Nutritional Composition of Beach-Cast Marine Algae from the Brazilian Coast: Added Value for Algal Biomass Considered as Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Species Identification
2.2. Proximate Composition
2.3. Protein Solubility
2.4. Statistical Analysis
3. Results and Discussion
3.1. Sample Collection and Species Identification
3.2. Proximate Composition
3.2.1. Dietary Fibers
3.2.2. Mineral Composition
3.2.3. Free Amino Acids
3.2.4. Fatty Acids
3.3. Protein Solubility
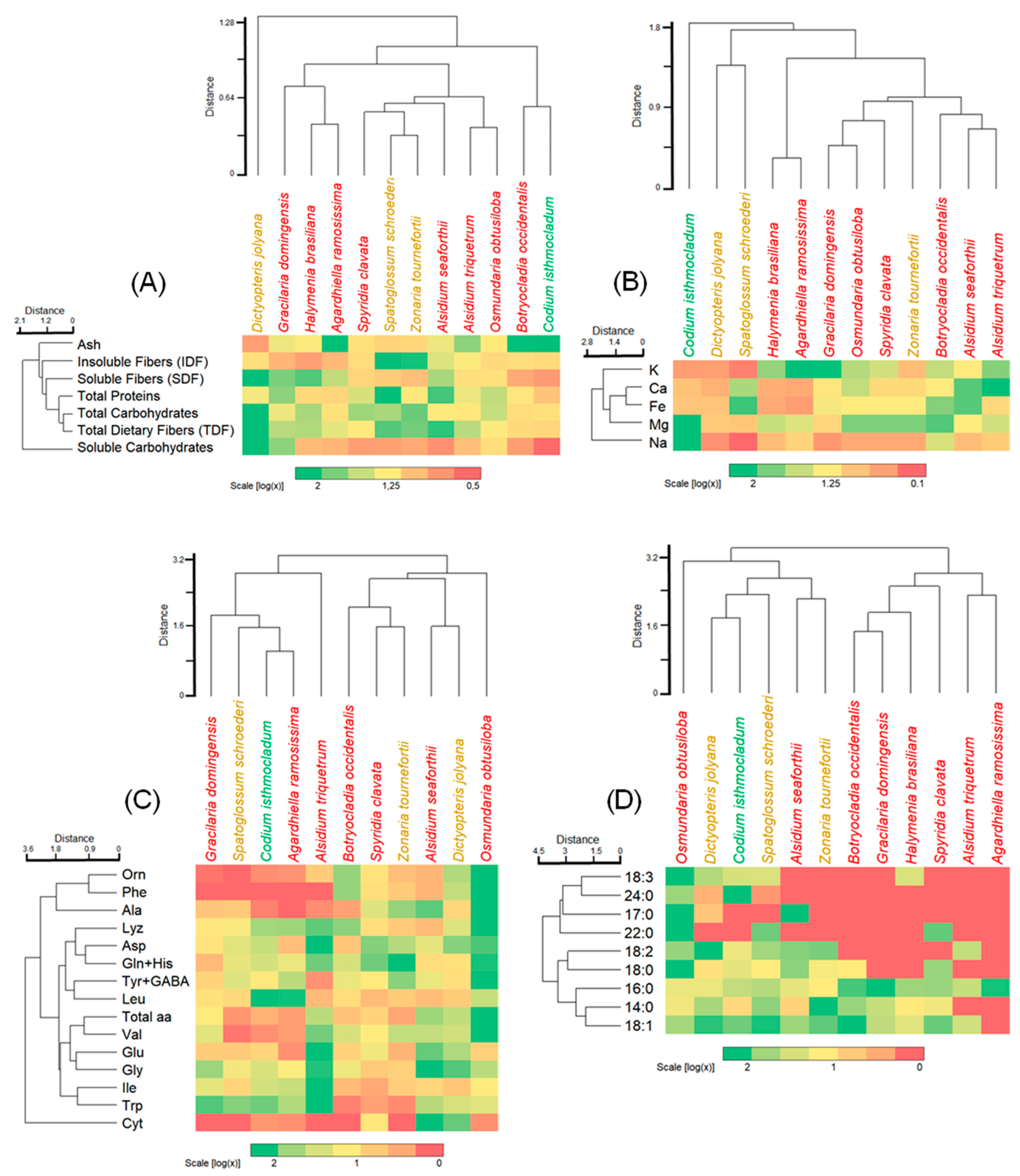
3.4. Integrated Cluster Analyses and Heatmaps
- (a)
- an isolated set by the brown alga Dictyopteris jolyana with high amounts of TDF, SDF, and carbohydrates, and moderate amounts of IDF and proteins;
- (b)
- a set including red and brown algae with variable number of parameters from high, moderate, and low;
- (c)
- a set comprising the red alga Botryocladia occidentalis and green alga Codium isthmocladum with moderate to low values, except for ash content.
