Analysis of Alternative Shelf Life-Extending Protocols and Their Effect on the Preservation of Seafood Products
Abstract
:1. Introduction
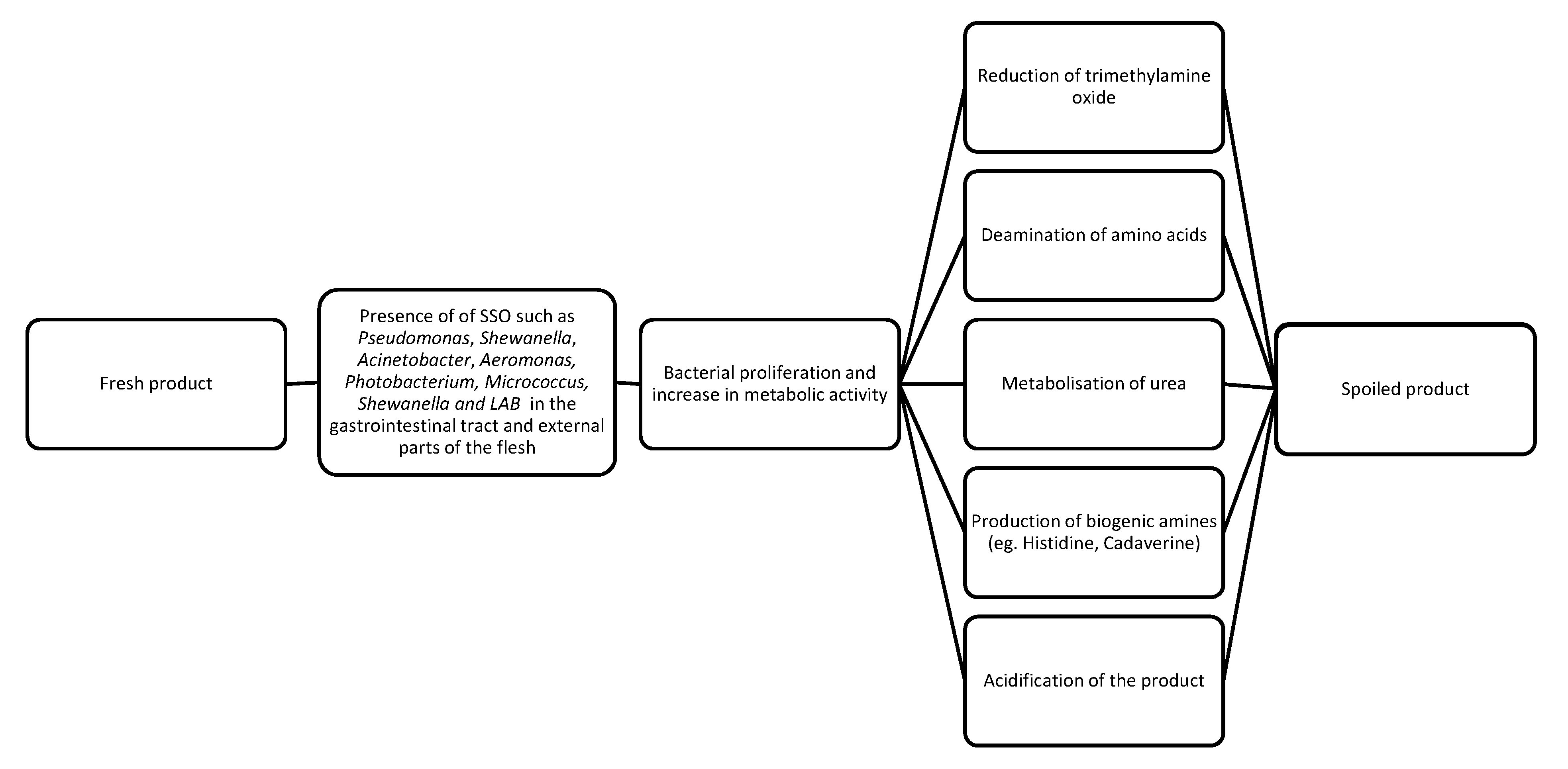
2. Degradation and Spoilage of Seafood Products
3. Strategies for Preservation of Seafood Products
3.1. Biodegradable Films, Edible Coatings, and Natural Preservatives
3.2. Superchilling
3.3. Ozonation
3.4. Irradiation Techniques
3.5. High-Pressure Processing and Hyperbaric Storage
3.6. Biopreservation
3.7. Comparative Analysis of Alternative Shelf Life-Extending Protocols
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, S.; Deshmukh, R. Seafood Market by Type (Fish, Crustaceans, Mollusca, Others), and by Application (Retail, Institutions and Food Service): Global Opportunity Analysis and Industry Forecast, 2020–2027. Allied Mark. Res. 2020, A02360, 51–64. Available online: https://www.alliedmarketresearch.com/seafood-market (accessed on 25 August 2021).
- Møretrø, T.; Moen, B.; Heir, E.; Hansen, A.; Langsrud, S. Contamination of salmon fillets and processing plants with spoilage bacteria. Int. J. Food Microbiol. 2016, 237, 98–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Pu, H.; Sun, D.W. Novel techniques for evaluating freshness quality attributes of fish: A review of recent developments. Trends Food Sci. Technol. 2019, 83, 259–273. [Google Scholar] [CrossRef]
- Fidalgo, L.G.; Lemos, Á.T.; Delgadillo, I.; Saraiva, J.A. Microbial and physicochemical evolution during hyperbaric storage at room temperature of fresh Atlantic salmon (Salmo salar). Innov. Food Sci. Emerg. Technol. 2018, 45, 264–272. [Google Scholar] [CrossRef]
- Anagnostopoulos, D.A.; Parlapani, F.F.; Boziaris, I.S. The evolution of knowledge on seafood spoilage microbiota from the 20th to the 21st century: Have we finished or just begun? Trends Food Sci. Technol. 2022, 120, 236–247. [Google Scholar] [CrossRef]
- He, Q.; Gong, B.; He, J.; Xiao, K. A novel superchilling storage-ice glazing (SS-IG) approach using anti-oxidative and antimicrobial essential oil (EO) for freshness-keeping of sea bass (Dicentrarchus labrax). Aquaculture 2019, 500, 243–249. [Google Scholar] [CrossRef]
- Getu, A.; Misganaw, K. Post-harvesting and Major Related Problems of Fish Production. Fish. Aquac. J. 2015, 6, 1000154. [Google Scholar] [CrossRef]
- Mesnildrey, L.; Lesueur, M.; Gouin, S. Behaviour, Motivations and Needs of Consumers of Fresh Seafood Products: New Opportunities and Marketing Strategies. In Proceedings of the IIFET 2010 Montpellier Proceedings, Montpellier, France, 13–16 July 2010. [Google Scholar]
- EUR-Lex-32002R0178-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2002/178/oj (accessed on 7 February 2022).
