Thinned Nectarines, an Agro-Food Waste with Antidiabetic Potential: HPLC-HESI-MS/MS Phenolic Characterization and In Vitro Evaluation of Their Beneficial Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sample Collection and Sample Preparation for HPLC Analyses
2.3. HPLC Analyses of Samples
2.3.1. Qualitative Polyphenols by HPLC-HESI-MS/MS
2.3.2. Quantitative Polyphenols Analysis by HPLC-DAD-FLD
2.3.3. HPLC-DAD-FLD Method Validation
2.4. Total Phenol Content (TPC)
2.5. Antioxidant Activity
2.5.1. DPPH• Radical Scavenging Assay
2.5.2. TEAC (Trolox Equivalent Antioxidant Capacity) Assay
2.5.3. Ferric Reducing/Antioxidant Power (FRAP) Assay
2.6. Antidiabetic Activity
2.6.1. α-Amylase Inhibitory Assay
2.6.2. Advanced Glycation End-Product (AGE) Inhibition
2.7. Statistics
3. Results
3.1. Qualitative Polyphenols Analysis by HPLC-HESI-MS/MS
3.1.1. Phenolic Acid Identification
3.1.2. Flavans Identification
3.1.3. Flavonols Identification
3.2. Quantitative Polyphenols Analysis by HPLC-DAD-FLD
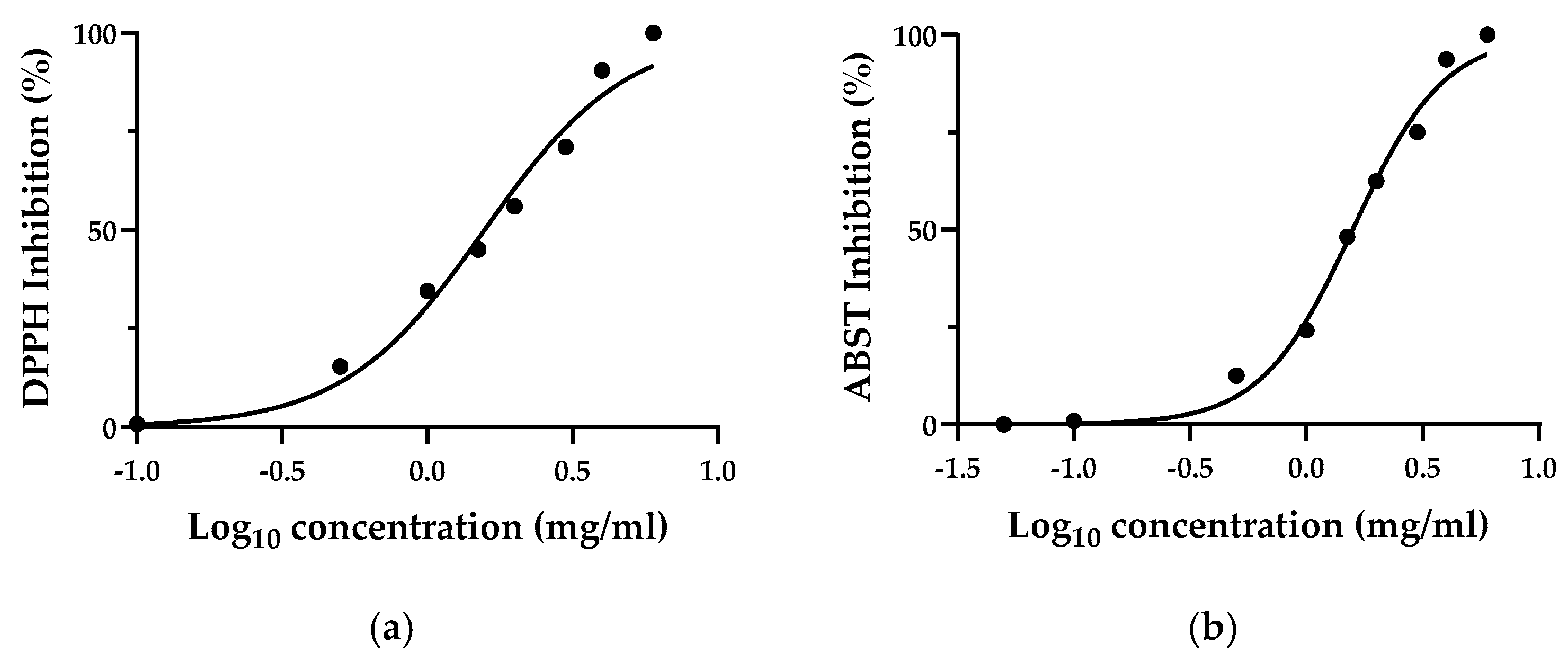
3.3. Total Polyphenols and In Vitro Antioxidant Activity of Thinned Nectarine
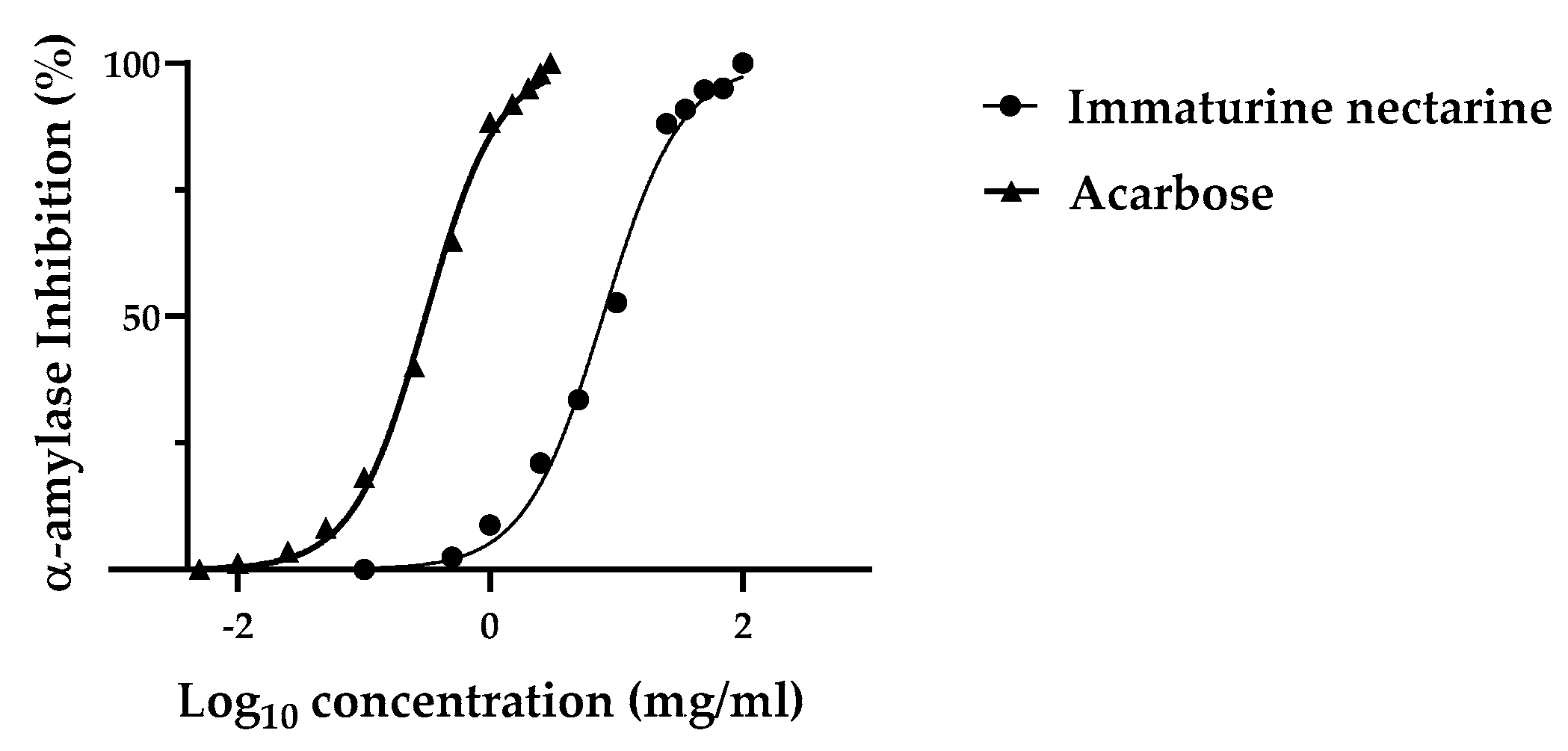
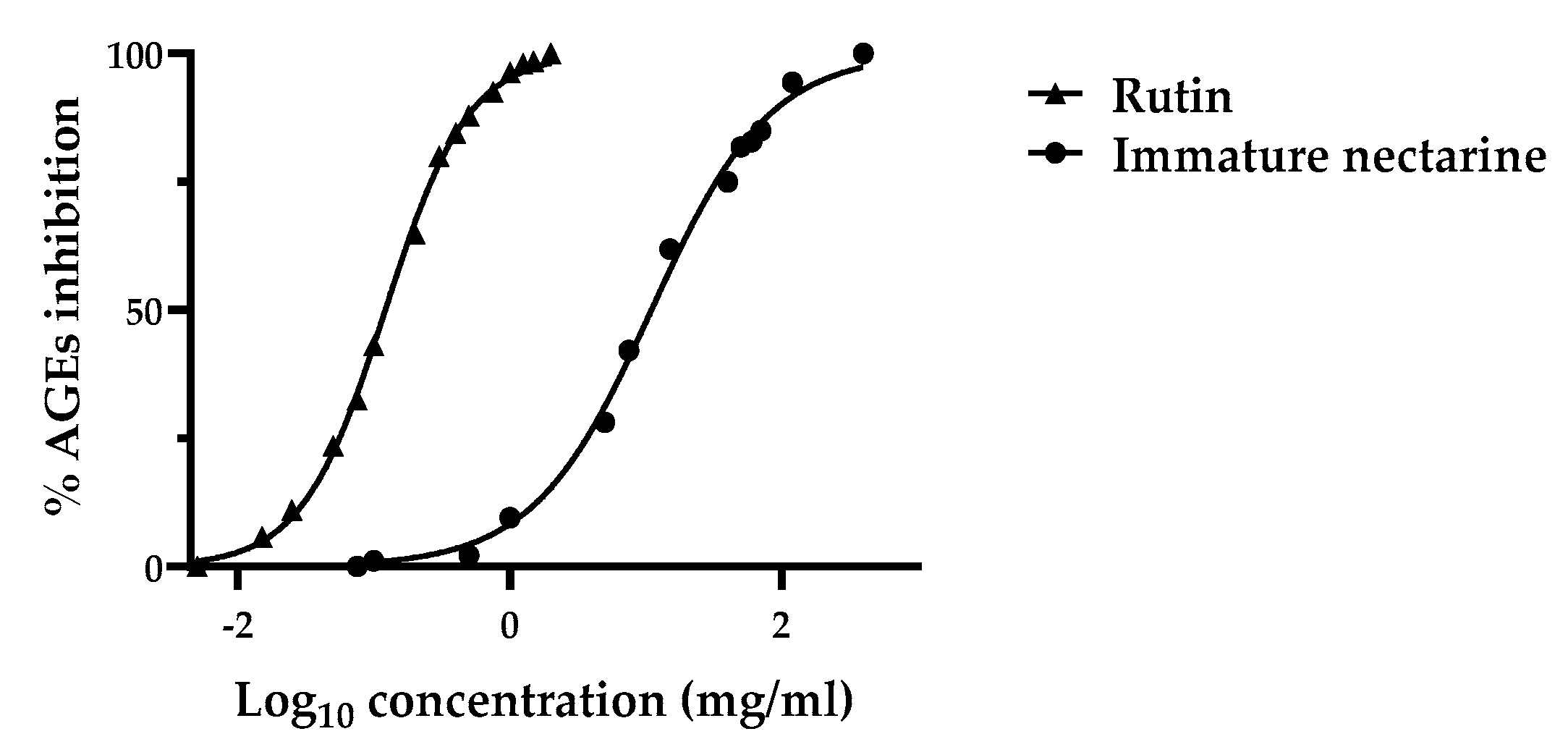
3.4. In Vitro Antidiabetic Activity
3.4.1. α-Amylase Inhibitory Assay
3.4.2. Advanced Glycation End-Product (AGE) Inhibition Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Aschner, P. New IDF clinical practice recommendations for managing type 2 diabetes in primary care. Diabetes Res. Clin. Pract. 