- (a)
- one with relatively small amounts of amino acids, such as Gracilaria domingensis, Spatoglossum schoroederi, Codium isthmocladum, and Agardhiella ramosissima;
- (b)
- an alone set by Alsidium triquetrum with high levels of half of the amino acids;
- (c)
- a major set with moderate to a high concentration of almost all amino acids, and
- (d)
- an alone set by Osmundaria obstusiloba exhibiting high levels of almost all amino acids.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Angell, A.R.; Angell, S.F.; de Nys, R.; Paul, N.A. Seaweed as a protein source for mono-gastric livestock. Trends Food Sci. Technol. 2016, 54, 74–84. [Google Scholar] [CrossRef]
- Angell, A.R.; Mata, L.; de Nys, R.; Paul, N.A. The protein content of seaweeds: A universal nitrogen-to-protein conversion factor of five. J. Appl. Phycol. 2016, 28, 511–524. [Google Scholar] [CrossRef]
- Bolton, J.J.; Robertson-Andersson, D.V.; Shuuluka, D.; Kandjengo, L. Growing Ulva (chlorophyta) in integrated systems as a commercial crop for abalone feed in South Africa: A swot analysis. J. Appl. Phycol. 2009, 21, 575–583. [Google Scholar] [CrossRef]
- Mata, L.; Magnusson, M.; Paul, N.A.; de Nys, R. The intensive land-based production of the green seaweeds Derbesia tenuissima and Ulva ohnoi: Biomass and bioproducts. J. Appl. Phycol. 2016, 28, 365–375. [Google Scholar] [CrossRef]
- Domínguez, H. 1-Algae as a source of biologically active ingredients for the formulation of functional foods and nutraceuticals. In Functional Ingredients from Algae for Foods and Nutraceuticals; Woodhead Publishing: Philadelphia, PA, USA, 2013; pp. 1–19. ISBN 978-0-85709-512-1. [Google Scholar]
- Fleurence, J. Seaweed proteins: Biochemical, nutritional aspects and potential uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Fleurence, J. The enzymatic degradation of algal cell walls: A useful approach for improving protein accessibility? J. Appl. Phycol. 1999, 11, 313–314. [Google Scholar] [CrossRef]
- Fleurence, J.; Le Coeur, C.; Mabeau, S.; Maurice, M.; Landrein, A. Comparison of different extractive procedures for proteins from the edible seaweeds Ulva rigida and Ulva rotundata. J. Appl. Phycol. 1995, 7, 577–582. [Google Scholar] [CrossRef]
- Bähr, M.; Fechner, A.; Hasenkopf, K.; Mittermaier, S.; Jahreis, G. Chemical composition of dehulled seeds of selected lupin cultivars in comparison to pea and soya bean. LWT-Food Sci. Technol. 2014, 59, 587–590. [Google Scholar] [CrossRef]
- Tenorio, A.T.; Kyriakopoulou, K.E.; Suarez-Garcia, E.; van den Berg, C.; van der Goot, A.J. Understanding differences in protein fractionation from conventional crops, and herbaceous and aquatic biomass-consequences for industrial use. Trends Food Sci. Technol. 2018, 71, 235–245. [Google Scholar] [CrossRef]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Kumar, C.S.; Ganesan, P.; Suresh, P.; Bhaskar, N. Seaweeds as a source of nutritionally beneficial compounds—A review. J. Food Sci. Technol. 2008, 45, 1–13. [Google Scholar]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [Green Version]
- Fleurence, J. Seaweeds as food. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 149–167. ISBN 978-0-12-802772-1. [Google Scholar]
- Harnedy, P.A.; FitzGerald, R.J. Extraction and enrichment of protein from red and green macroalgae. In Natural Products from Marine Algae: Methods and Protocols; Stengel, D.B., Connan, S., Eds.; Springer: New York, NY, USA, 2015; pp. 103–108. ISBN 978-1-4939-2684-8. [Google Scholar]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez-Hernández, J.; Bozzo, C.; Navarrete, E.; Osorio, A.; Rios, A. Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Patarra, R.F.; Paiva, L.; Neto, A.I.; Lima, E.; Baptista, J. Nutritional value of selected macroalgae. J. Appl. Phycol. 2011, 23, 205–208. [Google Scholar] [CrossRef] [Green Version]