- Rezaei, F.; Shahbazi, Y. Shelf-life extension and quality attributes of sauced silver carp fillet: A comparison among direct addition, edible coating and biodegradable film. LWT-Food Sci. Technol. 2018, 87, 122–133. [Google Scholar] [CrossRef]
- Giarratana, F.; Muscolino, D.; Beninati, C.; Ziino, G.; Giuffrida, A.; Panebianco, A. Activity of R(+) limonene on the maximum growth rate of fish spoilage organisms and related effects on shelf-life prolongation of fresh gilthead sea bream fillets. Int. J. Food Microbiol. 2016, 237, 109–113. [Google Scholar] [CrossRef]
- Montiel, R.; Alba, M.; Bravo, D.; Gaya, P.; Medina, M. Effect of high pressure treatments on smoked cod quality during refrigerated storage. Food Control 2012, 23, 429–436. [Google Scholar] [CrossRef]
- Eliasson, S.; Arason, S.; Margeirsson, B.; Bergsson, A.B.; Palsson, O.P. The effects of superchilling on shelf-life and quality indicators of whole Atlantic cod and fillets. LWT-Food Sci. Technol. 2019, 100, 426–434. [Google Scholar] [CrossRef]
- Huang, H.W.; Hsu, C.P.; Yang, B.B.; Wang, C.Y. Potential Utility of High-Pressure Processing to Address the Risk of Food Allergen Concerns. Compr. Rev. Food Sci. Food Saf. 2014, 13, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Rode, T.M.; Rotabakk, B.T. Extending shelf life of desalted cod by high pressure processing. Innov. Food Sci. Emerg. Technol. 2021, 69, 102476. [Google Scholar] [CrossRef]
- Ebadi, Z.; Khodanazary, A.; Hosseini, S.M.; Zanguee, N. The shelf life extension of refrigerated Nemipterus japonicus fillets by chitosan coating incorporated with propolis extract. Int. J. Biol. Macromol. 2019, 139, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, M.; Jami, M.; Domig, K.J.; Kneifel, W. Seafood biopreservation by lactic acid bacteria—A review. LWT-Food Sci. Technol. 2013, 54, 315–324. [Google Scholar] [CrossRef]
- Pan, H.; Yu, Q.; Qian, C.; Shao, H.; Han, J.; Li, Y.; Lou, Y. Effects of different doses of electron beam irradiation on bacterial community of Portunus trituberculatus. Food Biosci. 2021, 42, 101198. [Google Scholar] [CrossRef]
- Gram, L.; Huss, H.H. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 1996, 33, 121–137. [Google Scholar] [CrossRef]
- Kuuliala, L.; Abatih, E.; Ioannidis, A.G. Multivariate statistical analysis for the identification of potential seafood spoilage indicators. Food Control 2018, 84, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Erkmen, O.; Bozoglu, T.F. Chapter 18 Spoilage of Fish and other Seafoods. In Food Microbiology: Principles into Practice. Volume 1: Microorganisms Related to Foods, Foodborne Diseases, and Food Spoilage; Erkmen, O., Bozoglu, T.F., Eds.; John Wiley & Sons, Ltd.: West Sussex, UK, 2016; pp. 301–306. [Google Scholar] [CrossRef]
- Odeyemi, O.A.; Burke, C.M.; Bolch, C.C.J.; Stanley, R. Seafood spoilage microbiota and associated volatile organic compounds at different storage temperatures and packaging conditions. Int. J. Food Microbiol. 2018, 280, 87–99. [Google Scholar] [CrossRef]
- Boziaris, I.S.; Parlapani, F.F. Specific Spoilage Organisms (SSOs) in Fish. In The Microbiological Quality of Food: Foodborne Spoilers; Bevilaqua, A., Corbo, M.R., Singaglia, M., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 61–98. [Google Scholar] [CrossRef]
- Leroi, F.; Joffraud, J.J. Microbial degradation of seafood. In Aquaculture Microbiology and Biotechnology; Montet, D., Ray, R., Eds.; CRC Press: Boca Ranton, FL, USA, 2011; pp. 47–72. [Google Scholar] [CrossRef]
- Lou, X.; Zhai, D.; Yang, H. Changes of metabolite profiles of fish models inoculated with Shewanella baltica during spoilage. Food Control 2021, 123, 107697. [Google Scholar] [CrossRef]
- Keow, C.M.; Abu Bakar, F.; Salleh, A.B.; Heng, L.Y.; Wagiran, R.; Bean, L.S. An amperometric biosensor for the rapid assessment of histamine level in tiger prawn (Penaeus monodon) spoilage. Food Chem. 2007, 105, 1636–1641. [Google Scholar] [CrossRef]
- Comas-Basté, O.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.; Vidal-Carou, M.D.C. Histamine Intolerance: The Current State of the Art. Biomolecules 2020, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- la Bella, G.; Martella, V.; Basanisi, M.G.; Nobili, G.; Terio, V.; la Salandra, G. Food-Borne Viruses in Shellfish: Investigation on Norovirus and HAV Presence in Apulia (SE Italy). Food Environ. Virol. 2017, 9, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.; Parisi, A.; Conversano, M.C. Food-Borne Bacteria Associated with Seafoods: A Brief Review. J. Food Qual. Hazards Control 2020, 7, 4–10. [Google Scholar] [CrossRef]
- Iwamoto, M.; Ayers, T.; Mahon, B.E.; Swerdlow, D.L. Epidemiology of Seafood-Associated Infections in the United States. Clin. Microbiol. Rev. 2010, 23, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529. [Google Scholar] [CrossRef]
- Aibinu, I.E.; Smooker, P.M.; Lopata, A.L. Anisakis Nematodes in Fish and Shellfish—From infection to allergies. Int. J. Parasitol. Parasites Wildl. 2019, 9, 384. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.A. Escherichia coli in seafood: A brief overview. Adv. Biosci. Biotechnol. 2013, 4, 450–454. [Google Scholar] [CrossRef] [Green Version]
- Vongkamjan, K.; Benjakul, S.; Kim Vu, H.T.; Vuddhakul, V. Longitudinal monitoring of Listeria monocytogenes and Listeria phages in seafood processing environments in Thailand. Food Microbiol. 2017, 66, 11–19. [Google Scholar] [CrossRef]
- Bekhit, A.; Holman, B.; Giteru, S.; Hopkins, D. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Papastergiadis, A.; Mubiru, E.; van Langenhove, H.; de Meulenaer, B. Malondialdehyde Measurement in Oxidized Foods: Evaluation of the Spectrophotometric Thiobarbituric Acid Reactive Substances (TBARS) Test in Various Foods. J. Agric. Food Chem. 2012, 60, 9589–9594. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dermiş, S.; Can, S.; Doru, B. Determination of Peroxide Values of Some Fixed Oils by Using the mFOX Method. Spectrosc. Lett. 2012, 45, 359–363. [Google Scholar] [CrossRef]
- Thakur, M.S.; Ragavan, K. Biosensors in food processing. J. Food Sci. Technol. 2013, 50, 625. [Google Scholar] [CrossRef] [Green Version]
- Gumpu, M.; Nesakumar, N.; Sethuraman, S.; Krishnan, U.; Rayappan, J. Determination of Putrescine in Tiger Prawn Using an Amperometric Biosensor Based on Immobilization of Diamine Oxidase onto Ceria Nanospheres. Food Bioprocess Technol. 2016, 9, 717–724. [Google Scholar] [CrossRef]
- Shi, C.; Yang, X.; Han, S.; Fan, B.; Zhao, Z.; Wu, X.; Qian, J. Nondestructive Prediction of Tilapia Fillet Freshness during Storage at Different Temperatures by Integrating an Electronic Nose and Tongue with Radial Basis Function Neural Networks. Food Bioprocess Technol. 2018, 11, 1840–1852. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, M.; Bhandari, B.; Adhikari, B. Application of electronic tongue for fresh foods quality evaluation: A review. Food Rev. Int. 2018, 34, 746–769. [Google Scholar] [CrossRef]
- Wu, D.; Sun, D.; He, Y. Novel non-invasive distribution measurement of texture profile analysis (TPA) in salmon fillet by using visible and near infrared hyperspectral imaging. Food Chem. 2014, 145, 417–426. [Google Scholar] [CrossRef]
