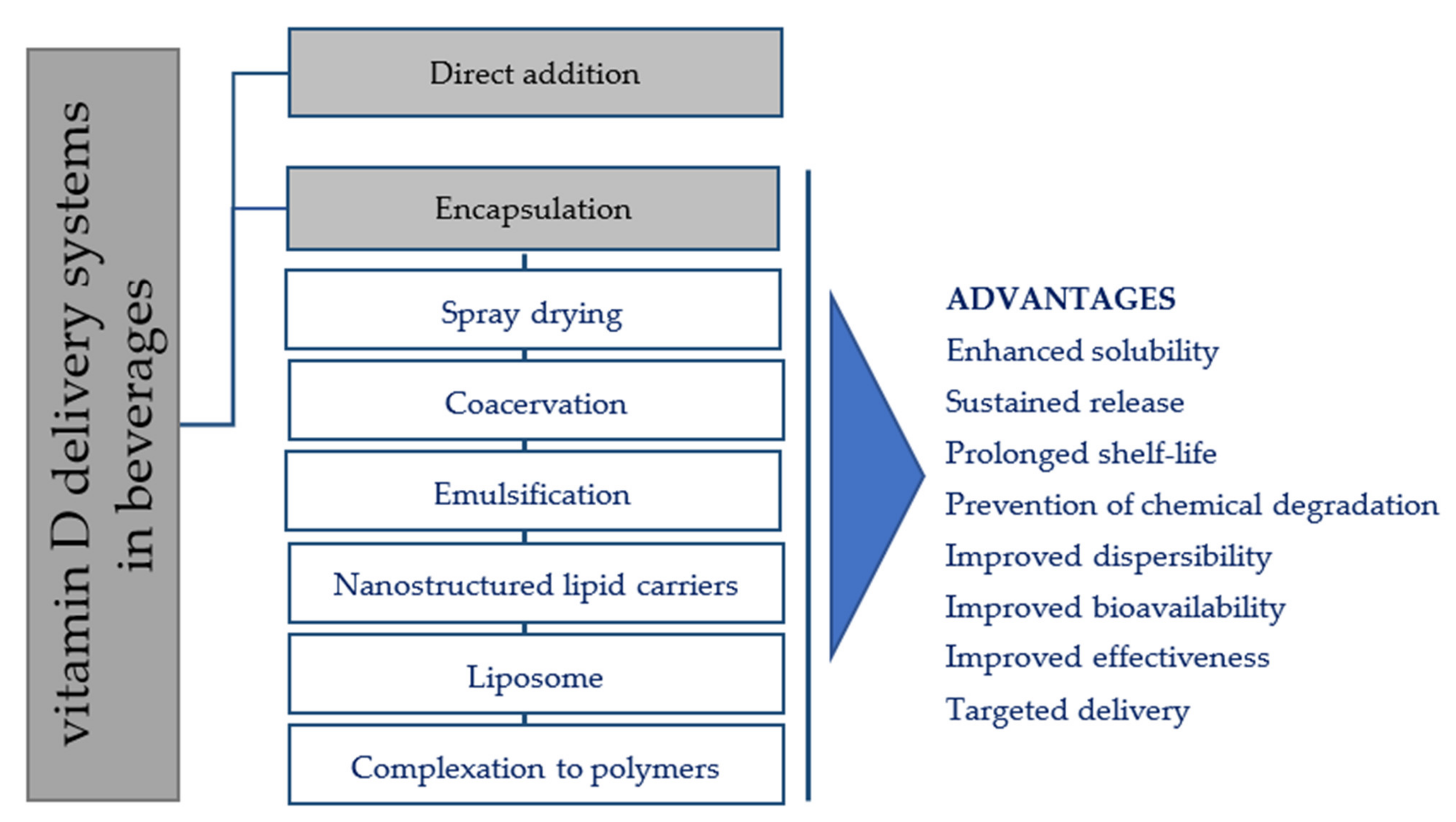
3. Fortification Strategies of Vitamin D in Beverages
3.1. Direct Addition of Vitamin D
3.2. Vitamin D Encapsulation Techniques
3.2.1. Spray Drying Technique
3.2.2. Coacervation Technique
3.2.3. Emulsification Technique
3.2.4. Nanostructured Lipid Carriers (NLC)
3.2.5. Liposome
3.3. Vitamin D Polymers Complexation
4. Stability, Bioaccessibility, and Bioavailability of Vitamin D-Fortified Beverages
5. Regulation of Vitamin D-Fortified Beverages
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization and Food and Agriculture Organization of the United Nations. Joint FAO/WHO expert consultation on human vitamin and mineral requirements. In Vitamin and Mineral Requirements for Human Nutrition, 2nd ed.; World Health Organization: Bangkok, Thailand, 2004. [Google Scholar]
- Nair, R.; Maseeh, A. Vitamin D: The “sunshine” vitamin. J. Pharmacol. Pharmacother. 2012, 3, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; de Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.E.; Abrams, S.A.; Aloia, J.; Bergeron, G.; Bourassa, M.W.; Brown, K.H.; Calvo, M.S.; Cashman, K.D.; Combs, G.; De-Regil, L.M.; et al. Global prevalence and disease burden of vitamin D deficiency: A roadmap for action in low-and middle-income countries. Ann. N. Y. Acad. Sci. 2018, 1430, 44–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, S.; Livney, Y.D. Potato protein based nanovehicles for health promoting hydrophobic bioactives in clear beverages. Food Hydrocoll. 2016, 57, 229–235. [Google Scholar] [CrossRef]
- Pilz, S.; März, W.; Cashman, K.D.; Kiely, M.E.; Whiting, S.J.; Holick, M.F.; Grant, W.B.; Pludowski, P.; Hiligsmann, M.; Trummer, C.; et al. Rationale and plan for vitamin D food fortification: A review and guidance paper. Front Endocrinol. 2018, 9, 373. [Google Scholar] [CrossRef]
- Gupta, R.; Behera, C.; Paudwal, G.; Rawat, N.; Baldi, A.; Gupta, P. Recent advances in formulation strategies for efficient delivery of vitamin D. AAPS PharmSciTech 2019, 20, 11. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee to Review. Dietary Reference Intakes for Vitamin D and Calcium. In Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., del Valle, H.B., Eds.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Jannasari, N.; Fathi, M.; Moshtaghian, S.J.; Abbaspourrad, A. Microencapsulation of vitamin D using gelatin and cress seed mucilage: Production, characterization and in vivo study. Int. J. Biol. Macromol. 2019, 129, 972–979. [Google Scholar] [CrossRef]
- Jakobsen, J.; Melse-Boonstra, A.; Rychlik, M. Challenges to quantify total vitamin activity: How to combine the contribution of diverse vitamers? Curr. Dev. Nutr. 2019, 3, nzz086. [Google Scholar] [CrossRef]
- Riccardi, C.; Perrone, L.; Filomena Napolitano, F.; Sampaolo, S.; Melone, M.A.B. Understanding the biological activities of vitamin D in type 1 neurofibromatosis: New insights into disease pathogenesis and therapeutic design. Cancers 2020, 12, 2965. [Google Scholar] [CrossRef]
- Lavelli, V.; D’Incecco, P.; Pellegrino, L. Vitamin D incorporation in foods: Formulation strategies, stability, and bioaccessibility as affected by the food matrix. Foods 2021, 10, 1989. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Berisha, A.T.; Martucci, G.; Pilz, S. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Vitamin D and osteoporosis. In Vitamin D-Biochemical, Chemical and Clinical Aspects Related to Calcium Metabolism: Proceedings of the Third Workshop on Vitamin D, Asilomar, Pacific Grove, California, USA, January 1977; Norman, A.W., Schaefer, K., Coburn, J.W., de Luca, H.F., Fraser, D., Grigoleit, H.G., Herrath, D.V., Eds.; Walter de Gruyter GmbH & Co. KG: Pacific Grove, CA, USA, 2020; pp. 627–634. [Google Scholar]