- Baweja, P.; Kumar, S.; Sahoo, D.; Levine, I. Biology of seaweeds. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 41–106. ISBN 978-0-12-802772-1. [Google Scholar]
- Ferdouse, F.; Holdt, S.L.; Smith, R.; Murúa, P.; Yang, Z. The global status of seaweed production, trade and utilization. In Globefish Research Programme; FAO: Rome, Italy, 2018; Volume 124. [Google Scholar]
- Pardilhó, S.; Machado, S.; Bessada, S.; Almeida, M.; Oliveira, M.; Dias, J. Marine macroalgae waste from Northern Portugal: A potential source of natural pigments? Waste Biomass Valorization 2021, 12, 239–249. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- De Bhowmick, G.; Hayes, M. In vitro protein digestibility of selected seaweeds. Foods 2022, 11, 289. [Google Scholar] [CrossRef]
- Pereira, L. A review of the nutrient composition of selected edible seaweeds. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses; Pomin, V.H., Ed.; Nova Science Publishers: Athens, GA, USA, 2011; pp. 15–47. ISBN 978-1-61470-878-0. [Google Scholar]
- Mabeau, S.; Fleurence, J. Seaweed in food products: Biochemical and nutritional aspects. Trends Food Sci. Technol. 1993, 4, 103–107. [Google Scholar] [CrossRef]
- Nunes, N.; Ferraz, S.; Valente, S.; Barreto, M.C.; De Carvalho, M.P. Biochemical composition, nutritional value, and antioxidant properties of seven seaweed species from the Madeira Archipelago. J. Appl. Phycol. 2017, 29, 2427–2437. [Google Scholar] [CrossRef]
- Harb, T.B.; Pereira, M.S.; Cavalcanti, M.I.L.; Fujii, M.T.; Chow, F. Antioxidant activity and related chemical composition of extracts from brazilian beach-cast marine algae: Opportunities of turning a waste into a resource. J. Appl. Phycol. 2021, 33, 3383–3395. [Google Scholar] [CrossRef]
- do Nascimento Santos, G.; do Nascimento, O.S.; dos Anjos Pedreira, F.; Ríos, G.I.; Vasconcelos, J.N.C.; de Castro Nunes, J.M. Análise quali-quantitativa das algas arribadas do Norte do Estado da Bahia, Brasil. Qualitative and quantitative analysis of arribadas algae North of Bahia State, Brazil. Acta Bot. Malacit. 2013, 38, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Lourenço, S.O.; Barbarino, E.; De-Paula, J.C.; Pereira, L.O.d.S.; Marquez, U.M.L. Amino acid composition, protein content and calculation of nitrogen-to-protein conversion factors for 19 tropical seaweeds. Phycol. Res. 2002, 50, 233–241. [Google Scholar] [CrossRef]
- Gressler, V.; Fujii, M.T.; Martins, A.P.; Colepicolo, P.; Mancini-Filho, J.; Pinto, E. Biochemical composition of two red seaweed species grown on the Brazilian Coast. J. Sci. Food Agric. 2011, 91, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Gressler, V.; Yokoya, N.S.; Fujii, M.T.; Colepicolo, P.; Filho, J.M.; Torres, R.P.; Pinto, E. Lipid, fatty acid, protein, amino acid and ash contents in four brazilian red algae species. Food Chem. 2010, 120, 585–590. [Google Scholar] [CrossRef]
- Barbot, Y.N.; Al-Ghaili, H.; Benz, R. A review on the valorization of macroalgal wastes for biomethane production. Mar. Drugs 2016, 14, 120. [Google Scholar] [CrossRef] [Green Version]
- Critchley, A.; Hurtado, A.; Pereira, L.; Cornish, M.; Largo, D.; Paul, N. Seaweed resources of the world: A 2020 vision. Bot. Mar. 2019, 62, 191–193. [Google Scholar] [CrossRef] [Green Version]