- Tito, N.; Rodemann, T.; Powell, S. Use of near infrared spectroscopy to predict microbial numbers on Atlantic salmon. Food Microbiol. 2012, 32, 431–436. [Google Scholar] [CrossRef]
- Karsli, B.; Caglak, E.; Prinyawiwatkul, W. Effect of high molecular weight chitosan coating on quality and shelf life of refrigerated channel catfish fillets. LWT-Food Sci. Technol. 2021, 142, 111034. [Google Scholar] [CrossRef]
- Farsanipour, A.; Khodanazary, A.; Hosseini, S.M. Effect of chitosan-whey protein isolated coatings incorporated with tarragon Artemisia dracunculus essential oil on the quality of Scomberoides commersonnianus fillets at refrigerated condition. Int. J. Biol. Macromol. 2020, 155, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Zarandona, I.; López-Caballero, M.E.; Montero, M.P.; Guerrero, P.; de la Caba, K.; Gómez-Guillén, M.C. Horse mackerel (Trachurus trachurus) fillets biopreservation by using gallic acid and chitosan coatings. Food Control 2021, 20, 107511. [Google Scholar] [CrossRef]
- Korkmaz, F.; Kocaman, E.M.; Alak, G. Using of Quinoa Based Film to Extend the Shelf Life of Rainbow Trout Fillets under Cold Storage (4 ± 1 °C) Condition. Mar. Sci. Technol. Bull. 2019, 8, 76–84. [Google Scholar] [CrossRef]
- Esmaeili, M.; Khodanazary, A. Effects of pectin/chitosan composite and bi-layer coatings combined with Artemisia dracunculus essential oil on the mackerel’s shelf life. J. Food Meas. Charact. 2021, 15, 3367–3375. [Google Scholar] [CrossRef]
- Yerlikaya, P.; Yatmaz, H.A.; Topuz, O.K. Applications of Edible Films and Coatings in Aquatic Foods. In Innovative Technologies in Seafood Processing; Ozogul, Y., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 71–91. [Google Scholar] [CrossRef]
- Alejandra Bertuzzic, M.; Marcelo Slavutsky, A. Standard and New Processing Techniques Used in the Preparation of Films and Coatings at the Lab Level and Scale-Up. In Edible Films and Coatings: Fundamentals and Applications; Montero Garcia, M.P., Gómez-Guillén, M., López-Caballero, M.E., Barbosa-Cánovas, G.V., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 21–42. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Effects of chitosan coating on shelf-life of ready-to-cook meat products during chilled storage. LWT-Food Sci. Technol. 2013, 53, 321–326. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; Elnesr, S.S. A review on the beneficial effect of thymol on health and production of fish. Rev. Aquac. 2021, 13, 632–641. [Google Scholar] [CrossRef]
- Han, Y.; Sun, Z.; Chen, W. Antimicrobial susceptibility and antibacterial mechanism of limonene against Listeria monocytogenes. Molecules 2020, 25, 33. [Google Scholar] [CrossRef] [Green Version]
- Andrade Pizarro, R.D.; Pérez Cervera, C.E.; Lujan Rhenals, D.E. Development and application of edible coatings in minimally processed fruit. Vitae 2016, 23, 9–10. [Google Scholar] [CrossRef] [Green Version]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Antimicrobial chitosan and chitosan derivatives: A review of the structure–activity relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Qu, B.; Luo, Y. Chitosan-based hydrogel beads: Preparations, modifications and applications in food and agriculture sectors—A review. Int. J. Biol. Macromol. 2020, 152, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, R.; Rhim, J.W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Hu, Z.; Gänzle, M.G. Challenges and opportunities related to the use of chitosan as a food preservative. J. Appl. Microbiol. 2019, 126, 1318–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz de Elguea-Culebras, G.; Bourbon, A.I.; Costa, M.J.; Muñoz-Tebar, N.; Carmona, M.; Molina, A.; Sánchez-Vioque, R.; Berruga, M.I.; Vicente, A.A. Optimization of a chitosan solution as potential carrier for the incorporation of Santolina chamaecyparissus L. solid by-product in an edible vegetal coating on ‘Manchego’ cheese. Food Hydrocoll. 2019, 89, 272–282. [Google Scholar] [CrossRef] [Green Version]
- Raeisi, M.; Tajik, H.; Aliakbarlu, J.; Mirhosseini, S.H.; Hosseini, S. Enhancing safety and quality of shrimp by nanoparticles of sodium alginate-based edible coating containing grapefruit seed extract. Int. J. Biol. Macromol. 2021, 189, 84–90. [Google Scholar] [CrossRef]
- Raeisi, M.; Tajik, H.; Aliakbarlu, J.; Mirhosseini, S.H.; Hosseini, S. Effect of carboxymethyl cellulose-based coatings incorporated with Zataria multiflora Boiss. essential oil and grape seed extract on the shelf life of rainbow trout fillets. LWT-Food Sci. Technol. 2015, 64, 898–904. [Google Scholar] [CrossRef]
- Yousuf, B.; Sun, Y.; Wu, S. Lipid and Lipid-containing composite edible coatings and films. Food Rev. Int. 2021. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial properties of propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [Green Version]
- Casquete, R.; Castro, S.M.; Jácome, S.; Teixeira, P. Antimicrobial activity of ethanolic extract of propolis in “Alheira”, a fermented meat sausage. Cogent Food Agric. 2016, 2, 1125773. [Google Scholar] [CrossRef]
- Bahram, S.; Rezaie, M.; Soltani, M.; Kamali, A.; Abdollahi, M.; Khezri Ahmadabad, M.; Nemati, M. Effect of whey protein concentrate coating cinamon oil on quality and shelf life of refrigerated beluga sturegeon (Huso huso). J. Food Qual. 2016, 39, 743–749. [Google Scholar] [CrossRef]
- Esmaeli, F.; Tajik, H.; Mehdizadeh, T.; Mayeli, M. Effect of combined application of Pimpinella affinis essential oil and extract in zein edible coating on vacuum packaged rainbow trout fillet quality. Vet. Res. Forum 2019, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Effect of fish gelatin coating enriched with oregano essential oil on the quality of refrigerated rainbow Trout fillet. J. Aquat. Food Prod. Technol. 2016, 25, 835–842. [Google Scholar] [CrossRef]
- Hu, J.; Xu, Y.; Majura, J.J.; Qiu, Y.; Ding, J.; Hatab, S.; Miao, W.; Gao, Y. Combined effect of the essential oil and collagen film on the quality of pacific mackerel (Pneumatophorus japonicus) fillet during cold storage. Foodborne Pathog. Dis. 2021, 18, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Memar, M.Y.; Raei, P.; Alizadeh, N.; Aghdam, M.A.; Kafil, H.S. Carvacrol and thymol: Strong antimicrobial agents against resistant isolates. Rev. Med. Microbiol. 2017, 28, 63–68. [Google Scholar] [CrossRef]
- Himour, S.; Yahia, A.; Belattar, H. Oleuropein and antibacterial activities of Olea europaea L. leaf extract. Eur. Sci. J. 2017, 13, 342. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Ozogul, Y.; Kuley Boğa, E.; Akyol, I.; Durmus, M.; Ucar, Y.; Regenstein, J.M.; Köşker, A.R. Antimicrobial activity of thyme essential oil nanoemulsions on spoilage bacteria of fish and food-borne pathogens. Food Biosci. 2020, 36, 100635. [Google Scholar] [CrossRef]
- Marinelli, L.; di Stefano, A.; Cacciatore, I. Carvacrol and its derivatives as antibacterial agents. Phytochem. Rev. 2018, 17, 903–921. [Google Scholar] [CrossRef]
- EUR-Lex-32012R0872-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32012R0872 (accessed on 19 February 2022).