- Latic, N.; Erben, R.G. Vitamin D and cardiovascular disease, with emphasis on hypertension, atherosclerosis, and heart failure. Int. J. Mol. Sci. 2020, 21, 6483. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Perrelli, A.; Ragni, A. Vitamin D deficiency and the risk of cerebrovascular disease. Antioxidants 2020, 9, 327. [Google Scholar] [CrossRef]
- Hong, S.-H.; Bin Kim, Y.; Choi, H.S.; Jeong, T.-D.; Kim, J.T.; Sung, Y.A. Association of vitamin D deficiency with diabetic nephropathy. Endocrinol. Metab. 2021, 36, 106–113. [Google Scholar] [CrossRef]
- Fernández-Barral, A.; Bustamante-Madrid, P.; Ferrer-Mayorga, G.; Barbáchano, A.; Larriba, M.J.; Muñoz, A. Vitamin D effects on cell differentiation and stemness in cancer. Cancers 2020, 12, 2413. [Google Scholar] [CrossRef]
- Menon, V.; Kar, S.K.; Suthar, N.; Nebhinani, N. Vitamin D and depression: A critical appraisal of the evidence and future directions. Indian J. Psychol. Med. 2020, 42, 11–21. [Google Scholar] [CrossRef]
- Christodoulou, S.; Goula, T.; Ververidis, A.; Drosos, G. Vitamin D and bone disease. Biomed. Res. Int. 2013, 2013, 396541. [Google Scholar] [CrossRef] [Green Version]
- Allen, L.; de Benoist, B.; Dary, O.; Hurrell, R. Guidelines on Food Fortification with Micronutrients; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Lam, I.T.Y.; Keller, H.H.; Pfisterer, K.; Duizer, L.; Stark, K.; Duncan, A.M. Micronutrient food fortification for residential care: A scoping review of current interventions. J. Am. Med. Dir. Assoc. 2016, 17, 588–595. [Google Scholar] [CrossRef]
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.; Bianchi, M.L.; Stepan, J.; Fuleihan, G.E.-H.; Bouillon, R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: A position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, 23–54. [Google Scholar] [CrossRef] [Green Version]
- Marques, M.F.; Marques, M.M.; Xavier, E.R. Food fortification: An alternative to meet the needs of micronutrients in the contemporary world. HU Rev. 2013, 38, 29–36. [Google Scholar]
- Maurya, V.K.; Bashir, K.; Aggarwal, M. Vitamin D microencapsulation and fortification: Trends and technologies. J. Steroid Biochem. Mol. Biol. 2020, 196, 105489. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, J.; Knuthsen, P. Stability of vitamin D in foodstuffs during cooking. Food Chem. 2014, 148, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Pezeshki, A.; Abbasi, M.M.; Ghanbarzadeh, B.; Hamishehkar, H. Vitamin D(3)-loaded nanostructured lipid carriers as a potential approach for fortifying food beverages; in vitro and in vivo evaluation. Adv. Pharm. Bull. 2017, 7, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Öztürk, B. Nanoemulsions for food fortification with lipophilic vitamins: Production challenges, stability, and bioavailability. Eur. J. Lipid. Sci. Technol. 2017, 119, 1500539. [Google Scholar] [CrossRef]
- Itkonen, S.T.; Erkkola, M.; Lamberg-Allardt, C.J. Vitamin D fortification of fluid milk products and their contribution to vitamin D intake and vitamin D status in observational studies—A review. Nutrients 2018, 10, 1054. [Google Scholar] [CrossRef] [Green Version]
- Calame, W.; Street, L.; Hulshof, T. Vitamin D serum levels in the UK Population, including a mathematical approach to evaluate the impact of vitamin D fortified ready-to-eat breakfast cereals: Application of the NDNS Database. Nutrients 2020, 12, 1868. [Google Scholar] [CrossRef]
- Souza, S.V.; Borges, N.; Vieira, E.F. Vitamin d-fortified bread: Systematic review of fortification approaches and clinical studies. Food Chem. 2022, 372, 131325. [Google Scholar] [CrossRef]
- Zahedirad, M.; Asadzadeh, S.; Nikooyeh, B.; Neyestani, T.R.; Khorshidian, N.; Yousefi, M.; Mortazavian, A.M. Fortification aspects of vitamin D in dairy products: A review study. Int. Dairy J. 2019, 94, 53–64. [Google Scholar] [CrossRef]
- Hanson, A.L.; Metzger, L.E. Evaluation of increased vitamin D fortification in high-temperature, short-time-processed 2% milk, UHT-processed 2% fat chocolate milk, and low-fat strawberry yogurt. J. Dairy Sci. 2010, 93, 801–807. [Google Scholar] [CrossRef] [Green Version]