2017, 132, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Krass, I.; Schieback, P.; Dhippayom, T. Adherence to diabetes medication: A systematic review. Diabet. Med. 2015, 32, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Mei Wong, J.L.; Sim, Y.J.; Wong, S.S.; Mohamed Elhassan, S.A.; Tan, S.H.; Ling Lim, G.P.; Rong Tay, N.W.; Annan, N.C.; Bhattamisra, S.K.; et al. Type 1 and 2 diabetes mellitus: A review on current treatment approach and gene therapy as potential intervention. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Ota, A.; Ulrih, N.P. An overview of herbal products and secondary metabolites used for management of type two diabetes. Front. Pharmacol. 2017, 8, 436. [Google Scholar] [CrossRef]
- Magnone, M.; Sturla, L.; Guida, L.; Spinelli, S.; Begani, G.; Bruzzone, S.; Fresia, C.; Zocchi, E. Abscisic acid: A conserved hormone in plants and humans and a promising aid to combat prediabetes and the metabolic syndrome. Nutrients 2020, 12, 1724. [Google Scholar] [CrossRef]
- Magnone, M.; Emionite, L.; Guida, L.; Vigliarolo, T.; Sturla, L.; Spinelli, S.; Buschiazzo, A.; Marini, C.; Sambuceti, G.; De Flora, A.; et al. Insulin-independent stimulation of skeletal muscle glucose uptake by low-dose abscisic acid via AMPK activation. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Brookbank, B.P.; Patel, J.; Gazzarrini, S.; Nambara, E. Role of basal aba in plant growth and development. Genes 2021, 12, 1936. [Google Scholar] [CrossRef]
- Leng, P.; Yuan, B.; Guo, Y.; Chen, P. The role of abscisic acid in fruit ripening and responses to abiotic stress. J. Exp. Bot. 2014, 65, 4577–4588. [Google Scholar] [CrossRef]