- Ibáñez, M.D.; Sanchez-Ballester, N.M.; Blázquez, M.A. Encapsulated limonene: A Pleasant Lemon-Like Aroma with Promising Application in the Agri-Food Industry. A Review. Molecules 2020, 25, 2598. [Google Scholar] [CrossRef]
- Khanzadi, S.; Keykhosravy, K.; Hashemi, M.; Azizzadeh, M. Alginate coarse/nanoemulsions containing Zataria multiflora Boiss essential oil as edible coatings and the impact on microbial quality of trout fillet. Aquac. Res. 2020, 51, 873–881. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Kopel, P.; Balková, R.; Hynek, D.; Bytesnikova, Z.; Gagic, M.; Milosavljevic, V.; Adam, V. Intelligent and active composite films based on furcellaran: Structural characterization, antioxidant and antimicrobial activities. Food Packag. Shelf Life 2019, 22, 100405. [Google Scholar] [CrossRef]
- Alsaggaf, M.S.; Moussa, S.H.; Tayel, A.A. Application of fungal chitosan incorporated with pomegranate peel extract as edible coating for microbiological, chemical and sensorial quality enhancement of Nile tilapia fillets. Int. J. Biol. Macromol. 2017, 99, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, C.; Liu, S.; Gao, J.; Cui, S.W.; Xia, W. Coating white shrimp (Litopenaeus vannamei) with edible fully deacetylated chitosan incorporated with clove essential oil and kojic acid improves preservation during cold storage. Int. J. Biol. Macromol. 2020, 162, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hong, W.S.; Oh, S.W. Effect of layer-by-layer antimicrobial edible coating of alginate and chitosan with grapefruit seed extract for shelf-life extension of shrimp (Litopenaeus vannamei) stored at 4 °C. Int. J. Biol. Macromol. 2018, 120, 1468–1473. [Google Scholar] [CrossRef]
- Nie, X.; Gao, Z.; Ren, X.; Jiang, Q.; Li, S.; Jiang, C.; Liu, B.; Liu, X.; He, F. Effect of pectin coating infused with gallic acid on the quality and shelf life of Japanese sea bass (Lateolabrax japonicas) fFillets. Food Bioprocess Technol. 2020, 13, 300–307. [Google Scholar] [CrossRef]
- Šimat, V.; Mekinić, I.G. Advances in Chilling. In Innovative Technologies in Seafood Processing; Ozogul, Y., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–25. [Google Scholar] [CrossRef]
- Banerjee, R.; Naveena, B.M. Superchilling of muscle foods: Potential alternative for chilling and freezing. Crit. Rev. Food Sci. Nutr. 2019, 59, 1256–1263. [Google Scholar] [CrossRef]
- Skírnisdóttir, S.; Knobloch, S.; Lauzon, H.L.; Ólafsdóttir, A.; Steinþórsson, P.; Bergsten, P.; Marteinsson, V.Þ. Impact of onboard chitosan treatment of whole cod (Gadus morhua) on the shelf life and spoilage bacteria of loins stored superchilled under different atmospheres. Food Microbiol. 2021, 97, 103723. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, P.; Fang, S.; Liu, W.; Mei, J.; Xie, J. Preservative effects of gelatin active coating enriched with eugenol emulsion on Chinese seabass (Lateolabrax maculatus) during superchilling (−0.9 °C) storage. Coatings 2019, 9, 489. [Google Scholar] [CrossRef] [Green Version]
- Ye, T.; Chen, X.; Chen, Z.; Liu, R.; Wang, Y.; Lin, L.; Lu, J. Quality characteristics of shucked crab meat (Eriocheir sinensis) processed by high pressure during superchilled storage. J. Food Biochem. 2021, 45, e13708. [Google Scholar] [CrossRef]
- Kuley, E.; Özoğul, F.; Polat, A. Advances in Packaging. In Innovative Technologies in Seafood Processing; Ozogul, Y., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 45–69. [Google Scholar] [CrossRef]
- Bouletis, A.D.; Arvanitoyannis, I.S.; Hadjichristodoulou, C. Application of modified atmosphere packaging on aquacultured fish and fish products: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2263–2285. [Google Scholar] [CrossRef]
- Mehrzadeh, S.; Roomiani, L. Effect of gamma irradiation and modified atmosphere packaging on the shelf-life of white shrimp (Metapenaeus affinis). Iran. J. Fish. Sci. 2021, 20, 1004–1021. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Haroutounian, S.A.; Nychas, G.J.E.; Boziaris, I.S. Microbiological spoilage and volatiles production of gutted European sea bass stored under air and commercial modified atmosphere package at 2 °C. Food Microbiol. 2015, 50, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Esteves, E.; Guerra, L.; Aníbal, J. Effects of vacuum and modified atmosphere packaging on the quality and shelf-life of gray triggerfish (Balistes capriscus) fillets. Foods 2021, 10, 250. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Liu, F.; Fang, S.; Lan, W.; Xie, J. High-CO2 Modified Atmosphere Packaging with Superchilling (−1.3 °C) Inhibit Biochemical and Flavor Changes in Turbot (Scophthalmus maximus) during Storage. Molecules 2020, 25, 2826. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhao, Y.; Yu, J.; Ling, J.; Shang, H.; Liu, Z. The Combined Efficacy of Superchilling and High CO2 Modified Atmosphere Packaging on Shelf Life and Quality of Swimming Crab (Portunus trituberculatus). J. Aquat. Food Prod. Technol. 2017, 26, 655–664. [Google Scholar] [CrossRef]
- Gonçalves, A.A.; Lira Santos, T.C. Improving quality and shelf-life of whole chilled Pacific white shrimp (Litopenaeus vannamei) by ozone technology combined with modified atmosphere packaging. LWT-Food Sci. Technol. 2019, 99, 568–575. [Google Scholar] [CrossRef]
- Sørensen, J.S.; Bøknæs, N.; Mejlholm, O.; Dalgaard, P. Superchilling in combination with modified atmosphere packaging resulted in long shelf-life and limited microbial growth in Atlantic cod (Gadus morhua L.) from capture-based-aquaculture in Greenland. Food Microbiol. 2020, 88, 103405. [Google Scholar] [CrossRef]
- Kaale, L.D.; Eikevik, T.M.; Rustad, T.; Nordtvedt, T.S. Changes in water holding capacity and drip loss of Atlantic salmon (Salmo salar) muscle during superchilled storage. LWT-Food Sci. Technol. 2014, 55, 528–535. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Jin, Z.; Liu, Y.; Chen, Y.; Konno, K.; Zhu, B.; Dong, X. Effects of super-chilling storage on shelf-life and quality indicators of Coregonus peled based on proteomics analysis. Food Res. Int. 2021, 143, 110229. [Google Scholar] [CrossRef]
- Liu, Q.; Kong, B.; Han, J.; Chen, Q.; He, X. Effects of superchilling and cryoprotectants on the quality of common carp (Cyprinus carpio) surimi: Microbial growth, oxidation, and physiochemical properties. LWT-Food Sci. Technol. 2014, 57, 165–171. [Google Scholar] [CrossRef]
- Gonçalves, A.A. Ozone Application in Seafood Processing. In Innovative Technologies in Seafood Processing; Ozogul, Y., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 191–217. [Google Scholar] [CrossRef]
- Brodowska, A.J.; Nowak, A.; Śmigielski, K. Ozone in the food industry: Principles of ozone treatment, mechanisms of action, and applications: An overview. Crit. Rev. Food Sci. Nutr. 2017, 58, 2176–2201. [Google Scholar] [CrossRef]
- Campos, C.A.; Rodríguez, Ó.; Losada, V.; Aubourg, S.P.; Barros-Velázquez, J. Effects of storage in ozonised slurry ice on the sensory and microbial quality of sardine (Sardina pilchardus). Int. J. Food Microbiol. 2005, 103, 121–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, C.A.; Losada, V.; Rodríguez, Ó.; Aubourg, S.P.; Barros-Velázquez, J. Evaluation of an ozone–slurry ice combined refrigeration system for the storage of farmed turbot (Psetta maxima). Food Chem. 2006, 97, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Gelman, A.; Sachs, O.; Khanin, Y.; Drabkin, V.; Glatman, L. Effect of ozone pretreatment on fish storage life at low temperatures. J. Food Prot. 2005, 68, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Nerantzaki, A.; Tsiotsias, A.; Paleologos, E.K.; Savvaidis, I.N.; Bezirtzoglou, E.; Kontominas, M.G. Effects of ozonation on microbiological, chemical and sensory attributes of vacuum-packaged rainbow trout stored at 4 ± 0.5 °C. Eur. Food Res. Technol. 2005, 221, 675–683. [Google Scholar] [CrossRef]
- Gonçalves, A.A. Ozone as a safe and environmentally friendly tool for the seafood industry. J. Aquat. Food Prod. Technol. 2016, 25, 210–229. [Google Scholar] [CrossRef]
- Gonçalves, A.A. Ozone—An emerging technology for the seafood industry. Braz. Arch. Biol. Technol. 2009, 52, 1527–1539. [Google Scholar] [CrossRef]