- Neyestani, T.R.; Hajifaraji, M.; Omidvar, N.; Nikooyeh, B.; Eshraghian, M.R.; Shariatzadeh, N.; Kalayi, A.; Khalaji, N.; Zahedirad, M.; Abtahi, M.; et al. Calcium-vitamin D-fortified milk is as effective on circulating bone biomarkers as fortified juice and supplement but has less acceptance: A randomised controlled school-based trial. J. Hum. Nutr. Diet. 2014, 27, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Khadgawat, R.; Marwaha, R.K.; Garg, M.K.; Ramot, R.; Oberoi, A.K.; Sreenivas, V.; Gahlot, M.; Mehan, N.; Mathur, P.; Gupta, N. Impact of vitamin D fortified milk supplementation on vitamin D status of healthy school children aged 10–14 years. Osteoporos. Int. 2013, 24, 2335–2343. [Google Scholar] [CrossRef] [PubMed]
- Syama, M.A.; Arora, S.; Gupta, C.; Sharma, A.; Sharma, V. Enhancement of vitamin D2 stability in fortified milk during light exposure and commercial heat treatments by complexation with milk proteins. Food Biosci. 2019, 29, 17–23. [Google Scholar] [CrossRef]
- Kaushik, R.; Sachdeva, B.; Arora, S. Vitamin D2 stability in milk during processing, packaging and storage. LWT-Food Sci. Technol. 2014, 56, 421–426. [Google Scholar] [CrossRef]
- Maurya, V.K.; Aggarwal, M. Fabrication of nano-structured lipid carrier for encapsulation of vitamin D3 for fortification of ‘Lassi’; A milk based beverage. J. Steroid Biochem. Mol. Biol. 2019, 193, 105429. [Google Scholar] [CrossRef]
- Fauziyyah, F.; Panunggal, B.; Afifah, D.N.; Rustanti, N.; Anjani, G. Microbiological characteristic and nutrition quality of goat milk kefir based on vitamin D3 fortification time. IOP Conf. Ser. Earth Environ. Sci. 2018, 116, 012040. [Google Scholar] [CrossRef]
- Tangpricha, V.; Koutkia, P.; Rieke, S.M.; Chen, T.C.; Perez, A.A.; Holick, M.F. Fortification of orange juice with vitamin D: A novel approach for enhancing vitamin D nutritional health. Am. J. Clin. Nutr. 2003, 77, 1478–1483. [Google Scholar] [CrossRef] [Green Version]
- Biancuzzo, R.M.; Young, A.; Bibuld, D.; Cai, M.H.; Winter, M.R.; Klein, E.K.; Ameri, A.; Reitz, R.; Salameh, W.; Chen, T.C.; et al. Fortification of orange juice with vitamin D(2) or vitamin D(3) is as effective as an oral supplement in maintaining vitamin D status in adults. Am. J. Clin. Nutr. 2010, 91, 1621–1626. [Google Scholar] [CrossRef] [Green Version]
- Economos, C.D.; Moore, C.E.; Hyatt, R.R.; Kuder, J.; Chen, T.; Meydani, S.N.; Meydani, M.; Klein, E.; Biancuzzo, R.M.; Holick, M.F. Multinutrient-fortified juices improve vitamin D and vitamin E status in children: A randomized controlled trial. J. Acad. Nutr. Diet 2014, 114, 709–717. [Google Scholar] [CrossRef]
- Dima, C.; Milea, A.S.; Constantin, O.E.; Stoica, M.; Stela Ivan, A.S.; Alexe, P.; Stanciuc, N. Fortification of pear juice with vitamin D3 encapsulated in polymer microparticles: Physico-chemical and microbiolgical characterization. J. Agroaliment. Process Technol. 2020, 26, 140–148. [Google Scholar]
- Zhang, H.; Önning, G.; Triantafyllou, A.Ö.; Öste, R. Nutritional properties of oat-based beverages as affected by processing and storage. J. Sci. Food Agric. 2007, 87, 2294–2301. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, J.; Dai, T.; Mundo, J.L.M.; Tan, Y.; Bai, L.; McClements, D.J. The gastrointestinal fate of inorganic and organic nanoparticles in vitamin D-fortified plant-based milks. Food Hydrocoll. 2021, 112, 106310. [Google Scholar] [CrossRef]
- Grant, J.; Ryland, D.; Isaak, C.K.; Prashar, S.; Siow, Y.L.; Taylor, C.G.; Aliani, M. Effect of Vitamin D(3) Fortification and saskatoon berry syrup addition on the flavor profile, acceptability, and antioxidant properties of rooibos tea (Aspalathus linearis). J. Food Sci. 2017, 82, 807–817. [Google Scholar] [CrossRef]
- Mehmood, T.; Ahmed, A. Tween 80 and soya-lecithin-based food-grade nanoemulsions for the effective delivery of vitamin D. Langmuir 2020, 36, 2886–2892. [Google Scholar] [CrossRef]