- Tenore, G.C.; Caruso, D.; D’avino, M.; Buonomo, G.; Caruso, G.; Ciampaglia, R.; Schiano, E.; Maisto, M.; Annunziata, G.; Novellino, E. A pilot screening of agro-food waste products as sources of nutraceutical formulations to improve simulated postprandial glycaemia and insulinaemia in healthy subjects. Nutrients 2020, 12, 1292. [Google Scholar] [CrossRef]
- Mengyuan, W.; Haoli, W.; Tingting, M.; Qian, G.; Yulin, F.; Xiangyu, S. Comprehensive Utilization of Thinned Unripe Fruits from Horticultural Crops. Foods 2021, 10, 2043. [Google Scholar]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Daliu, P.; Narciso, V.; Tenore, G.C.; Novellino, E. Colon bioaccessibility and antioxidant activity of white, green and black tea polyphenols extract after in vitro simulated gastrointestinal digestion. Nutrients 2018, 10, 1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maisto, M.; Annunziata, G.; Schiano, E.; Piccolo, V.; Iannuzzo, F.; Santangelo, R.; Ciampaglia, R.; Tenore, G.C.; Novellino, E.; Grieco, P. Potential Functional Snacks: Date Fruit Bars Supplemented by Different Species of Lactobacillus spp. Foods 2021, 10, 1760. [Google Scholar] [CrossRef]
- Carlo Tenore, G.; Caruso, D.; Buonomo, G.; D’avino, M.; Ciampaglia, R.; Maisto, M.; Schisano, C.; Bocchino, B.; Novellino, E. Lactofermented annurca apple puree as a functional food indicated for the control of plasma lipid and oxidative amine levels: Results from a randomised clinical trial. Nutrients 2019, 11, 122. [Google Scholar] [CrossRef] [Green Version]
- ICH Official. Available online: https://www.ich.org/ (accessed on 17 February 2022).