- Greene, A.K.; Güzel-Seydim, Z.B.; Seydim, A.C. Chemical and Physical Properties of Ozone. In Ozone in Food Processing; O’Donnell, C., Tiwari, B.K., Cullen, P.J., Rice, R.G., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2012; pp. 19–32. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Subhashini, S.; Banuu Priya, E.P.; Kothakota, A.; Ramesh, S.; Shahir, S. Ozone based food preservation: A promising green technology for enhanced food safety. J. Int. Ozone Assoc. 2018, 41, 17–34. [Google Scholar] [CrossRef]
- Crapo, C.; Himelbloom, B.; Vitt, S.; Pedersen, L. Ozone efficacy as a bactericide in seafood processing. J. Aquat. Food Prod. Technol. 2008, 13, 111–123. [Google Scholar] [CrossRef]
- Kontominas, M.G.; Badeka, A.; Kosma, I.S.; Nathanailides, C.I. Innovative seafood preservation technologies: Recent developments. Animals 2021, 11, 92. [Google Scholar] [CrossRef]
- Chen, J.; Huang, J.; Deng, S.; Huang, Y. Combining ozone and slurry ice to maximize shelf-life and quality of bighead croaker (Collichthys niveatus). J. Food Sci. Technol. 2016, 53, 3651–3660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okpala, C.O.R. Investigation of quality attributes of ice-stored Pacific white shrimp (Litopenaeus vannamei) as affected by sequential minimal ozone treatment. LWT-Food Sci. Technol. 2014, 57, 538–547. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, K.; Gao, M. Inactivation of Vibrio parahaemolyticus by aqueous ozone. J. Microbiol. Biotechnol. 2018, 28, 1233–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louppis, A.P.; Katikou, P.; Georgantelis, D.; Badeka, A.; Kontominas, M.G. Effect of ozonation and γ-irradiation on post-harvest decontamination of mussels (Mytillus galloprovincialis) containing diarrhetic shellfish toxins. Food Addit. Contam. 2011, 28, 1735–1744. [Google Scholar] [CrossRef] [Green Version]
- Ceylan, Z.; Özoğul, Y. Irradiation Technology. In Innovative Technologies in Seafood Processing; Ozogul, Y., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 115–129. [Google Scholar] [CrossRef]
- Sage, E.; Shikazono, N. Radiation-induced clustered DNA lesions: Repair and mutagenesis. Free. Radic. Biol. Med. 2017, 107, 125–135. [Google Scholar] [CrossRef]
- Maherani, B.; Hossain, F.; Criado, P.; Ben-Fadhel, Y.; Salmieri, S.; Lacroix, M. World market development and consumer acceptance of irradiation technology. Foods 2016, 5, 79. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Wang, H.; Wang, W.; Qi, W.; Yue, L.; Ye, Q. Effect of 10 MeV E-beam irradiation combined with vacuum-packaging on the shelf life of Atlantic salmon fillets during storage at 4 °C. Food Chem. 2014, 145, 535–541. [Google Scholar] [CrossRef]
- Suklim, K.; Flick, G.J.; Vichitphan, K. Effects of gamma irradiation on the physical and sensory quality and inactivation of Listeria monocytogenes in blue swimming crab meat (Portunas pelagicus). Radiat. Phys. Chem. 2014, 103, 22–26. [Google Scholar] [CrossRef]
- Yu, Q.; Pan, H.; Qian, C.; Shao, H.; Han, J.; Li, Y.; Lou, Y. Determination of the optimal electron beam irradiation dose for treating shrimp (Solenocera melantho) by means of physical and chemical properties and bacterial communities. LWT-Food Sci. Technol. 2022, 153, 112539. [Google Scholar] [CrossRef]
- Harrell, C.R.; Djonov, V.; Fellabaum, C.; Volarevic, V. Risks of using sterilization by gamma radiation: The other side of the coin. Int. J. Med. Sci. 2018, 15, 274. [Google Scholar] [CrossRef] [Green Version]
- Metta, V.A.; Mameesh, M.S.; Johnson, B.C. Vitamin K deficiency in rats induced by the feeding of irradiated beef. J. Nutr. 1959, 69, 18–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tritsch, G.L. Food irradiation. Nutrition 2000, 16, 698–701. [Google Scholar] [CrossRef]
- Al-Kuraieef, A.N. Microbiological, chemical and organoleptic evaluation of fresh fish and its products irradiated by gamma rays. Potravin. Slovak J. Food Sci. 2021, 15, 95–100. [Google Scholar] [CrossRef]
- Alivand, N.; Roomiani, L. Effect of gamma irradiation on the shelf-life of vacuum-packaged silver carp surimi in 4 °C. Iran. J. Aquat. Anim. Health 2019, 5, 67–82. [Google Scholar] [CrossRef]
- de Alba, M.; Pérez-Andrés, J.M.; Harrison, S.M.; Brunton, N.P.; Burgess, C.M.; Tiwari, B.K. High pressure processing on microbial inactivation, quality parameters and nutritional quality indices of mackerel fillets. Innov. Food Sci. Emerg. Technol. 2019, 55, 80–87. [Google Scholar] [CrossRef]
- Rode, T.M.; Hovda, M.B. High pressure processing extend the shelf life of fresh salmon, cod and mackerel. Food Control 2016, 70, 242–248. [Google Scholar] [CrossRef]
- Modugno, C.; Peltier, C.; Simonin, H.; Dujourdy, L.; Capitani, F.; Sandt, C.; Perrier-Cornet, J.M. Understanding the effects of high pressure on bacterial spores using synchrotron infrared spectroscopy. Front. Microbiol. 2020, 10, 3122. [Google Scholar] [CrossRef]
- Woldemariam, H.W.; Emire, S.A. High pressure processing of foods for microbial and mycotoxins control: Current trends and future prospects. Cogent Food Agric. 2019, 5, 1622184. [Google Scholar] [CrossRef]
- Ucak, İ.; Toepfl, S. High-Pressure Processing of Seafood. In Innovative Technologies in Seafood Processing; Ozogul, Y., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 93–114. [Google Scholar] [CrossRef]
- Arnaud, C.; de Lamballerie, M.; Pottier, L. Effect of high pressure processing on the preservation of frozen and re-thawed sliced cod (Gadus morhua) and salmon (Salmo salar) fillets. High Press. Res. 2018, 38, 62–79. [Google Scholar] [CrossRef]
- Yagiz, Y.; Kristinsson, H.G.; Balaban, M.O.; Welt, B.A.; Ralat, M.; Marshall, M.R. Effect of high pressure processing and cooking treatment on the quality of Atlantic salmon. Food Chem. 2009, 116, 828–835. [Google Scholar] [CrossRef]
- Christensen, L.B.; Hovda, M.B.; Rode, T.M. Quality changes in high pressure processed cod, salmon and mackerel during storage. Food Control 2017, 72, 90–96. [Google Scholar] [CrossRef]
- Giannoglou, M.; Dimitrakellis, P.; Efthimiadou, A.; Gogolides, Ε.; Katsaros, G. Comparative study on the effect of cold atmospheric plasma, ozonation, pulsed electromagnetic fields and high-pressure technologies on sea bream fillet quality indices and shelf life. Food Eng. Rev. 2021, 13, 175–184. [Google Scholar] [CrossRef]
- Rastogi, N.K.; Raghavarao, K.S.M.S.; Balasubramaniam, V.M.; Niranjan, K.; Knorr, D. Opportunities and challenges in high pressure processing of foods. Crit. Rev. Food Sci. Nutr. 2010, 47, 69–112. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, A.; Kaur, B.P.; Rao, P.S. Effect of high pressure processing and thermal treatment on quality of hilsa (Tenualosa ilisha) fillets during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2015, 29, 151–160. [Google Scholar] [CrossRef]
- Otero, L.; Pérez-Mateos, M.; López-Caballero, M.E. Hyperbaric cold storage versus conventional refrigeration for extending the shelf-life of hake loins. Innov. Food Sci. Emerg. Technol. 2017, 41, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Moreira, S.A.; Duarte, R.V.; Fernandes, P.A.R.; Alves, S.P.; Bessa, R.J.; Delgadillo, I.; Saraiva, J.A. Hyperbaric storage preservation at room temperature using an industrial-scale equipment: Case of two commercial ready-to-eat pre-cooked foods. Innov. Food Sci. Emerg. Technol. 2015, 32, 29–36. [Google Scholar] [CrossRef]
- Fidalgo, L.G.; Simões, M.M.Q.; Casal, S.; Lopes-da-Silva, J.A.; Delgadillo, I.; Saraiva, J.A. Enhanced preservation of vacuum-packaged Atlantic salmon by hyperbaric storage at room temperature versus refrigeration. Sci. Rep. 2021, 11, 1668. [Google Scholar] [CrossRef]
- Otero, L.; Pérez-Mateos, M.; Holgado, F.; Márquez-Ruiz, G.; López-Caballero, M.E. Hyperbaric cold storage: Pressure as an effective tool for extending the shelf-life of refrigerated mackerel (Scomber scombrus, L.). Innov. Food Sci. Emerg. Technol. 2019, 51, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Fidalgo, L.G.; Simões, M.M.Q.; Casal, S.; Lopes-da-Silva, J.A.; Carta, A.M.; Delgadillo, I.; Saraiva, J.A. Physicochemical parameters, lipids stability, and volatiles profile of vacuum-packaged fresh Atlantic salmon (Salmo salar) loins preserved by hyperbaric storage at 10 °C. Food Res. Int. 2020, 127, 108740. [Google Scholar] [CrossRef]