- Ron, N.; Zimet, P.; Bargarum, J.; Livney, Y.D. Beta-lactoglobulin–polysaccharide complexes as nanovehicles for hydrophobic nutraceuticals in non-fat foods and clear beverages. Int. Dairy J. 2010, 20, 686–693. [Google Scholar] [CrossRef]
- Sosa Henríquez, M.; Gómez de Tejada Romero, M.J. Cholecalciferol or calcifediol in the management of vitamin D deficiency. Nutrients 2020, 12, 1617. [Google Scholar] [CrossRef] [PubMed]
- Nikooyeh, B.; Neyestani, T.R. Poor vitamin D status increases the risk of anemia in school children: National Food and Nutrition Surveillance. Nutrition 2018, 47, 69–74. [Google Scholar] [CrossRef]
- Hohman, E.E.; Lachcik, P.J.; Gordon, D.T.; Fleet, J.C.; Weaver, C.M. Bioavailability and efficacy of vitamin D-2 from UV-irradiated yeast in growing, vitamin D-deficient rats. J. Agric. Food Chem. 2011, 59, 2341–2346. [Google Scholar] [CrossRef] [Green Version]
- Santos, M.B.; de Carvalho, C.W.P.; Garcia-Rojas, E.E. Microencapsulation of vitamin D3 by complex coacervation using carboxymethyl tara gum (Caesalpinia spinosa) and gelatin A. Food Chem. 2021, 343, 128529. [Google Scholar] [CrossRef]
- Golfomitsou, J.; Mitsou, E.; Xenakis, A.; Papadimitriou, V. Development of food grade O/W nanoemulsions as carriers of vitamin D for the fortification of emulsion based food matrices: A structural and activity study. J. Mol. Liq. 2018, 268, 734–742. [Google Scholar] [CrossRef]
- Seo, T.R.; Lee, I.; Chun, Y.-G.; Park, D.-J.; Lee, S.-H.; Kim, B.-K. Improved Stability of polyglycerol polyricinoleate-substituted nanostructured lipid carrier cholecalciferol emulsions with different carrier oils. J. Food Sci. 2019, 84, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Ghanbarzadeh, B.; Hamishehkar, H. Formulation of nanoliposomal vitamin D3 for potential application in beverage fortification. Adv. Pharm. Bull. 2014, 4, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Haham, M.; Ish-Shalom, S.; Nodelman, M. Stability and bioavailability of vitamin D nanoencapsulated in casein micelles. Food Funct. 2012, 3, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Levi, M.; Lesmes, U.; Margier, M.; Reboul, E.; Livney, Y.D. Re-assembled casein micelles improve in vitro bioavailability of vitamin D in a Caco-2 cell model. Food Funct. 2017, 8, 2133–2141. [Google Scholar] [CrossRef]
- Markman, G.; Livney, Y.D. Maillard-conjugate based core-shell co-assemblies for nanoencapsulation of hydrophobic nutraceuticals in clear beverages. Food Funct. 2012, 3, 262–270. [Google Scholar] [CrossRef]
- Abbasi, A.; Emam-Djomeh, Z.; Mousavi, M.A.E.; Davoodi, D. Stability of vitamin D3 encapsulated in nanoparticles of whey protein isolate. Food Chem. 2014, 143, 379–383. [Google Scholar] [CrossRef]
- Diarrassouba, F.; Garrait, G.; Remondetto, G.; Alvarez, P.; Beyssac, E.; Subirade, M. Increased stability and protease resistance of the β-lactoglobulin/vitamin D3 complex. Food Chem. 2014, 145, 646–652. [Google Scholar] [CrossRef]
- Lamsen, M.R.L.; Wang, T.; D’Souza, D.; Dia, V.; Chen, G.; Zhong, Q. Encapsulation of vitamin D3 in gum arabic to enhance bioavailability and stability for beverage applications. J. Food Sci. 2020, 85, 2368–2379. [Google Scholar] [CrossRef]
- Pedersen, J.N.; Frislev, H.S.; Pedersen, J.S.; Otzen, D.E. Using protein-fatty acid complexes to improve vitamin D stability. J. Dairy Sci. 2016, 99, 7755–7767. [Google Scholar] [CrossRef]
- Verduin, J.; Uijl, M.J.D.; Peters, R.J.B.; Bommel, M.R.V. Photodegradation products and their analysis in food. J. Food Sci. Nutr. 2020, 6, 1–16. [Google Scholar] [CrossRef]