- Moon, J.K.; Shibamoto, T. Antioxidant assays for plant and food components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef]
- Babbar, N.; Oberoi, H.S.; Uppal, D.S.; Patil, R.T. Total phenolic content and antioxidant capacity of extracts obtained from six important fruit residues. Food Res. Int. 2011, 44, 391–396. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Surveswaran, S.; Cai, Y.Z.; Corke, H.; Sun, M. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chem. 2007, 102, 938–953. [Google Scholar] [CrossRef]
- Schisano, C.; Narciso, V.; Maisto, M.; Annunziata, G.; Grieco, P.; Sommella, E.M.; Tenore, G.C.; Novellino, E. In vitro effects of protein fractions from Controne beans (Phaseolus vulgaris L. ecotype Controne) on intestinal permeability, ACE and α-amylase activities. Eur. Food Res. Technol. 2019, 245, 2311–2322. [Google Scholar] [CrossRef]
- Poongunran, J.; Perera, H.K.I.; Jayasinghe, L.; Fernando, I.T.; Sivakanesan, R.; Araya, H.; Fujimoto, Y. Bioassay-guided fractionation and identification of α-amylase inhibitors from Syzygium cumini leaves. Pharm. Biol. 2017, 55, 206–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Justino, A.B.; Franco, R.R.; Silva, H.C.G.; Saraiva, A.L.; Sousa, R.M.F.; Espindola, F.S. B procyanidins of Annona crassiflora fruit peel inhibited glycation, lipid peroxidation and protein-bound carbonyls, with protective effects on glycated catalase. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Hong, Y.; Wang, Z.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. High-throughput screening and characterization of phenolic compounds in stone fruits waste by lc-esi-qtof-ms/ms and their potential antioxidant activities. Antioxidants 2021, 10, 234. [Google Scholar] [CrossRef] [PubMed]
- Sheng, F.; Hu, B.; Jin, Q.; Wang, J.; Wu, C.; Luo, Z. The analysis of phenolic compounds in walnut husk and pellicle by uplc-q-orbitrap hrms and hplc. Molecules 2021, 26, 3013. [Google Scholar] [CrossRef]
- Anton, D.; Bender, I.; Kaart, T.; Roasto, M.; Heinonen, M.; Luik, A.; Püssa, T. Changes in Polyphenols Contents and Antioxidant Capacities of Organically and Conventionally Cultivated Tomato (Solanum lycopersicum L.) Fruits during Ripening. Int. J. Anal. Chem. 2017, 2017, 2367453. [Google Scholar] [CrossRef] [Green Version]
- Fathoni, A.; Saepudin, E.; Cahyana, A.H.; Rahayu, D.U.C.; Haib, J. Identification of nonvolatile compounds in clove (Syzygium aromaticum) from Manado. AIP Conf. Proc. 2017, 1862, 030079. [Google Scholar] [CrossRef] [Green Version]
- Ncube, E.N.; Mhlongo, M.I.; Piater, L.A.; Steenkamp, P.A.; Dubery, I.A.; Madala, N.E. Analyses of chlorogenic acids and related cinnamic acid derivatives from Nicotiana tabacum tissues with the aid of UPLC-QTOF-MS/MS based on the in-source collision-induced dissociation method. Chem. Cent. J. 2014, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yuzuak, S.; Ballington, J.; Xie, D.Y. HPLC-qTOF-MS/MS-based profiling of flavan-3-ols and dimeric proanthocyanidins in berries of two muscadine grape hybrids FLH 13-11 and FLH 17-66. Metabolites 2018, 8, 57. [Google Scholar] [CrossRef] [Green Version]
- Rue, E.A.; Rush, M.D.; van Breemen, R.B. Procyanidins: A comprehensive review encompassing structure elucidation via mass spectrometry. Phytochem. Rev. 2018, 17, 1–16. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Jungfer, E.; Ritter, C.; Santiago-Schübel, B.; Thiele, B.; Fett, R.; Galensa, R. Characterization of flavan-3-ols in seeds of grape pomace by CE, HPLC-DAD-MS n and LC-ESI-FTICR-MS. Food Res. Int. 2012, 48, 848–855. [Google Scholar] [CrossRef] [Green Version]
- Barros, L.; Dueñas, M.; Pinela, J.; Carvalho, A.M.; Buelga, C.S.; Ferreira, I.C.F.R. Characterization and Quantification of Phenolic Compounds in Four Tomato (Lycopersicon esculentum L.) Farmers’ Varieties in Northeastern Portugal Homegardens. Plant Foods Hum. Nutr. 2012, 67, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Liu, R.; Liu, S.; Zhang, X.; Wang, Z.; Zhang, J.; Lu, J. HPLC-LTQ-orbitrap MSn profiling method to comprehensively characterize multiple chemical constituents in xiao-er-qing-jie granules. Anal. Methods 2015, 7, 7511–7526. [Google Scholar] [CrossRef]