- Corsetti, A.; Valmorri, S. Lactic Acid Bacteria|Lactobacillus spp.: Lactobacillus plantarum. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 111–118. [Google Scholar] [CrossRef]
- Nes, I.F.; Brede, D.A.; Diep, D.B. Class II Non-Lantibiotic Bacteriocins. In Handbook of Biologically Active Peptides; Kastin, A., Ed.; Academic Press: Cambridge MA, USA, 2013; pp. 85–92. [Google Scholar] [CrossRef]
- Pinto, A.L.; Fernandes, M.; Pinto, C. Characterization of anti-Listeria bacteriocins isolated from shellfish: Potential antimicrobials to control non-fermented seafood. Int. J. Food Microbiol. 2009, 129, 50–58. [Google Scholar] [CrossRef]
- Baños, A.; García-López, J.D.; Núñez, C.; Martínez-Bueno, M.; Maqueda, M.; Valdivia, E. Biocontrol of Listeria monocytogenes in fish by enterocin AS-48 and Listeria lytic bacteriophage P100. LWT-Food Sci. Technol. 2016, 66, 672–677. [Google Scholar] [CrossRef]
- Woraprayote, W.; Malila, Y.; Sorapukdee, S.; Swetwiwathana, A.; Benjakul, S.; Visessanguan, W. Bacteriocins from lactic acid bacteria and their applications in meat and meat products. Meat Sci. 2016, 120, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Delcarlo, S.B.; Parada, R.; Schelegueda, L.I.; Vallejo, M.; Marguet, E.R.; Campos, C.A. From the isolation of bacteriocinogenic LAB strains to the application for fish paste biopreservation. LWT-Food Sci. Technol. 2019, 110, 239–246. [Google Scholar] [CrossRef]
- Ghanbari, M.; Jami, M.; Kneifel, W.; Domig, K.J. Antimicrobial activity and partial characterization of bacteriocins produced by lactobacilli isolated from Sturgeon fish. Food Control 2013, 32, 379–385. [Google Scholar] [CrossRef]
- López de Lacey, A.M.; López-Caballero, M.E.; Montero, P. Agar films containing green tea extract and probiotic bacteria for extending fish shelf-life. LWT-Food Sci. Technol. 2014, 55, 559–564. [Google Scholar] [CrossRef]
- Ceylan, Z.; Uslu, E.; İspirli, H.; Meral, R.; Gavgalı, M.; Tahsin’Yilmaz, M.; Dertli, E. A novel perspective for Lactobacillus reuteri: Nanoencapsulation to obtain functional fish fillets. LWT-Food Sci. Technol. 2019, 115, 108427. [Google Scholar] [CrossRef]
- Tomé, E.; Gibbs, P.A.; Teixeira, P.C. Growth control of Listeria innocua 2030c on vacuum-packaged cold-smoked salmon by lactic acid bacteria. Int. J. Food Microbiol. 2008, 121, 285–294. [Google Scholar] [CrossRef]
- Jo, D.M.; Park, S.K.; Khan, F.; Kang, M.G.; Lee, J.H.; Kim, Y.M. An approach to extend the shelf life of ribbonfish fillet using lactic acid bacteria cell-free culture supernatant. Food Control 2021, 123, 107731. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Shi, G.; Chang, J.; Liu, Z.; Zeng, M. Cooperation of lactic acid bacteria regulated by the AI-2/LuxS system involve in the biopreservation of refrigerated shrimp. Food Res. Int. 2019, 120, 679–687. [Google Scholar] [CrossRef]
- Wiernasz, N.; Leroi, F.; Chevalier, F.; Cornet, J.; Cardinal, M.; Rohloff, J.; Passerini, D.; Skırnisdóttir, S.; Pilet, M.F. Salmon gravlax biopreservation with lactic acid bacteria: A polyphasic approach to assessing the impact on organoleptic properties, microbial ecosystem and volatilome composition. Front. Microbiol. 2020, 10, 3103. [Google Scholar] [CrossRef]
- Wiernasz, N.; Cornet, J.; Cardinal, M.; Pilet, M.F.; Passerini, D.; Leroi, F. Lactic acid bacteria selection for biopreservation as a part of hurdle technology approach applied on seafood. Front. Mar. Sci. 2017, 4, 119. [Google Scholar] [CrossRef] [Green Version]
- Coffey, B.; Mills, S.; Coffey, A.; McAuliffe, O.; Paul Ross, R. Phage and their lysins as biocontrol agents for food safety applications. Annu. Rev. Food Sci. Technol. 2010, 1, 449–468. [Google Scholar] [CrossRef] [PubMed]
- Połaska, M.; Sokołowska, B. Review bacteriophages—A new hope or a huge problem in the food industry. AIMS Microbiol. 2019, 5, 324–347. [Google Scholar] [CrossRef] [PubMed]
- Lasagabaster, A.; Jiménez, E.; Lehnherr, T.; Miranda-Cadena, K.; Lehnherr, H. Bacteriophage biocontrol to fight Listeria outbreaks in seafood. Food Chem. Toxicol. 2020, 145, 111682. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lin, H.; Khan, M.N.; Wang, J.; Kong, L. Effects of bacteriophage on the quality and shelf life of Paralichthys olivaceus during chilled storage. J. Sci. Food Agric. 2014, 94, 1657–1662. [Google Scholar] [CrossRef]
- Yamaki, S.; Kawai, Y.; Yamazaki, K. Biocontrol of Morganella morganii subsp. morganii and histamine accumulation in tuna meat by treatment with a lytic bacteriophage. Food Sci. Technol. Res. 2018, 24, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Hernández, I. Bacteriophages against Serratia as Fish Spoilage Control Technology. Front. Microbiol. 2017, 8, 449. [Google Scholar] [CrossRef] [Green Version]
- Guttman, B.; Raya, R.; Kutter, E. Chapter 3: Basic Phage Biology. In Bacteriophages. Biology and Application; Kutter, E., Sulakvelidze, A., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 29–64. [Google Scholar] [CrossRef]
- Zulkarneev, E.R.; Aleshkin, A.V.; Kiseleva, I.A.; Rubalsky, E.O.; Rubalsky, O.V. Bacteriophage cocktail effectively prolonging the shelf-life of chilled fish. Bull. Exp. Biol. Med. 2019, 167, 818–822. [Google Scholar] [CrossRef]
- Shamloo, E.; Hosseini, H.; Moghadam, A.Z.; Larsen, H.M.; Haslberger, A.; Alebouyeh, M. Importance of Listeria monocytogenes in food safety: A review of its prevalence, detection, and antibiotic resistance. Iran. J. Vet. Res. 2019, 20, 241–254. [Google Scholar]
- Nath, S.; Chowdhury, S. Effect of lactic acid bacteria application on shelf life and safety of fish fillet at 6 ± 1 °C. Int. J. Adv. Res. 2014, 2, 201–207. [Google Scholar]
- Mei, J.; Shen, Y.; Liu, W.; Lan, W.; Li, N.; Xie, J. Effectiveness of sodium alginate active coatings containing bacteriocin EFL4 for the quality improvement of ready-to-eat fresh salmon fillets during cold storage. Coatings 2020, 10, 506. [Google Scholar] [CrossRef]
| Compound | Additional Treatment | Species Tested | Results | Reference |
|---|---|---|---|---|
| Chitosan coating | Aspartic acid | Channel catfish (Ictalurus punctatus) | 2 log cycles of reduction after 6 days. Regulation of pH and TVB-N values. | [45] |
| Chitosan coating | Whey protein and tarragon essential oil | Talang queenfish (Scomberoides commersonnianus) | Extension of TVB-N values under 30 mg/100 g from 8 to 16 days. pH changes contained. Over 2 log cycles of psychrotrophic and mesophilic bacteria reduction after 8 days. | [46] |
| Chitosan coating | Gallic acid | Horse mackerel (Trachurus trachurus) | 4 days of extension of shelf life when nanoparticles and gallic acid were used. Regulation of pH and TVB-N values. Total inhibition of H2S-producing microorganisms. | [47] |
| Chitosan coating | Propolis extract | Japanese threadfin bream (Nemipterus japonicus) | Reduced lipid oxidation. Reduced TVB-N and pH values. Over 10 days of extension of shelf life. Improved sensorial characteristics. | [16] |
| Sodium alginate coating | Zataria multiflora Boiss essential oil | Trout * | Inhibition of total viable bacteria, total psychrophilic bacteria, hydrogen sulfide producing bacteria, and Enterobacteriaceae. | [78] |
| Furcellaran film | Green tea extract and synthetized selenium nanoparticles | Common carp (Cyprinus carpio) | Enhanced antimicrobial activity against E. coli, S. aureus, and MRSA. Great antioxidant activity. | [79] |
| Chitosan coating | Pomegranate peel extract | Nile tilapia (Oreochromis niloticu) | Inhibition of Enterobacteriaceae, coliform bacteria, Salmonella spp., E. coli, yeast and mold, and Staphylococcus aureus growth to undetectable levels. Control of TVB-N values under acceptable limits. Shelf life extension from <15 to >30 days. Preservation of sensorial characteristics for over 30 days. | [80] |
| Chitosan coating | Clove essential oil and kojic acid | White prawn shrimp (Litopenaeus vannamei) | Over 3 log cycles of total aerobic bacteria growth inhibition. Shelf life extension. Reduced TVB-N and pH increase. Preservation of sensorial characteristics. Reduced weight loss. | [81] |