- Yeh, E.B.; Barbano, D.M.; Drake, M. Vitamin fortification of fluid milk. J. Food Sci. 2017, 82, 856–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suganya, V.; Anuradha, V. Microencapsulation and nanoencapsulation: A review. Int. J. Pharm. Clin. Res. 2017, 9, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Do Amaral, P.H.R.; Andrade, P.L.; de Conto, L.C. Microencapsulation and its uses in food science and technology: A review. In Microencapsulation: Processes, Technologies and Industrial Applications; Salaün, F., Ed.; BoD–Books on Demand: Norderstedt, Germany, 2019; pp. 93–102. [Google Scholar]
- McClements, D.J.; Decker, E.A.; Park, Y.; Weiss, J. Structural design principles for delivery of bioactive components in nutraceuticals and functional foods. Crit. Rev. Food Sci. Nutr. 2009, 49, 577–606. [Google Scholar] [CrossRef] [PubMed]
- Nahum, V.; Domb, A.J. Recent developments in solid lipid microparticles for food ingredients delivery. Foods 2021, 10, 400. [Google Scholar] [CrossRef]
- Lovett, M.D. Calcium Chloride and Vitamin D Fortified Beverages: Bioavailability in Wistar Rats. Master’s Thesis, Faculty of North Carolina State University, Raleigh, CA, USA, 2007. Available online: https://www.lib.ncsu.edu/resolver/1840.16/2901 (accessed on 22 February 2022).
- Cerqueira, M.Â.; Pinheiro, A.C.; Ramos, O.L.; Silva, H.; Bourbon, A.I.; Vicente, A.A. Advances in food nanotechnology. In Emerging Nanotechnologies in Food Science; Busquets, R., Ed.; Elsevier: Boston, MA, USA, 2017; pp. 11–38. [Google Scholar] [CrossRef]
- Komaiko, J.S.; McClements, D.J. Formation of food-grade nanoemulsions using low-energy preparation methods: A review of available methods. Compr. Rev. Food Sci. Food Saf. 2016, 15, 331–352. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Sahani, S.; Sharma, Y.C. Advancements in applications of nanotechnology in global food industry. Food Chem. 2021, 342, 128318. [Google Scholar] [CrossRef]
- Akkam, Y.; Rababah, T.; Costa, R.; Almajwal, A.; Feng, H.; Laborde, J.E.A.; Abulmeaty, M.M.; Razak, S. Pea protein nanoemulsion effectively stabilizes vitamin D in food products: A potential supplementation during the COVID-19 pandemic. Nanomaterials 2021, 11, 887. [Google Scholar] [CrossRef]
- Park, S.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Development of nanostructured lipid carriers for the encapsulation and controlled release of vitamin D3. Food Chem. 2017, 225, 213–219. [Google Scholar] [CrossRef]
- Marsanasco, M.; Márquez, A.L.; Wagner, J.R.; Alonso, S.d.V.; Chiaramoni, N.S. Liposomes as vehicles for vitamins E and C: An alternative to fortify orange juice and offer vitamin C protection after heat treatment. Food Res. Int. 2011, 44, 3039–3046. [Google Scholar] [CrossRef]
- Ajeeshkumar, K.K.; Aneesh, P.A.; Raju, N.; Suseela, M.; Ravishankar, C.N.; Benjakul, S. Advancements in liposome technology: Preparation techniques and applications in food, functional foods, and bioactive delivery: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1280–1306. [Google Scholar] [CrossRef] [PubMed]
- Zarrabi, A.; Abadi, M.A.A.; Khorasani, S. Nanoliposomes and tocosomes as multifunctional nanocarriers for the encapsulation of nutraceutical and dietary molecules. Molecules 2020, 25, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiting, S.J.; Calvo, M.S. Vitamin D fortification and supplementation policies to correct vitamin D insufficiency/deficiency globally. In Vitamin D, 4th ed.; Feldman, D., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 2, pp. 91–108. [Google Scholar] [CrossRef]
- FDA Food Additives Status List. Available online: https://www.fda.gov/food/food-additives-petitions/food-additive-status-list (accessed on 22 February 2022).
- List of the Vitamins and Minerals Which May Be Added to Foods as Listed in Annex I of Regulation (EC) No 1925/2006 and as Amended by Commission Regulation (EC) No 1170/2009, Commission Regulation (EU) No 1161/2011, Commission Regulation (EU) No 119/2014 and Commission Regulation (EU) 2017/1203. Available online: https://ec.europa.eu/food/system/files/2021-01/labelling_nutrition-vitamins_minerals-comm_reg_en.pdf (accessed on 22 February 2022).