- López-Mosquera, M.E.; Fernández-Lema, E.; Villares, R.; Corral, R.; Alonso, B.; Blanco, C. Composting fish waste and seaweed to produce a fertilizer for use in organic agriculture. Procedia Environl. Sci. 2011, 9, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Harb, T.B.; Chow, F. An overview of beach-cast seaweeds: Potential and opportunities for the valorization of underused waste biomass. Algal Res. 2022, 62, 102643. [Google Scholar] [CrossRef]
- Cavalcanti, L.G.M.I.; González Sánchez, P.M.; Fuji, M.T. Comparison of the diversity and biomass of beach-cast seaweeds from Ne and Se Brazil. Eur. J. Phycol. 2022, 1–10. [Google Scholar] [CrossRef]
- Nunes, N.; Valente, S.; Ferraz, S.; Barreto, M.C.; de Carvalho, M.A.P. Biochemical study of attached macroalgae from the Madeira Archipelago and beach-cast macroalgae from the Canary Islands: Multivariate analysis to determine bioresource potential. Bot. Mar. 2020, 63, 283–298. [Google Scholar] [CrossRef]
- AOAC. Ash of flour. In Official Methods of Analysis of the Association of Official Analytcal Chemists (AOCA); Method, 923.03; Horwitz, W.A., Ed.; AOAC International: Gaithersburg, MD, USA, 2003. [Google Scholar]
- AOAC. Protein (crude) in animal feed. In Official Methods of Analysis of the Association of Official Analytical Chemists (AOAC); Method 968.06; AOAC International: Gaithersburg, MD, USA, 2003. [Google Scholar]
- Bjarnadóttir, M.; Aðalbjörnsson, B.V.; Nilsson, A.; Slizyte, R.; Roleda, M.Y.; Hreggviðsson, G.Ó.; Friðjónsson, Ó.H.; Jónsdóttir, R. Palmaria palmata as an alternative protein source: Enzymatic protein extraction, amino acid composition, and nitrogen-to-protein conversion factor. J. Appl. Phycol. 2018, 30, 2061–2070. [Google Scholar] [CrossRef]
- Hayes, M. Measuring protein content in food: An overview of methods. Foods 2020, 9, 1340. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, S.; Hayes, M. Algal proteins: Extraction, application, and challenges concerning production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Mæhre, H.K.; Dalheim, L.; Edvinsen, G.K.; Elvevoll, E.O.; Jensen, I.-J. Protein determination—Method matters. Foods 2018, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Barbarino, E.; Lourenço, S.O. An evaluation of methods for extraction and quantification of protein from marine macro- and microalgae. J. Appl. Phycol. 2005, 17, 447–460. [Google Scholar] [CrossRef]
- Biancarosa, I.; Espe, M.; Bruckner, C.; Heesch, S.; Liland, N.; Waagbø, R.; Torstensen, B.; Lock, E. Amino acid composition, protein content, and nitrogen-to-protein conversion factors of 21 seaweed species from Norwegian Waters. J. Appl. Phycol. 2017, 29, 1001–1009. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.-I.; Lee, Y.C. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef]
- Santa-Catarina, C.; Silveira, V.; Balbuena, T.S.; Viana, A.M.; Estelita, M.E.M.; Handro, W.; Floh, E.I. IAA, ABA, polyamines and free amino acids associated with zygotic embryo development of Ocotea catharinensis. Plant Growth Regul. 2006, 49, 237–247. [Google Scholar] [CrossRef]
- Egydio, A.P.M.; Santa Catarina, C.; Floh, E.I.S.; dos Santos, D.Y.A.C. Free amino acid composition of annona (annonaceae) fruit species of economic interest. Ind. Crops Prod. 2013, 45, 373–376. [Google Scholar] [CrossRef]
- Morr, C.V.; German, B.; Kinsella, J.E.; Regenstein, J.M.; Buren, J.P.v.; Kilara, A.; Lewis, B.A.; Mangino, M.E. A collaborative study to develop a standardized food protein solubility procedure. J. Food Sci. 1985, 50, 1715–1718. [Google Scholar] [CrossRef]