| Sodium alginate and chitosan coating | Grapefruit seed extract | White prawn shrimp (Litopenaeus vannamei) | Extension of TVB-N values under acceptable limits from 8 to 12 days. Improved sensorial characteristics. Inhibition of psychrophilic and mesophilic bacteria. Reduced melanosis. | [82] |
| Sodium alginate coating | Grapefruit seed extract | Shrimp * | Reduced weight loss. Extension of TVB-N values under acceptable limits from 4 to over 8 days. Delay in chemical decay. Reduced melanosis. Enhanced overall acceptability. | [62] |
| Quinoa starch film | - | Rainbow trout (Oncorhynchus mykiss) | Chemical and biological protective effect. Resulted in slight but significant inhibition of bacterial growth and chemical decay. | [48] |
| Pectin/chitosan coating | Tarragon essential oil (Artemisia dracunculus) | Narrow-barred Spanish mackerel (Scomberomorus commerson) | Significant reduction in lipid oxidation. Lower bacterial counts. Reduced TVB-N and TBARS values. Extension of shelf life from 8 to over 16 days of storage. | [49] |
| Pectin coating | Gallic acid | Japanese sea bass (Lateolabrax japonicas) | Regulation of TVB-N and pH values. Some acidification of the matrix was observed. Considerable reduction in TBARS values. Sensorial characteristics remained acceptable for at least 5 days longer. | [83] |
| Carboxymethyl cellulose coating | Zataria multiflora Boiss essential oil and grape seed extract | Rainbow trout (Oncorhynchus mykiss) | Better microbial and sensorial scores in treated samples. Organoleptic properties remained acceptable through more extended periods of storage. Decrease in lactic acid bacteria and pseudomonas counts. Regulation of TVB-N increase. | [63] |
| Additional Treatment | Storage Conditions (°C) | Species Tested | Results | Reference |
|---|---|---|---|---|
| - | −1.7 | Atlantic salmon (Salmo salar) | Significant decrease in liquid loss after 1 day of superchilled storage. No significant differences after this point. | [98] |
| - | −1 | Atlantic cod (Gadus morhua) | Extension of 2–4 days of freshness period and 3 days of shelf life. Lower microbial growth, H2S-producing bacteria, and total volatile basic nitrogen in superchilled samples. | [13] |
| - | −2 | Peled (Coregonus peled) | Lower collagen degradation and extended texture retention period in superchilled samples. Colony-forming units per gram below FAO standard in superchilled samples after 6 days. | [99] |
| Cryoprotectants | −1; −3; −3 with cryoprotectants | Common carp (Cyprinus carpio) | Reduced microbial growth, total volatile basic nitrogen, and moisture for samples stored at superchilled conditions with cryoprotectants. Increased preservative impact of superchilling storage at −3 °C, especially when combined with cryoprotectants. | [100] |
| Clove essential oil enriched ice glazing | −1 | Sea bass (Dicentrarchus labrax) | Considerable preservation of sensorial attributes during 24 days, when compared to control samples. Lower microbial and chemical degradation when superchilled. The preservation potential of the process increases with the concentration of essential oil. | [6] |
| Modified atmosphere (high CO2) (MAP) | −3 | Swimming crab (Portunus trituberculatus) | Shelf life of crab was increased from 10–15 days, in conventionally superchilled samples, to 15–20 days in samples stored in superchilling under a modified atmosphere of 60–80% CO2. Lower bacterial growth and total volatile basic nitrogen in samples stored in MAP. | [95] |
| Modified atmosphere (high CO2 and N2) (MAP) | −1.7 | Atlantic cod (Gadus morhua) | Shelf life: iced storage, 15 days; MAP iced storage, 21 days; air superchilling storage and MAP superchilling storage, >32 days. Total volatile basic nitrogen values remained below the EU limit after 34 days in superchilled samples. Lower aerobic viable counts but higher CFU/g of Photobacterium spp. in MAP samples. | [97] |
| Modified atmosphere (MAP) and chitosan treatment | −1 | Atlantic cod (Gadus morhua) | Chitosan did not alter the sensory characteristics, freshness, or shelf life of the product. Decrease in total viable counts and total specific spoilage organism counts immediately after application of chitosan. Lower bacterial diversity in chitosan-treated samples. Lower total volatile basic nitrogen in MAP samples. Extension of 3–4 days of shelf life in MAP samples. | [86] |
| Modified atmosphere (high CO2) (MAP) | −1.3 | Turbot (Scophthalmus maximus) | Superchilling storage with high CO2 (60–70% CO2) maintained better results in organoleptic, microbiological, and chemical parameters during storage. | [94] |
| Gelatin active coating with eugenol emulsion | −0.9 | Chinese seabass (Lateolabrax maculatus) | Lower values of total volatile basic nitrogen, total viable count, H2S-producing bacteria, Pseudomonas spp., and psychrophilic bacteria in superchilled samples. The presence of eugenol in the coating showed improved efficiency in inhibiting product deterioration. | [87] |
| High-pressure processing (300 MPa) | −4 | Mitten crab (Eriocheir sinensis) | High drip loss. Aerobic plate counts below the high-quality upper limit of 5 log CFU/g after 4 weeks. Total volatile basic nitrogen under 30 mg/100 g (maximum recommended) for 3 weeks. Extension of shelf life from 7 days (when refrigerated at 4 °C) to 3 weeks when superchilled and processed with high pressure. | [88] |
| Radiation Dose/Type | Food Matrix | Results | Reference |
|---|---|---|---|
| 2, 4, 6, 8, and 10 kGy/EBI | Shrimp (Solenocera melantho) | Weight loss. Decrease in chewiness with increasing radiation. Reduced concentration of polyphenol oxidase. Strong bactericidal effect observed, increasing alongside radiation dose. Destruction of shrimp muscle above 6 kGy. | [122] |
| 2, 4, 6, 8, and 10 kGy/EBI | Gazami crab (Portunus trituberculatus) | Changes in the composition of microbial communities. Decrease in bacterial variety. Proteobacteria dominated microflora above 4 kGy. Psychrobacter only inhibited above 8 kGy. The recommended dose to achieve bactericidal aims defined at 6 kGy. | [18] |
| 1.5, 3, and 4.5 kGy/gamma | Nile tilapia (Oreochromis niloticus), herring *, mackerel * | Decrease in total viable bacteria. At 4.5 kGy, reduction in Streptococcus, Staphylococcus, yeasts, and molds below detectable values. Superior bactericidal activity at higher radiation doses. Considerably higher values of peroxide and TBA in irradiated samples. Reduced organoleptic score in samples irradiated with 4.5 kGy. | [126] |
| 1, 3, 5, and 7 kGy/gamma | Silver carp (Hypophthalmichthys molitrix) | Reduced peroxide, TBA, and TVB-N values in irradiated samples. Up to 2 log CFU/g of reduction in irradiated samples after 15 days of storage. Increase in lipid oxidation and development of unpleasant odors. Reduced lightning index and superior yellowish color in treated samples. Increased softness, reduced chewiness, and hardness. Up to 3 days of shelf life-extension. | [127] |
| 0.5, 1, 2, and 3 kGy/EBI | Atlantic salmon * | Reduced TVB-N values in irradiated samples. Increased TBA values are higher in treated samples. Inhibition of bacterial growth proportional to the radiation dose. Unpleasant color and odor at higher doses. No significant sensorial changes in doses below 2 kGy. Inhibition of bacterial growth. | [120] |
| 1, 2, 4, and 6 kGy/gamma | Blue swimming crab (Portunus pelagicus) | Reduction in total viable counts. Elimination of Vibrio cholerae and Vibrio vulnificus. Inactivation of Listeria monocytogenes. | [121] |
| Pressure Applied | Food Matrix | Results | Reference |
|---|---|---|---|
| 150, 300, and 450 MPa | Cod (Gadus morhua) and salmon (Salmo salar) | Efficient microbial reduction in samples treated with 450 MPa. Greater impact on color and cooked appearance when 300 and 450 MPa were used. Higher doses produced changes in all sensorial criteria. Increased lipid oxidation in salmon. | [133] |
| 400, 500, and 600 MPa | Atlantic cod * | Reduction in total viable counts. Increased antibacterial activity at higher pressures. Extension of shelf life in all HPP-treated samples beyond 49 days of storage. HPP increased drip loss of product. | [15] |