- Sinosaki, N.B.M.; Tonin, A.P.P.; Ribeiro, M.A.S.; Poliseli, C.B.; Roberto, S.B.; da Silveira, R.; Visentainer, J.V.; Santos, O.O.; Meurer, E.C. Structural study of phenolic acids by triple quadrupole mass spectrometry with electrospray ionization in negative mode and H/D isotopic exchange. J. Braz. Chem. Soc. 2020, 31, 402–408. [Google Scholar] [CrossRef]
- Kuhnert, N.; Jaiswal, R.; Matei, M.F.; Sovdat, T.; Deshpande, S. How to distinguish between feruloyl quinic acids and isoferuloyl quinic acids by liquid chromatography/tandem mass spectrometry. RAPID Commun. MASS Spectrom. 2010, 24, 1575–1582. [Google Scholar] [CrossRef]
- Grieman, M.M.; Greaves, J.; Saltzman, E.S. A method for analysis of vanillic acid in polar ice cores. Clim. Past 2015, 11, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, R.; Kuhnert, N. How to identify and discriminate between the methyl quinates of chlorogenic acids by liquid chromatography-tandemmass spectrometry. J. Mass Spectrom. 2011, 46, 269–281. [Google Scholar] [CrossRef]
- Elsadig Karar, M.G.; Kuhnert, N. UPLC-ESI-Q-TOF-MS/MS Characterization of Phenolics from Crataegus monogyna and Crataegus laevigata (Hawthorn) Leaves, Fruits and their Herbal Derived Drops (Crataegutt Tropfen). J. Chem. Biol. Ther. 2016, 1. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Liang, F.; Bin, Y.; Li, P.; Duan, C. Screening non-colored phenolics in red wines using liquid chromatography/ultraviolet and mass spectrometry/mass spectrometry libraries. Molecules 2007, 12, 679–693. [Google Scholar] [CrossRef] [Green Version]
- Ben Said, R.; Hamed, A.I.; Mahalel, U.A.; Al-Ayed, A.S.; Kowalczyk, M.; Moldoch, J.; Oleszek, W.; Stochmal, A. Tentative characterization of polyphenolic compounds in the male flowers of Phoenix dactylifera by liquid chromatography coupled with mass spectrometry and DFT. Int. J. Mol. Sci. 2017, 18, 512. [Google Scholar] [CrossRef]
- De Beer, D.; Schulze, A.E.; Joubert, E.; Villiers, A.; Malherbe, C.J.; Stander, M.A. Food ingredient extracts of cyclopia subternata (Honeybush): Variation in phenolic composition and antioxidant capacity. Molecules 2012, 17, 14602–14624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza Mesquita, L.M.; e Paiva Caria, C.R.; Santos, P.S.; Ruy, C.C.; Da Silva Lima, N.; Taketa Moreira, D.K.; Da Rocha, C.Q.; Murador, D.C.; De Rosso, V.V.; Gambero, A.; et al. Modulatory effect of polyphenolic compounds from the mangrove tree rhizophora mangle l. On non-alcoholic fatty liver disease and insulin resistance in high-fat diet obese mice. Molecules 2018, 23, 2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.H.; Guo, H.; Xu, W.B.; Ge, J.; Li, X.; Alimu, M.; He, D.J. Rapid Identification of Flavonoid Constituents Directly from PTP1B Inhibitive Extract of Raspberry (Rubus idaeus L.) Leaves by HPLC-ESI-QTOF-MS-MS. J. Chromatogr. Sci. 2016, 54, 805–810. [Google Scholar] [CrossRef] [Green Version]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Singh, A.; Kumar, B. Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal. 2017, 7, 214–222. [Google Scholar] [CrossRef]
- Kang, J.; Price, W.E.; Ashton, J.; Tapsell, L.C.; Johnson, S. Identification and characterization of phenolic compounds in hydromethanolic extracts of sorghum wholegrains by LC-ESI-MSn. Food Chem. 2016, 211, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xiong, H.; Xu, X.; Xue, X.; Liu, M.; Xu, S.; Liu, H.; Gao, Y.; Zhang, H.; Li, X. Compounds identification in semen cuscutae by ultra-high-performance liquid chromatography (uplcs) coupled to electrospray ionization mass spectrometry. Molecules 2018, 23, 1199. [Google Scholar] [CrossRef] [Green Version]