| Fortified Beverage | Country | Formulation | Fortification Level | Processing | Vitamin Stability and Bioaccessibility | Effects on Sensory Properties | Effects on Health | Ref. |
|---|---|---|---|---|---|---|---|---|
| HTST 2% fat milk | USA | Water dispersible VD3 | 250 IU/240 mL | HTST (73 °C for 15 s) and storage at 4 °C for 21, 42 and 60 days | Tolerate HTST No loss of VD3 during storage at 4 °C | No significant changes in composition and sensory attributes | NE | [34] |
| UHT 2% fat chocolate milk | USA | Water dispersible VD3 | 100 IU/240 mL | UHT (138 °C for 2 s) and storage at 4 °C for 21, 42 and 60 days | Tolerate UHT No loss of VD3 during storage at 4 °C | No significant changes in composition and sensory attributes | NE | [34] |
| Milk | Iran | ND | 100 IU/200 mL | NE | NE | Lower acceptance compared to orange juice | ↑[25(OH)D] serum levels | [35] |
| Milk | India | VD3 Spray Drying | 600 IU or 1000 IU/200 mL | NE | Stability loss <10% after 12 weeks of storage period | NE | ↑[25(OH)D] serum levels | [36] |
| UHT 3% and 8.5% fat milk | India | VD2-protein complexes (NaCas-VD, SNaCas-VD, RNaCas-VD and RSNaCas-VD) | 500 IU/L | Pasteurization (63 °C/30 min), boiling and sterilization (121 °C for 15 min at 15 psi) | Higher stability during storage at −20 °C, followed by 4 °C and 37 °C | NE | NE | [37] |
| Cow and buffalo milk | India | VD2 Encapsulation | 600 IU/L | Pasteurization (63 °C/30 min), boiling and sterilization (121 °C for 15 min at 15 psi) | Stable during pasteurization, boiling, sterilization, packaging, and storage conditions | NE | NE | [38] |
| “Lassi” milk-based beverage | India | VD3-NLC | 400 IU/100 mL | Environmental stress conditions of temperature and humidity, pH, and ionic strength | High physicochemical stability against temperature, pH, and ionic strength | No significant changes in composition and sensory attributes | NE | [39] |
| Goat milk kefir | Indonesia | VD3 | 42 IU/100 mL | Pasteurization at 72 °C for 15 s and cooling to 25 °C Different times of fermentation tested: 0, 6, 12, 18, and 24 h | The highest level of VD3 was found after 6 h of fermentation | Higher viscosity after 24 h of fermentation | NE | [40] |
| Orange juice and milk | USA | VD3 | 1000 IU/240 mL | NE | No loss of VD3 during 30 days of storage at 4 °C. The fat content of milk did not affect the bioavailability of VD3 | NE | ↑[25(OH)D] serum levels | [41] |
| Orange juice | USA | Water dispersible VD3 or VD2 | 1000 IU VD3 or VD2/240 mL orange juice or capsule | NE | VD2 and VD3 were equally bioavailable in orange juice and capsules | NE | ↑[25(OH)D] serum levels | [42] |
| Orange juice | USA | ND | 100 IU/240 mL | NE | NE | NE | ↑[25(OH)D] serum levels | [43] |
| Orange juice | Iran | ND | 100 IU/200 mL | NE | NE | Higher acceptance compared to orange juice | ↑[25(OH)D] serum levels | [35] |
| Pear juice | Romania | VD3-gum arabic- chitosan complex Spray drying | 0.002 g/100 mL | NE | No loss of VD3 during 7 days of storage at 4 °C | NE | NE | [44] |
| Oat-based beverage | Sweden | VD3 | 23 IU/100 g of liquid | Sterilization at 140 °C for 5 or 20 s | Stability loss of 60% | NE | NE | [45] |
| Almond and oat milks | ND | VD3 nanocellulose or TiO2 nanoemulsion | 0.4 wt% | NE | Low bioaccessibility (~20%) of VD3 loaded in VD3-nanocellulose or TiO2 nanoemulsion | Nanocellulose increased the shear viscosity, while TiO2 particles increased the whiteness of fortified milks | NE | [46] |
| Rooibos Tea | Canada | Water dispersible VD3 | 10,000 IU/200 mL | NE | ND | No significant changes in composition and sensory attributes High sensorial acceptance | NE | [47] |
| Technique | Preparation Method | Matrix Composition | Physico- Chemical Attributes | Fortification Level in Beverage | Main Observations | Ref. |
|---|---|---|---|---|---|---|
| Coacervation | Microencapsulation (VD3-cress seed mucilage–gelatine complex) | Optimum conditions: -core to shell ratio: 0.76 cress seed mucilage-to -gelatine volume ratio: 0.36; pH 3.4 | PS (μm) 137.22 ± 3.21 EE (%) 67.93 LC (%) 50.9 | NE | 28 and 70% VD3 delivery to gastric and intestinal media after 2 and 6 h, respectively Increase of body height, weight and 25(OH)D serum levels in male albino rats (6-week treatment) | [10] |
| Microencapsulation (VD3-carboxymethyl tara gum– gelatine A complex) | Optimum conditions: core to shell ratio: 1:2; carboxymethyl tara gum- gelatine A ratio: 6; pH 4.0 | PS (μm) 0.25 EE (%) 80 | NE | Bioaccessibility of 56% after in vitro digestion | [53] | |