- Tamayo Tenorio, A.; Boom, R.M.; van der Goot, A.J. Understanding leaf membrane protein extraction to develop a food-grade process. Food Chem. 2017, 217, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Dumay, J.; Morançais, M. Proteins and pigments. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 275–318. ISBN 978-0-12-802772-1. [Google Scholar]
- Vásquez, V.; Martínez, R.; Bernal, C. Enzyme-assisted extraction of proteins from the seaweeds Macrocystis pyrifera and Chondracanthus chamissoi: Characterization of the extracts and their bioactive potential. J. Appl. Phycol. 2019, 31, 1999–2010. [Google Scholar] [CrossRef]
- Harrysson, H.; Konasani, V.R.; Toth, G.B.; Pavia, H.; Albers, E.; Undeland, I. Strategies for improving the protein yield in ph-shift processing of Ulva lactuca linnaeus: Effects of ulvan lyases, ph-exposure time, and temperature. ACS Sustain. Chem. Eng. 2019, 7, 12688–12691. [Google Scholar] [CrossRef]
- Rioux, L.-E.; Turgeon, S.L. Seaweed carbohydrates. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 141–192. ISBN 978-0-12-418697-2. [Google Scholar]
- Stiger-Pouvreau, V.; Bourgougnon, N.; Deslandes, E. Chapter 8 carbohydrates from seaweeds. In Seaweed in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2016; pp. 223–274. ISBN 978-0-12-802772-1. [Google Scholar]
- Hurtado, A.Q.; Magdugo, R.; Critchley, A.T. Selected red seaweeds from the Philippines with emerging high-value applications. Adv. Bot. Res. 2020, 95, 19–56. [Google Scholar]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Lahaye, M. Marine algae as sources of fibres: Determination of soluble and insoluble dietary fibre contents in some ‘sea vegetables’. J. Sci. Food Agric. 1991, 54, 587–594. [Google Scholar] [CrossRef]
- Lahaye, M.; Kaeffer, B. Seaweed dietary fibres: Structure, physico-chemical and biological properties relevant to intestinal physiology. Sci. Aliments 1997, 17, 562–584. [Google Scholar]
- Suzuki, T.; Ohsugi, Y.; Yoshie, Y.; Shirai, T.; Hirano, T. Dietary fiber content, water-holding capacity and binding capacity of seaweeds. Fish. Sci. 1996, 62, 454–461. [Google Scholar] [CrossRef] [Green Version]
- Mišurcová, L. Chemical composition of seaweeds. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; Kim, S.-K., Ed.; Wiley Online Library: Chichester, UK, 2012; pp. 173–186. ISBN 978-0-470-97918-1. [Google Scholar]
- Gelli, V.; Barbieri, E. Cultivo e aproveitamento da macroalga Kappaphycus alvarezii para pequenos maricultores. Aquicultura no Brasil: Novas perspectivas. Pedro João 2015, 641–658. [Google Scholar] [CrossRef]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Kraan, S. Pigments and minor compounds in algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Woodhead Publishing: Philadelphia, PA, USA, 2013; pp. 205–251. ISBN 978-0-85709-512-1. [Google Scholar]
- Kato, H.; Rhue, M.R.; Nishimura, T. Role of free amino acids and peptides in food taste. Flavor Chem. 1989, 388, 158–174. [Google Scholar]
- Fleurence, J. Seaweed proteins. In Proteins in Food Processing; Yada, R.Y., Ed.; Woodhead Publishing: Cambridge, UK, 2004; pp. 197–213. ISBN 978-1-85573-723-5. [Google Scholar]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive proteins, peptides, and amino acids from macroalgae. J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Conde, E.; Balboa, E.M.; Parada, M.; Falqué, E. Algal proteins, peptides and amino acids. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Philadelphia, PA, USA, 2013; pp. 135–180. ISBN 978-0-85709-512-1. [Google Scholar]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Kumar, M.; Gupta, V.; Reddy, C.R.K.; Jha, B. Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem. 2010, 120, 749–757. [Google Scholar] [CrossRef]