- Koolen, H.H.F.; da Silva, F.M.A.; Gozzo, F.C.; de Souza, A.Q.L.; de Souza, A.D.L. Antioxidant, antimicrobial activities and characterization of phenolic compounds from buriti (Mauritia flexuosa L. f.) by UPLC-ESI-MS/MS. Food Res. Int. 2013, 51, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Masike, K.; Mhlongo, M.I.; Mudau, S.P.; Nobela, O.; Ncube, E.N.; Tugizimana, F.; George, M.J.; Madala, N.E. Highlighting mass spectrometric fragmentation differences and similarities between hydroxycinnamoyl-quinic acids and hydroxycinnamoyl-isocitric acids. Chem. Cent. J. 2017, 11, 1–7. [Google Scholar] [CrossRef]
- Bittner, K.; Rzeppa, S.; Humpf, H. Distribution and Quanti fi cation of Flavan-3-ols and Procyanidins with Low Degree of Polymerization in Nuts, Cereals, and Legumes. J. Agric. Food Chem. 2013, 61, 9148–9154. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem.-Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, R.H.; Halim, L. Antioxidant and antiproliferative activities of common edible nut seeds. LWT-Food Sci. Technol. 2009, 42, 1–8. [Google Scholar] [CrossRef]
- Guo, C.; Bi, J.; Li, X.; Lyu, J.; Zhou, M.; Wu, X. Antioxidant profile of thinned young and ripe fruits of Chinese peach and nectarine varieties. Int. J. Food Prop. 2020, 23, 1272–1286. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Tipton, K.F. Molecules Assessment of Enzyme Inhibition: A Review with Examples from the Development of Monoamine Oxidase and Cholinesterase Inhibitory Drugs. Molecules 2017, 22, 1192. [Google Scholar] [CrossRef] [Green Version]
- Kelley, D.E. Sugars and starch in the nutritional management of diabetes mellitus. Am. J. Clin. Nutr. 2003, 78, 858–864. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 541–555. [Google Scholar] [CrossRef]
- Sun, L.; Warren, F.J.; Gidley, M.J. Natural products for glycaemic control: Polyphenols as inhibitors of alpha-amylase. Trends Food Sci. Technol. 2019, 91, 262–273. [Google Scholar] [CrossRef]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxid. Med. Cell. Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef] [Green Version]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Review Article Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef] [Green Version]
- Singh Jaggi, A.; Parkash Singh, V.; Bali, A.; Singh, N. Advanced Glycation End Products and Diabetic Complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [Green Version]
- González, I.; Morales, M.A.; Rojas, A. Polyphenols and AGEs/RAGE axis. Trends and challenges. Food Res. Int. 2020, 129, 108843. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.J.; Hsia, S.M.; Lee, W.H.; Wu, C.H. Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings. J. Food Drug Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, L.; Della Posta, S.; Fanali, C.; Dugo, L.; De Gara, L.; Gugliucci, A. Antioxidant and Antiglycation Effects of Polyphenol Compounds Extracted from Hazelnut Skin on Advanced Glycation End-Products (AGEs) Formation. Antioxidants 2021, 10, 424. [Google Scholar] [CrossRef] [PubMed]
| Number | Compound | m/z | Diagnostic Fragmentation | Reference |
|---|---|---|---|---|
| 1 | Hydroxycaffeic acid | 195.01 | 177.12 [M-H-H2O]−–167.08 [M-H-CO]−– 151.00 [M-H-CO2]−–133.10 [M-H-CO2-H2O]− | [25] |
| 2 | Dihydroxybenzoic acid | 152.97 | 134.93 [M-H-H2O]−–124.97 [M-H-CO]−– 108.95 [M-H-CO2]−–96.99 [M-H-2CO]− | [25] |
| 3 | Gallic acid | 169.07 | 151.20 [M-H-H2O]−–140.93 [M-H-CO]−– 124.85 [M-H-CO2]− | [26] |
| 4 | Homovanillic acid O-hexoside | 343.01 | 325.13 [M-H-H2O]−–298.99 [M-H-CO2]−– 297.06 [M-H-CO-H2O]−–181.21 [M-H-Hex]− | [27] |
| 5 | Quinic acid | 190.97 | 172.95 [M-H-H2O]−–154.94 [M-H-2H2O]−– 146.82 [M-H-CO2]−–110.83 [M-H-CO2-2H2O]− | [28] |
| 6 | Neochlorogenic acid | 353.30 | 334.89 [M-H-H2O]−–191.02 [QA-H]−– 178.98 [CA-H]−–134.85 [CA-H-CO2]− | [29] |
| 7 | Procyanidin B1 | 577.14 | 451.23 [M-H-C6H6O3]−–425.17 [M-H-C8H8O3]−– 288.98 [M-H-C15H12O6]−–287.07 [M-H-C15H14O6]− | [30,31] |