| Nanoemulsion | High pressure homogenization (VD3-tween 20-soybean lecithin complex) | 0.8% (w/w) VD3 90% (w/w) water 4% (w/w) tween 20/ lecithin (3:1) 6% (w/w) soybean oil | Two populations of droplets: PS (nm) 146 ± 7 due the presence of surfactant micelles PS (nm) 21 ± 1 due the presence of micelles | Whole-fat milk 600 IU VD3/250 mL | Droplet diameter and PS of milk were not affected by the presence of the O/W nanoemulsion The fortified milk was stable under particle growth and gravitational separation for at least 10 days | [54] |
| Ultrasonic homogenization (VD3-tween 80-soybean lecithin complex) | 5% VD2 8% (w/w) canola oil 3% (w/w) tween 80 1% (w/w) soybean lecithin | PS (nm): <200 | NE | PS of 140.15 nm (4 °C) and 155.5 nm (25 °C) Stability of 74.4% and 55.3% (30 days storage at 4 °C and 25 °C, respectively) | [48] | |
| Blend of the oil phase (10% w/v) and the aqueous phase (90% w/v), followed by microfluidization | 60% (w/w) corn oil 40% (w/w) VD3 1% (w/w) quillaja saponin 1% (w/w) nanocellulose/TiO2 | PS (nm): 140 (nanocellulose); 600 (TiO2) ζ-Potential (mV): -39.4 ± 3.2 (nanocellulose); −35.0 ± 1.3 (TiO2) | Almond and oat milks 10% VD3 (w/v) | TiO2 nanoparticles were most effective at increasing the whiteness of the fortified milk, whereas the nanocellulose ones were most effective at increasing the shear viscosity Low VD3 bioaccessibility (≈20%) | [46] | |
| Nano- structured lipid carrier (NLC) | Hot homogenization technique | VD3 2.92–4% (w/v) precirol 2.92–4% (w/v) compritol 0.4–1.48% (w/v) miglyol 2–6% (w/v) tween20 1–6% (w/v) tween80 1–6% (w/v) poloxamer407 | PS (nm): 77–2504 Span value: 0.77–3.65 | NE | An optimum concentration of 3% of Poloxamer407 or 2% of Tween20 was sufficient to prevent agglomeration during the homogenization process VD3 intestinal absorption was enhanced by incorporating NLCs | [28] |
| Phase inversion-based cold water titration method | 20% (v/v) kolliphor 20% (v/v) CCTG 60% (v/v) water 2.5% (w/v) leciva 2% (w/v) VD3 5% (w/v) sodium chloride | PS (nm): 48.61 ± 1.58 ζ-Potential (mV): −17.310 ± 0.501 EE (%): 96.82 ± 0.31 VD3 release (%): 22.54 ± 0.33 | Lassi 400 IU VD3/100 mL | High stability under different environmental stress conditions (temperature, pH, and ionic strength) Higher level of sensorial acceptability compared to control | [39] | |
| Hot homogenization technique | 100 mg VD3 3 g soybean lecithin 2.5 g MCT oil 4 g GMS or PGPR 5 g of Poloxamer | PS (nm): 300–430 ζ-Potential (mV): −39.5 to−67.8 EE (%): 85.2−90.4 | NE | Higher VD3 stability under different environmental stress conditions (temperature, pH, and ionic strength) compared to control | [55] | |
| Nanoliposomes | Thin film hydration–sonication technique | 60:0, 50:10, 40:20, 30:30 (w/w) mixtures of lecithin and cholesterol 15 mL (2:1 v/v) EOH/MeOH 10 mL distilled water | PS (nm): 82–90 Span value: 0.70–0.85 ζ-Potential (mV): −29 to −43 EE (%): 93 | NE | High protection against VD3 degradation | [56] |
| Polymer complexation | Ultra-high-pressure homogenization (VD3–casein complex) | 162.5 mg/mL VD3 solution 1.25% (v/v) EtOH 10 mg/mL caseins 0.009 M K2HPO4 0.004 M tri- potassium citrate 0.011 M CaCl2 | PS (nm): 91 ± 8 | 1% fat milk 50 000 IU VD3/100 mL | High stability during thermal treatment (80 °C, 1 min) and 28-day cold storage (≈10%) compared to Tween-80-VD3 complex and unencapsulated VD3 -Increased 25(OH)D serum levels in humans | [57] |
| Ultra-high-pressure homogenization (VD3–casein complex) | 6.11 mg/mL sodium caseinate 8 mg/mL VD3 solution 1.2% EOH | PS (nm): 95 ± 2 –89 ± 0.3 | NE | High stability in gastric and upper-intestinal conditions, High bioavailability in vitro | [58] | |
| Vortex stirring for 30 s at room temperature (VD3–casein-maltodextrin complex) | 0.02% (w/w) casein in water 0.25% (v/v) EOH | PS (nm): <30 nm EE%: 90% | NE | Provide protection against degradation at low pH, and during shelf life at neutral pH and 4 °C | [59] | |
| Add dropwise, homogenization and freeze-drying (VD2–sodium caseinate complex) | VD2-casein-complexes: -VD2-NaCas -VD2-SnaCas -VD2-RnaCas -VD2-SNaCas | Milk 500 IU VD2/1000 mL | Stability up to 78.9% (−20 °C), 74.0% (4 °C) and 21.4% (37 °C) Higher stability for VD2-casein complexes and free-VD2 fortified milk stored in transparent glass bottles upon exposure to different light intensities VD2 stability of 90 and 67% when submitted to pasteurization (63 °C/30 min), boiling and sterilization (121 °C/15 min/15 psi) treatments, respectively | [37] | ||