- Harrysson, H.; Hayes, M.; Eimer, F.; Carlsson, N.G.; Toth, G.B.; Undeland, I. Production of protein extracts from swedish red, green, and brown seaweeds, Porphyra umbilicalis (Kutzing), Ulva lactuca (Linnaeus), and Saccharina latissima (Linnaeus) j. V. Lamouroux using three different methods. J. Appl. Phycol. 2018, 30, 3565–3580. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Machado, D.I.; López-Cervantes, J.; López-Hernández, J.; Paseiro-Losada, P. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Fleurence; Massiani, L.; Guyader, O.; Mabeau, S. Use of enzymatic cell wall degradation for improvement of protein extraction from Chondrus crispus, Gracilaria verrucosa and Palmaria palmata. J. Appl. Phycol. 1995, 7, 393. [Google Scholar] [CrossRef]
- Vilg, J.V.; Undeland, I. Ph-driven solubilization and isoelectric precipitation of proteins from the brown seaweed Saccharina latissima—effects of osmotic shock, water volume and temperature. J. Appl. Phycol. 2017, 29, 585–593. [Google Scholar] [CrossRef] [Green Version]
- Muranyi, I.S.; Otto, C.; Pickardt, C.; Osen, R.; Koehler, P.; Schweiggert-Weisz, U. Influence of the isolation method on the technofunctional properties of protein isolates from Lupinus angustifolius L. J. Food Sci. 2016, 81, C2656–C2663. [Google Scholar] [CrossRef]
- Cornish, M.L.; Mouritsen, O.G.; Critchley, A.T. A mini-review on the microbial continuum: Consideration of a link between judicious consumption of a varied diet of macroalgae and human health and nutrition. J. Ocean Limnol. 2019, 37, 790–805. [Google Scholar] [CrossRef]
| Species | Dry Matter | Ash | Total Dietary Fibers (TDF) | Soluble Fibers (SDF) | Insoluble Fibers (IDF) | SDF/TDF | IDF/TDF | Total Proteins | Total Carbohydrates | Soluble Carbohydrates * |
|---|---|---|---|---|---|---|---|---|---|---|
| Rhodophyta (red algae) | ||||||||||
| Agardhiella ramosissima | 92.2 ± 0.2 cd | 57.3 ± 0.2 a | 40.1 ± 1.5 cd | 31.5 ± 2.1 d | 8.6 ± 0.7 d | 0.79 | 0.21 | 7.3 ± 0.1 i | 35.4 ± 0.3 i | 25.9 ± 0.1 de |
| Alsidium seaforthii | 94.5 ± 0.3 a | 34.7 ± 1.0 c | 61.7 ± 4.9 a | 33.5 ± 0.6 d | 28.2 ± 4.7 b | 0.54 | 0.46 | 19.7 ± 0.1 b | 45.6 ± 0.9 g | 11.9 ± 0.1 de |
| Alsidium triquetrum | 92.7 ± 0.1 bc | 46.5 ± 0.4 b | 45.2 ± 4.8 c | 19.3 ± 2.4 e | 25.9 ± 2.3 b | 0.43 | 0.57 | 12.8 ± 0.2 f | 40.7 ± 0.5 h | 32.3 ± 0.1 d |
| Botryocladia occidentalis | 92.7 ± 0.1 bc | 58.3 ± 0.4 a | 25.0 ± 0.2 e | 6.2 ± 0.4 h | 18.8 ± 0.6 c | 0.25 | 0.75 | 10.3 ± 0.2 g | 31.4 ± 0.5 j | 15.8 ± 0.1 de |
| Gracilaria domingensis | 94.9 ± 0.3 a | 35.2 ± 0.9 c | 45.9 ± 0.8 c | 37.5 ± 0.9 c | 8.3 ± 0.1 d | 0.82 | 0.18 | 16.8 ± 0.1 c | 47.9 ± 0.8 f | 113.5 ± 0.1 b |
| Halymenia brasiliana | 89.0 ± 0.1 h | 33.7 ± 1.0 c | 46.8 ± 0.3 c | 41.8 ± 1.1 b | 5.1 ± 0.9 d | 0.89 | 0.11 | 8.2 ± 0.4 h | 58.1 ± 1.4 d | 30.0 ± 0.1 d |
| Osmundaria obtusiloba | 92.9 ± 0.1 b | 31.2 ± 0.1 d | 36.9 ± 3.5 d | 19.8 ± 1.7 e | 17.1 ± 1.7 c | 0.54 | 0.46 | 14.6 ± 0.2 d | 54.1 ± 0.2 e | 58.9 ± 0.1 c |
| Spyridia clavata | 90.8 ± 0.2 g | 25.8 ± 0.2 e | 33.7 ± 0.2 d | 16.0 ± 0.9 ef | 17.8 ± 0.9 c | 0.47 | 0.53 | 13.6 ± 0.2 e | 60.6 ± 0.3 c | 15.8 ± 0.1 cde |
| Phaeophyceae (brown algae) | ||||||||||
| Dictyopteris jolyana | 92.0 ± 0.2 de | 6.5 ± 0.6 g | 65.8 ± 1.1 a | 46.4 ± 1.0 a | 19.4 ± 0.2 c | 0.71 | 0.29 | 12.5 ± 0.2 f | 81.0 ± 0.7 a | 146.0 ± 0.1 a |
| Spatoglossum schroederi | 91.3 ± 0.4 fg | 19.4 ± 0.4 f | 59.2 ± 0.4 ab | 13.1 ± 0.3 fg | 46.2 ± 0.3 a | 0.22 | 0.78 | 21.5 ± 0.2 a | 59.1 ± 0.2 cd | 16.6 ± 0.1 de |
| Zonaria tournefortii | 91.6 ± 0.4 ef | 20.4 ± 0.8 f | 54.5 ± 2.3 b | 10.2 ± 1.8 g | 44.3 ± 1.1 a | 0.19 | 0.81 | 10.9 ± 0.4 g | 68.7 ± 0.5 b | 19.5 ± 0.1 de |