| 8 | Procyanidin B3 | 577.45 | 451.19 [M-H-C6H6O3]−–425.25 [M-H-C8H8O3]−– 289.04 [M-H-C15H12O6]−–287.09 [M-H-C15H14O6]− | [30,31] |
| 9 | Catechin | 289.12 | 271.03 [M-H-H2O]−–245.02 [M-H-C2H4O]−– 205.00 [M-H-C4H4O2]−–137.00 [M-H-C8H8O3]− | [30,32] |
| 10 | Coumaric acid O-hexoside | 325.15 | 307.26 [M-H-H2O]−–289.03 [M-H-2H2O]−– 162.98 [M-H-Hex]−–118.97 [M-H-Hex-CO2]− | [33] |
| 11 | 3-O-Coumaroylquinic acid | 337.25 | 301.77 [M-H-2H2O]−–191.02 [QA-H]−– 173.02 [QA-H-H2O]−–162.92 [M-H-QA]− | [34] |
| 12 | Procyanidin C-type | 865.25 | 739.34 [M-H-C6H6O3]−–713.20 [M-H-C8H8O3]−– 695.19 [M-H-C8H8O3-H2O]−–286.94 [M-H-C30H26O12]− | [31] |
| 13 | Chlorogenic acid | 353.15 | 335.24 [M-H-H2O]−–190.97 [QA-H]−– 179.07 [CA-H]−–134.98 [CA-H-CO2]− | [29] |
| 14 | Caffeic acid | 178.92 | 160.96 [M-H-H2O]−–150.80 [M-H-CO]−– 134.95 [M-H-CO2]−–106.86 [M-H-CO-CO2]− | [35] |
| 15 | 3-O-Feruloylquinic acid | 367.18 | 193.04 [M-H-QA]−–191.13 [QA-H]−– 173.02 [QA-H-H2O]−–134.02 [M-H-QA-CH3-CO2]− | [36] |
| 16 | Vanillic acid | 167.14 | 151.90 [M-H-CH3]−–132.92 [M-H-CH3-H2O]−– 122.96 [M-H-CO2]−–107.90 [M-H-CH3-CO2]− | [37] |
| 17 | Methyl 3-O-Caffeoylquinate | 367.25 | 335.09 [M-H-2H2O]−–191.10 [QA-H]−– 161.00 [M-H-QA-CH3-H2O]−–134.97 [M-H-QA-CH3-CO2]− | [38] |
| 18 | 4-O-Coumaroylquinic acid | 337.12 | 191.13 [QA-H]−–172.86 [QA-H-H2O]−– 162.98 [M-H-QA]−–111.03 [QA-H-2H2O]− | [34] |
| 19 | Trans-5-O-Coumaroylquinic acid | 337.20 | 190.96 [QA-H]−–173.02 [QA-H-H2O]−– 162.94 [M-H-QA]−–111.05 [QA-H-2H2O]− | [34,39] |
| 20 | Syringic acid | 196.99 | 179.05 [M-H-H2O]−–168.97 [M-H-CO]−– 160.82 [M-H-2H2O]−–152.99 [M-H-CO2]− | [40] |
| 21 | Cis-5-O-Coumaroylquinic acid | 337.13 | 190.98 [QA-H]−–172.84 [QA-H-H2O]−– 163.05 [M-H-QA]−–145.02 [M-H-QA-H2O]− | [34,39] |
| 22 | Methyl 4-O-Caffeoylquinate | 367.18 | 296.85 [M-H-C3H2O2]−–191.13 [QA-H]−– 160.98 [M-H-QA-CH3-H2O]−–134.92 [M-H-QA-CH3-CO2]− | [38] |
| 23 | Caffeoylshikimic acid isomer 1 | 335.18 | 317.03 [M-H-H2O]−–179.01 [M-H-SA]−– 161.04 [M-H-SA-H2O]−–134.93 [M-H-SA-CO2]− | [41] |
| 24 | Procyanidin B2 | 577.25 | 451.13 [M-H-C6H6O3]−–425.07 [M-H-C8H8O3]−– 289.10 [M-H-C15H12O6]−–287.14 [M-H-C15H14O6]− | [30,31] |
| 25 | Caffeoylshikimic acid isomer 2 | 335.12 | 317.32 [M-H-H2O]−–178.99 [M-H-SA]−– 160.90 [M-H-SA-H2O]−–134.98 [M-H-SA-CO2]− | [41] |
| 26 | Caffeoylshikimic acid isomer 3 | 335.17 | 317.09 [M-H-H2O]−–179.04 [M-H-SA]−– 161.01 [M-H-SA-H2O]−–134.88 [M-H-SA-CO2]− | [41] |
| 27 | 5-O-Feruloylquinic acid | 367.19 | 349.23 [M-H-H2O]−–190.95 [QA-H]−– 172.97 [QA-H-H2O]−–134.09 [M-H-QA-CH3-CO2]− | [36] |
| 28 | Epicatechin | 288.92 | 271.06 [M-H-H2O]−–245.06 [M-H-C2H4O]−– 205.06 [M-H-C4H4O2]−–136.94 [M-H-C8H8O3]− | [30,32] |
| 29 | p-Coumaric acid | 162.88 | 144.92 [M-H-H2O]−–134.83 [M-H-CO]−– 118.97 [M-H-CO2]−–93.08 [M-H-CO2-C2H2]− | [35] |
| 30 | Procyanidin C-type linkage | 865.45 | 739.33 [M-H-C6H6O3]−–713.18 [M-H-C8H8O3]−– 695.28 [M-H-C8H8O3-H2O]−–287.11 [M-H-C30H26O12]− | [31] |
| 31 | Methyl 5-O-Caffeoylquinate | 367.19 | 191.02 [QA-H]−–179.00 [M-H-QA-CH3]−– 161.02 [M-H-QA-CH3-H2O]−–135.00 [M-H-QA-CH3-CO2]− | [38] |
| 32 | Procyanidin C1 | 865.32 | 739.19 [M-H-C6H6O3]−–713.19 [M-H-C8H8O3]−– 695.25 [M-H-C8H8O3-H2O]−–287.04 [M-H-C30H26O12]− | [31] |
| 33 | Procyanidin C-type linkage | 865.22 | 739.19 [M-H-C6H6O3]−–713.26 [M-H-C8H8O3]−– 695.32 [M-H-C8H8O3-H2O]−–287.11 [M-H-C30H26O12]− | [31] |
| 34 | Ferulic acid | 193.16 | 177.95 [M-H-CH3]−–160.02 [M-H-CH3-H2O]−– 148.94 [M-H-CO2]−–133.94 [M-H-CH3-CO2]− | [35] |
| 35 | Eriodyctiol O-hexoside | 449.20 | 431.11 [M-H-H2O]−–287.07 [M-H-Hex]−– 150.72 [M-H-Hex-C8H8O2]−–135.09 [M-H-Hex-C7H4O4]− | [42] |
| 36 | Rutin | 609.34 | 591.40 [M-H-H2O]−–463.22 [M-H-Rha]−– 301.16 [M-H-Glu-Rha]−–179.06 [M-H-Glu-Rha-C7H6O2]− | [43,44] |
| 37 | Naringenin | 271.04 | 253.07 [M-H-H2O]−–226.99 [M-H-CO2]−– 150.92 [M-H-C8H8O]−–106.92 [M-H-C8H8O-CO2]− | [45] |