| Girox method (VD3–whey protein isolate complex) | 8% (w/w) WPI solution 54 mg VD3/100 mL WPI solution 50 mM CaCl2 | PS (nm): 80.0–260 | NE | VD3 should be added to WIP solution before pH cycling. Presence of CaCl2 in nanoparticle composition reduces VD3 degradation during storage time. WPI–VD3 nanoparticles can be used for enriching of clear or non-clear drinks | [60] | |
| Homogenization (VD3–βLg complex) | 0.2% β-lactoglobulin solution 10 mg VD3 in 25 mL MeOH (2:1 βLg/VD3 complex) | VD3 release: 24.5 ± 0.73% and 40.9 ± 0.71% (absence and presence of pancreatin, respectively) | NE | Increased VD3 stability at 4 °C and UV light exposure Resistance to proteases in simulated GI digestion Increased 25(OH)D levels in rats fed with β-lactoglobulin-VD3 complex | [61] | |
| Add dropwise and vortexing (VD3–potato protein isolate complex) | Different concentrations of VD3 1 mg/mL potato protein stock solution (79 μM) | PS (nm): −33 to −116 | NE | Stability under different environmental stress conditions (during pasteurization, shelf life) Maintain optical clarity in aqueous solution (may be suitable for enrichment of clear beverages) | [6] | |
| Homogenization (VD2-βLg-low methoxyl pectin complex) | 0.05% (w/w) βLg solution 0–0.15% (w/w) low-methoxyl pectin solution 276 μL (5 mg/mL VD2 solution) per 100 mL protein solution | PS (nm): 49–88 ζ-Potential (mV): <−40 mV | NE | The lowest turbidity (0.035) was obtained at pH 4.25 and 0.05% pectin. The optimal system was transparent and suitable for enrichment of clear acid beverages. β-Lg-pectin nanocomplex provided higher protection against VD2 degradation and stability compared to the unprotected vitamin dispersion | [49] | |
| Sonication and spray drying (VD3–gum arabic–chitosan complex) | 9:1 (w/w) linseed oil/VD3 16% (w/w) gum arabic and chitosan as 9:1 (w/w) 1.5% (w/w) Tween 80 | PS (μm): 12.64 ± 1.14 EE%: 89.78 ± 3.88 | Pear juice 2 mg VD3/100 mL | Stability in quality parameters (antioxidant, physico-chemical and microbiological) after 7 days of storage at 4 °C | [44] | |
| Homogenization and freeze-drying (VD3–gum arabic complex) | 5.0% (w/v) gum arabic solution 5 mL VD3 at concentrations corresponding to 0.3, 0.6, 3.0 and 6.0% mass of gum arabic | PS (nm): 81.3 LC: 3.47% EE%: 61.24 ζ-Potential (mV): −3.1 to −31.0 mV (pH 2.0 to 7.4) | NE | Stability at pH 2.0–7.4 range (100 days at 3 °C) High bioaccessibility (95.76%) compared to nonencapsulated VD3 (68.98%) Increased 25(OH)D levels (>81 ng/mL) after 2-week supplementation (Sprague–Dawley rats) of 60 μg VD3/day | [62] | |
| Homogenization (VD3–αLa–oleate complex) | VD3 (280 μM) was mixed with 4 mg/mL of αLa-oleate complex | Complete solubilization of VD3, increase in stability under UV light 9-fold, and increase in long-term stability at 37 °C up to 1000-fold | NR | The liprotide was water soluble, transparent, and protected VD3 against elevated temperatures and UV light, but was not stable at ≤pH 6 -The liprotide was suitable for enrichment of clear beverages | [63] |
| Food (Serving) | USA | Canada | Finland | Australia |
|---|---|---|---|---|
| Vitamin D per Serving in μg (1 μg = 40 IU) | ||||
| Fluid cow’s milk (250 mL or 1 cup) | 2.5–5.0 a | 2.5–5.0 a | 2.5–5.0 a | 1.25 b |
| Orange juice with added calcium b (125 mL or 1/2 cup) | 1.25 | 1.25 | 1.25 | - |
| Plant-based milk (soy, oat, almond) b (250 mL or 1 cup) | 1.5–3.0 | 1.5–3.0 | 1.9–3.75 | - |
| Malted drink b (g powder) | 3.08 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, E.F.; Souza, S. Formulation Strategies for Improving the Stability and Bioavailability of Vitamin D-Fortified Beverages: A Review. Foods 2022, 11, 847. https://doi.org/10.3390/foods11060847
Vieira EF, Souza S. Formulation Strategies for Improving the Stability and Bioavailability of Vitamin D-Fortified Beverages: A Review. Foods. 2022; 11(6):847. https://doi.org/10.3390/foods11060847
Chicago/Turabian StyleVieira, Elsa F., and Suene Souza. 2022. "Formulation Strategies for Improving the Stability and Bioavailability of Vitamin D-Fortified Beverages: A Review" Foods 11, no. 6: 847. https://doi.org/10.3390/foods11060847
APA StyleVieira, E. F., & Souza, S. (2022). Formulation Strategies for Improving the Stability and Bioavailability of Vitamin D-Fortified Beverages: A Review. Foods, 11(6), 847. https://doi.org/10.3390/foods11060847