| Chlorophyta (green algae) | ||||||||||
| Codium isthmocladum | 93.1 ± 0.1 b | 59.3 ± 1.5 a | 22.1 ± 0.2 e | 4.7 ± 0.5 h | 17.4 ± 0.3 c | 0.21 | 0.79 | 5.1 ± 0.1 j | 35.6 ± 1.5 i | 5.3 ± 0.1 e |
| Species | Ca | K | Mg | Na | Fe * | Cd | Cu |
|---|---|---|---|---|---|---|---|
| Rhodophyta (red algae) | |||||||
| Agardhiella ramosissima | 0.50 ± 0.01 i | 11.18 ± 0.06 a | 0.96 ± 0.01 f | 2.83 ± 0.01 c | 112.72 ± 0.60 j | nd | nd |
| Alsidium seaforthii | 5.94 ± 0.05 b | 4.39 ± 0.01 h | 0.81 ± 0.01 h | 1.72 ± 0.01 e | 1879.26 ± 24.36 c | nd | nd |
| Alsidium triquetrum | 7.24 ± 0.01 a | 7.76 ± 0.03 c | 1.03 ± 0.01 e | 2.44 ± 0.01 d | 509.17 ± 2.99 h | nd | nd |
| Botryocladia occidentalis | 2.82 ± 0.03 e | 5.90 ± 0.01 f | 1.74 ± 0.01 b | 7.17 ± 0.06 b | 1613.59 ± 12.54 d | nd | nd |
| Gracilaria domingensis | 2.29 ± 0.03 f | 10.80 ± 0.01 b | 0.45 ± 0.01 i | 0.65 ± 0.01 i | 941.38 ± 14.01 e | nd | nd |
| Halymenia brasiliana | 0.54 ± 0.01 i | 7.24 ± 0.03 d | 1.12 ± 0.02 d | 1.62 ± 0.06 f | 153.21 ± 0.49 j | nd | nd |
| Osmundaria obtusiloba | 3.84 ± 0.03 d | 6.25 ± 0.02 e | 0.44 ± 0.01 i | 0.29 ± 0.01 k | 832.60 ± 2.24 g | nd | nd |
| Spyridia clavata | 1.70 ± 0.03 g | 5.43 ± 0.01 g | 1.51 ± 0.01 c | 1.04 ± 0.01 g | 878.81 ± 14.26 f | nd | nd |
| Phaeophyceae (brown algae) | |||||||
| Dictyopteris jolyana | 0.58 ± 0.01 i | 0.56 ± 0.01 j | 0.33 ± 0.01 j | 0.54 ± 0.01 j | 316.57 ± 1.88 i | nd | nd |
| Spatoglossum schroederi | 4.31 ± 0.05 c | 0.26 ± 0.01 k | 0.30 ± 0.01 k | 0.15 ± 0.01 l | 2021.13 ± 28.13 b | nd | nd |
| Zonaria tournefortii | 2.75 ± 0.01 e | 1.28 ± 0.01 i | 0.86 ± 0.01 g | 0.81 ± 0.01 h | 2306.33 ± 15.58 a | nd | nd |
| Chlorophyta (green algae) | |||||||
| Codium isthmocladum | 0.93 ± 0.01 h | 0.54 ± 0.01 j | 2.11 ± 0.01 a | 14.90 ± 0.01 a | 310.40 ± 10.84 i | nd | nd |
| Species | Ala | Arg | Asn | Asp | Cit | Gln + His | Glu | Gly | Ile | Leu |
|---|---|---|---|---|---|---|---|---|---|---|
| Rhodophyta (red algae) | ||||||||||
| Agardhiella ramosissima | 0.4 ± 0.1 d | 15.4 ± 0.3 c | 8.5 ± 0.8 b | 12.0 ± 0.8 g | 65.8 ± 1.7 d | 14.5 ± 0.2 c | 11.8 ± 1.1 e | 3.2 ± 0.2 c | 6.8 ± 0.3 b | 30.3 ± 0.2 b |
| Alsidium seaforthii | 36.8 ± 14.9 b | 4.9 ± 1.5 c | 35.7 ± 0.1 b | 42.7 ± 6.4 ef | 2878.4 ± 211.5 a | 8.2 ± 3.3 c | 327.1 ± 44.2 b | 19.0 ± 7.9 a | 2.4 ± 0.5 cd | 2.1 ± 0.6 de |
| Alsidium triquetrum | 1.2 ± 0.4 d | 1682.6 ± 76.7 a | 2.6 ± 1.1 b | 190.8 ± 5.5 a | 0.9 ± 0.5 d | 41.1 ± 4.7 b | 513.4 ± 9.9 a | 16.0 ± 1.7 a | 23.7 ± 2.0 a | 1.8 ± 0.4 e |
| Botryocladia occidentalis | 1.9 ± 0.4 d | 2.2 ± 0.4 c | 6.3 ± 0.2 b | 13.9 ± 1.5 g | 9.1 ± 0.9 d | 17.6 ± 3.3 c | 46.3 ± 3.4 e | 3.9 ± 0.6 c | 1.4 ± 0.3 d | 4.0 ± 0.6 de |
| Gracilaria domingensis | 4.0 ± 1.2 d | 293.0 ± 78.7 b | 4.4 ± 0.9 b | 26.3 ± 5.7 fg | 8.5 ± 1.6 d | 3.9 ± 0.6 c | 42.7 ± 9.7 e | 8.1 ± 1.9 bc | 5.3 ± 0.9 bc | 4.3 ± 1.5 d |
| Osmundaria obtusiloba | 56.1 ± 5.7 a | 2.1 ± 0.2 c | 6788.1 ± 751.8 a | 139.8 ± 17.4 b | 67.4 ± 15.2 d | 59.7 ± 8.3 ab | 45.8 ± 8.6 e | 6.1 ± 1.3 bc | 4.1 ± 0.1 bcd | 9.9 ± 0.7 c |
| Spyridia clavata | 13.6 ± 3.6 cd | 2.0 ± 0.5 c | 5.9 ± 0.6 b | 96.8 ± 5.4 c | 416.4 ± 16.7 c | 40.8 ± 1.1 b | 103.3 ± 3.1 d | 1.5 ± 1.9 c | 0.9 ± 0.2 d | 2.0 ± 0.6 de |
| Phaeophyceae (brown algae) | ||||||||||
| Dictyopteris jolyana | 5.8 ± 0.6 d | 2.4 ± 0.01 c | 51.7 ± 1.0 b | 96.0 ± 3.2 c | 1832.7 ± 0.1 b | 8.8 ± 0.01 c | 184.8 ± 0.1 c | 13.6 ± 0.01 ab | 1.7 ± 0.2 d | 2.8 ± 1.3 de |
| Spatoglossum schroederi | 4.6 ± 0.1 d | 1.3 ± 0.4 c | 5.0 ± 0.3 b | 44.6 ± 0.1 ef | 9.0 ± 4.5 d | 15.0 ± 0.01 c | 41.2 ± 0.1 e | 3.7 ± 0.9 c | 2.3 ± 1.5 cd | 3.6 ± 0.7 de |