| 300 MPa | Sea bass (Dicentrarchus labrax) | pH increased after treatment. Sensorial alterations, increased lightness and hardness. HPP reduced overall acceptability. No increase in lipid oxidation was detected. Reduction in total viable bacteria, Pseudomonas spp., Enterobacteriaceae, and lactic acid bacteria. The shelf life increased from 5 to 9 days, based on the sensorial evaluation. | [136] |
| 250 and 350 MPa | Hilsa (Tenualosa ilisha) | TBARS and TVB-N reduction in pressure-treated samples. Reduced lipid oxidation and TMA values. Lipid oxidation is higher at 350 MPa than 250 MPa. Modification and reduced acceptability of color characteristics of the product. Textural alterations. A 15-day increase in shelf life period. | [138] |
| 200 and 500 MPa | Cod (Gadus morhua), salmon (Salmo salar), and mackerel (Scomber scombrus) | Significant bacterial inhibition in cod and mackerel. Mackerel shelf life extended from 8 to over 19 days. Cod shelf life extended from 15 to 21 and over 26 days for samples treated with 200 and 500 MPa, respectively. Increased lipid oxidation in all pressurized matrixes, especially those treated with 500 MPa. | [129] |
| 100, 300, and 500 MPa | Mackerel (Scomber spp.) | Bacterial inhibition is proportional to the pressure applied. Decrease in total viable counts and H2S-producing bacteria. Negative impact on color. Increased hardiness in samples pressurized with 500 MPa. Changes in color and texture but no impact on lipid oxidation. | [128] |
| Product | Biopreservative Agent | Results | Reference |
|---|---|---|---|
| Hake * | Lacticaseibacillus paracasei L26 and Bifidobacterium lactis B94 | Lower total viable counts, H2S-producing bacteria, and total volatile basic nitrogen. TVB-N values below the limit of acceptability after 15 days. Over one week of extension of shelf life. Increase in probiotic cultures in the product. | [151] |
| Hake (Merluccius hubbsi) | Enterococcus mundtii STw38 | Low values of total mesophilic counts (1.5 log cycles) compared to control (4.0 log cycles). Decrease in enterococci population for the initial 3 days, with recovery to inoculation levels afterward. | [149] |
| Ribbonfish (Trichiurus lepturus) | Lactobacillus plantarum SKD4 cell-free supernatant and Pediococcus stilesii SKD11 cell-free supernatant | Slight acidification of the product. Significant inhibition of bacterial growth. Low trimethylamine (TMA) values during storage. Diminished changes in color values. Conservation of sensorial characteristics throughout storage. | [154] |
| Litopenaeus vannamei (Shrimp) | Lactobacillus plantarum AB-1 and Lactobacillus casei | Higher sensory scores in co-cultured samples. Total volatile basic nitrogen under 30 mg/100 g limit for 8 days (5 days in control samples). Lower pH. | [155] |
| Horse mackerel * | Lactobacillus sakei ATCC 15521 | Inhibition of bacterial growth, up to 1.5 log CFU/g. Typical bacteriostatic effect. Lower total volatile basic nitrogen and pH values. | [167] |
| Salmon * | Bacteriocin Enterococcus faecalis L04 | Foodborne pathogen and food spoilage bacteria inhibition. Reduced total viable counts, lipid oxidation, and TVB-N values. Better maintenance of product quality during storage in refrigerated conditions. Preservation of sensorial characteristics. | [168] |
| Salmon * | Carnobacterium maltaromaticum SF1944, Lactococcus piscium EU2229, Leuconostoc gelidum EU2249, Vagococcus fluvialis CD264, Carnobacterium inhibens MIP2551, and Aerococcus viridans SF1044 | Sensorial characteristics remained desirable for extended periods in samples treated with V. fluvialis. Strong, undesirable, acidification of samples inoculated with L. piscium or L. gelidum. Inhibited spoilage bacteria growth. Inhibition of Listeria monocytogenes growth. | [156] |
| Olive flounder (Paralichthys olivaceus) | Bacteriophage Spp001 | Shelf life-extension from <4 to 14 days. Inhibition of bacterial growth, both total viable count and specific spoilage organisms. Preservation of good sensorial characteristics | [161] |
| Tuna * | Bacteriophage FSP1 | No significant impact on total viable cell counts. Considerable inhibition of Morganella morganni cells. Reduced levels of histamine accumulation. | [162] |
| Atlantic horse mackerel (Trachurus trachurus) | Bacteriophage AZT6 | Reduction in Serratia population by up to 90% during fish storage. Similar total viable counts to control. | [163] |
| Rainbow trout (Salmo irideus) | Bacteriophages Ah1, Pf1, Psp6, Ro1, Cf1, and Lm1 | Inhibition of mesophilic aerobic bacteria growth. Samples treated with the cocktail remained under 105 CFU/g for 3 days longer than control samples. | [165] |
| Technique | Properties |
|---|---|
| Biodegradable films, edible coatings, and natural preservatives [10,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83] | +Strong antibacterial activity +Safe +Biodegradable +All-natural final product +Great variety of candidate compounds +Easy to implement +Can add nutritional value and health claims to the product −Can result in strong organoleptic changes |
| Superchilling [6,13,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100] | +Considerable increase in shelf life +Strong inhibition of bacterial growth +Preservation of most sensorial characteristics +Great potential if used in combination with other techniques such as MAP −Physical degradation if temperatures applied are non-optimal −Optimal temperature varies depending on matrix −Short optimal temperature interval |
| Ozonation [96,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116] | +Versatile disinfectant +Activity against bacterial spores +Various forms of application +Sensorial preservation +Potential to reduce the presence of toxins such as diarrhetic shellfish toxins +Becoming progressively cheaper −Few studies on its application in seafood or other solid foods −Demands the acquisition of specialized equipment −Increases product manufacture cost |
| Irradiation [18,117,118,119,120,121,122,123,124,125,126,127] | +Low-intensity radiation preserves product characteristics +High-intensity radiation has strong antibacterial activity −High-intensity radiation increases TBARS values and results in changes in color, taste, texture, cohesiveness, and resilience −Impact on consumer health perceived as negative −Very expensive equipment and maintenance |
| High-pressure processing [14,15,128,129,130,131,132,133,134,135,136,137,138] | +Antibacterial activity increases with higher pressures +Potential to inactivate spores +Inactivation of allergens +Significant shelf life-extension −Sensorial impact at high pressures −Optimal pressure depends on product type −Very expensive equipment and maintenance |
| Hyperbaric storage [139,140,141,142,143] | +Low operating costs +Energetically efficient +Significant shelf life-extension +Preservation of sensorial characteristics +Maintenance of muscular structure and conservation of water holding and drip loss properties −Few studies −No commercial equipment available |
| Biopreservation [17,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168] | +Lactic acid bacteria can add nutritional value to the product +Bacteriophages can be used to target specific bacteria +Beneficial bacteria is preserved −LAB activity might result in undesirable sensorial changes −Acidification of the product −Some doubts regarding the safety of bacteriophages exist |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto de Rezende, L.; Barbosa, J.; Teixeira, P. Analysis of Alternative Shelf Life-Extending Protocols and Their Effect on the Preservation of Seafood Products. Foods 2022, 11, 1100. https://doi.org/10.3390/foods11081100
Pinto de Rezende L, Barbosa J, Teixeira P. Analysis of Alternative Shelf Life-Extending Protocols and Their Effect on the Preservation of Seafood Products. Foods. 2022; 11(8):1100. https://doi.org/10.3390/foods11081100
Chicago/Turabian StylePinto de Rezende, Lourenço, Joana Barbosa, and Paula Teixeira. 2022. "Analysis of Alternative Shelf Life-Extending Protocols and Their Effect on the Preservation of Seafood Products" Foods 11, no. 8: 1100. https://doi.org/10.3390/foods11081100
APA StylePinto de Rezende, L., Barbosa, J., & Teixeira, P. (2022). Analysis of Alternative Shelf Life-Extending Protocols and Their Effect on the Preservation of Seafood Products. Foods, 11(8), 1100. https://doi.org/10.3390/foods11081100