| 38 | Quercetin-3-O-glucoside | 463.18 | 445.14 [M-H-H2O]−–343.04 [M-H-C4H8O4]−– 301.03 [M-H-Glu]−–179.09 [M-H-Hex-C7H6O2]− | [44] |
| 39 | Quercetin-O-glucoside isomer | 463.22 | 445.17 [M-H-H2O]−–343.17 [M-H-C4H8O4]−– 301.10 [M-H-Hex]−–178.97 [M-H-Hex-C7H6O2]− | [44] |
| 40 | Kaempferol-O-rutinoside isomer 1 | 593.32 | 447.21 [M-H-Pent]−–327.18 [M-H-Pent-C4H8O4]−– 285.10 [M-H-Hex-Pent]−–257.14 [M-H-Hex-Pent-CO]− | [46] |
| 41 | Naringenin O-hexoside | 433.23 | 415.26 [M-H-H2O]−–313.23 [M-H-C4H8O4]−– 271.11 [M-H-Hex]−–253.07 [M-H-Hex-H2O]− | [47] |
| 42 | Dicaffeoylquinic acid isomer 1 | 515.07 | 353.17 [M-H-CA]−–334.98 [M-H-CA-H2O]−– 317.22 [M-H-CA-2H2O]−–178.91 [M-H-CA-QA]− | [48] |
| 43 | Kaempferol-3-O-glucoside | 447.14 | 428.99 [M-H-H2O]−–327.07 [M-H-C4H8O4]−– 285.07 [M-H-Glu]−–255.01 [M-H-Glu-CH2O]− | [46] |
| 44 | Procyanidin dimer A-type linkage | 575.19 | 557.19 [M-H-H2O]−–449.13 [M-H-C6H6O3]−– 431.13 [M-H-C6H6O3-H2O]−–285.11 [M-H-C15H14O6]− | [30,31] |
| 45 | Quercetin | 301.18 | 273.17 [M-H-CO]−–257.07 [M-H-CO2]−– 179.11 [M-H-C7H6O2]−–150.88 [M-H-C8H6O3]− | [49] |
| 46 | Dicaffeoylquinic acid isomer 2 | 515.18 | 353.04 [M-H-CA]−–334.98 [M-H-CA-H2O]−– 317.09 [M-H-CA-2H2O]−–178.84 [M-H-CA-QA]− | [48] |
| 47 | Kaempferol-O-rutinoside isomer 2 | 593.34 | 447.15 [M-H-Pent]−–327.12 [M-H-Pent-C4H8O4]−– 284.95 [M-H-Hex-Pent]−–257.15 [M-H-Hex-Pent-CO]− | [46] |
| 48 | Kaempferol-O-rutinoside isomer 3 | 593.38 | 447.13 [M-H-Pent]−–327.09 [M-H-Pent-C4H8O4]−– 285.11 [M-H-Hex-Pent]−–256.83 [M-H-Hex-Pent-CO]− | [46] |
| Compound | Retention Time (min) | Mean Value ± SD (µg/g) |
|---|---|---|
| Gallic acid | 4.00 | 168.31 ± 1.51 |
| Neochlorogenic acid | 7.76 | 1456.98 ± 1.19 |
| Procyanidin B1 + Procyanidin B3 * | 12.70 | 8.41 ± 0.02 |
| Catechin | 13.34 | 128.32 ± 0.36 |
| Chlorogenic acid | 13.72 | 1496.85 ± 0.22 |
| Caffeic acid | 13.76 | 15.85 ± 0.06 |
| Vanillic acid | 14.78 | 19.28 ± 0.91 |
| Syringic acid | 17.20 | 115.16 ± 0.21 |
| Procyanidin B2 | 18.03 | 6.55 ± 0.01 |
| Epicatechin | 19.54 | 34.63 ± 0.83 |
| p-Coumaric acid | 20.65 | 5.05 ± 0.33 |
| Procyanidin C1 | 22.47 | 12.66 ± 0.01 |
| Ferulic acid | 24.39 | 10.59 ± 0.02 |
| Rutin | 28.27 | 48.86 ± 0.67 |
| Naringenin | 31.14 | 10.92 ± 0.42 |
| Quercetin-3-O-glucoside | 32.68 | 166.01 ± 3.35 |
| Kaempferol-3-O-glucoside | 36.84 | 63.65 ± 3.01 |
| Quercetin | 46.06 | 17.89 ± 0.41 |
| Antioxidant Activity (µmol TE/g TN ± SD) | ||
|---|---|---|
| DPPH Assay | ABTS Assay | FRAP Assay |
| 40.09 ± 0.14 | 63.26 ± 0.72 | 58.07 ± 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiano, E.; Piccolo, V.; Novellino, E.; Maisto, M.; Iannuzzo, F.; Summa, V.; Tenore, G.C. Thinned Nectarines, an Agro-Food Waste with Antidiabetic Potential: HPLC-HESI-MS/MS Phenolic Characterization and In Vitro Evaluation of Their Beneficial Activities. Foods 2022, 11, 1010. https://doi.org/10.3390/foods11071010
Schiano E, Piccolo V, Novellino E, Maisto M, Iannuzzo F, Summa V, Tenore GC. Thinned Nectarines, an Agro-Food Waste with Antidiabetic Potential: HPLC-HESI-MS/MS Phenolic Characterization and In Vitro Evaluation of Their Beneficial Activities. Foods. 2022; 11(7):1010. https://doi.org/10.3390/foods11071010
Chicago/Turabian StyleSchiano, Elisabetta, Vincenzo Piccolo, Ettore Novellino, Maria Maisto, Fortuna Iannuzzo, Vincenzo Summa, and Gian Carlo Tenore. 2022. "Thinned Nectarines, an Agro-Food Waste with Antidiabetic Potential: HPLC-HESI-MS/MS Phenolic Characterization and In Vitro Evaluation of Their Beneficial Activities" Foods 11, no. 7: 1010. https://doi.org/10.3390/foods11071010
APA StyleSchiano, E., Piccolo, V., Novellino, E., Maisto, M., Iannuzzo, F., Summa, V., & Tenore, G. C. (2022). Thinned Nectarines, an Agro-Food Waste with Antidiabetic Potential: HPLC-HESI-MS/MS Phenolic Characterization and In Vitro Evaluation of Their Beneficial Activities. Foods, 11(7), 1010. https://doi.org/10.3390/foods11071010