| Zonaria tournefortii | 24.9 ± 5.7 bc | 1.0 ± 0.3 c | 4.9 ± 2.0 b | 66.4 ± 0.1 d | 24.2 ± 0.1 d | 69.0 ± 20.6 a | 24.5 ± 0.1 e | 1.7 ± 0.4 c | 2.0 ± 0.8 cd | 2.8 ± 1.2 de |
| Chlorophyta (green algae) | ||||||||||
| Codium isthmocladum | 0.9 ± 0.2 d | 20.7 ± 0.1 c | 5.9 ± 2.3 b | 55.8 ± 2.8 de | 78.0 ± 0.1 d | 18.2 ± 0.9 c | 49.6 ± 0.5 e | 4.8 ± 1.2 c | 6.3 ± 2.5 b | 33.7 ± 0.1 a |
| Species | Lys | Met | Orn | Phe | Ser | Thr | Trp | Tyr + GABA | Val | |
| Rhodophyta (red algae) | ||||||||||
| Agardhiella ramosissima | 15.7 ± 2.7 bcd | 0.8 ± 0.2 b | 19.8 ± 1.9 e | 1.3 ± 0.2 de | 46.7 ± 1.6 cde | 1.1 ± 0.1 bc | 45.8 ± 1.6 cde | 2.7 ± 0.3 b | 0.7 ± 0.3 e | |
| Alsidium seaforthii | 2.2 ± 0.6 f | 1.0 ± 0.2 b | 42.8 ± 3.5 de | 7.6 ± 0.4 de | 43.9 ± 9.1 cde | nd | 43.9 ± 9.1 cde | 1.8 ± 0.1 b | 6.7 ± 1.3 c | |
| Alsidium triquetrum | 24.6 ± 1.1 b | 3.3 ± 1.3 b | 67.3 ± 3.9 de | 1.0 ± 0.1 de | 1128.3 ± 5.2 a | 10.1 ± 5.5 a | 128.3 ± 5.2 a | 0.2 ± 0.1 b | 13.0 ± 1.4 b | |
| Botryocladia occidentalis | 18.0 ± 2.9 bc | 0.5 ± 0.2 b | 361.7 ± 27.2 b | 70.2 ± 9.2 b | 2.1 ± 0.5 g | 0.2 ± 0.1 c | 2.1 ± 0.5 f | 1.8 ± 0.5 b | 5.8 ± 1.5 cd | |
| Gracilaria domingensis | 5.4 ± 0.6 ef | 732.3 ± 212.7 a | 10.8 ± 2.1 e | 0.5 ± 0.1 de | 91.6 ± 34.6 b | 0.9 ± 0.6 bc | 91.6 ± 34.6 b | 0.8 ± 0.1 b | 5.1 ± 1.5 cd | |
| Osmundaria obtusiloba | 37.9 ± 8.3 a | 1.2 ± 0.2 b | 814.6 ± 68.2 a | 156.2 ± 17.0 a | 29.9 ± 4.9 ef | 5.6 ± 1.3 ab | 29.9 ± 4.9 ef | 8.4 ± 1.6 a | 22.4 ± 1.0 a | |
| Spyridia clavata | 4.6 ± 1.4 f | 0.5 ± 0.2 b | 108.7 ± 0.9 d | 18.6 ± 3.9 d | 4.2 ± 0.4 g | 0.7 ± 0.4 bc | 4.2 ± 0.4 f | 1.5 ± 0.2 b | 3.6 ± 0.6 d | |
| Phaeophyceae (brown algae) | ||||||||||
| Dictyopteris jolyana | 9.0 ± 0.5 cdef | 0.7 ± 0.1 b | 289.5 ± 21.4 c | 46.8 ± 5.6 c | 32.7 ± 5.0 def | 1.9 ± 0.1 bc | 32.7 ± 5.0 def | 1.0 ± 0.1 b | 6.0 ± 0.7 cd | |
| Spatoglossum schroederi | 7.1 ± 4.3 def | 1.3 ± 0.7 b | 7.3 ± 5.1 e | 0.4 ± 0.3 e | 64.5 ± 0.0 bcd | 0.4 ± 0.3 c | 64.5 ± 0.1 bcd | 2.0 ± 2.5 b | 0.3 ± 0.1 e | |
| Zonaria tournefortii | 3.1 ± 0.8 f | 0.2 ± 0.1 b | 50.9 ± 15.9 de | 12.7 ± 0.6 de | 2.6 ± 1.2 g | 1.2 ± 0.2 bc | 2.6 ± 1.2 f | 4.1 ± 0.5 b | 6.7 ± 0.1 c | |
| Chlorophyta (green algae) | ||||||||||
| Codium isthmocladum | 15.0 ± 4.8 bcde | 3.6 ± 3.3 b | 17.6 ± 8.5 e | 0.7 ± 0.7 de | 74.8 ± 5.3 bc | 1.0 ± 0.5 bc | 74.8 ± 5.3 bc | 1.3 ± 0.8 b | 0.5 ± 0.4 e | |
| Species | 14:0 | 16:0 | 17:0 | 18:0 | 22:0 | 24:0 | 18:1 | 18:2 | 18:3 |
|---|---|---|---|---|---|---|---|---|---|
| Rhodophyta (red algae) | |||||||||
| Agardhiella ramosissima | nd | 62.77 ± 10.63 a | nd | nd | nd | nd | nd | nd | nd |
| Alsidium seaforthii | 1.92 ± 0.21 g | 21.65 ± 0.47 d | 3.56 ± 1.43 | 2.63 ± 0.20 | nd | nd | 10.40 ± 0.87 bcd | 4.68 ± 1.05 | nd |
| Alsidium triquetrum | nd | 27.36 ± 0.33 cd | nd | nd | nd | nd | 7.50 ± 0.31 d | 3.24 ± 0.10 | nd |
| Botryocladia occidentalis | 7.32 ± 0.08 bc | 53.58 ± 0.78 b | nd | 1.11 ± 1.93 | nd | nd | 20.09 ± 0.63 a | nd | nd |
| Gracilaria domingensis | 5.53 ± 0.19 d | 68.16 ± 1.15 a | nd | nd | nd | nd | 8.38 ± 0.10 cd | nd | nd |
| Halymenia brasiliana | 3.32 ± 0.13 f | 35.32 ± 1.08 c | nd | nd | nd | nd | 6.62 ± 0.34 d | nd | 2.01 ± 1.74 |
| Osmundaria obtusiloba | 4.11 ± 0.64 e | 21.27 ± 2.50 d | 3.52 ± 1.50 | 4.67 ± 1.05 | 5.18 ± 2.04 | 3.14 ± 1.63 | 9.82 ± 1.69 cd | 4.21 ± 1.41 | 5.86 ± 0.48 |
| Spyridia clavata | 4.13 ± 0.32 e | 35.50 ± 3.81 c | nd | 2.45 ± 2.27 | 3.64 ± 0.00 | nd | 13.99 ± 1.45 abcd | nd | nd |
| Phaeophyceae (brown algae) | |||||||||
| Dictyopteris jolyana | 6.89 ± 0.35 c | 21.05 ± 1.05 d | 0.34 ± 0.58 | 0.91 ± 0.05 | nd | 0.37 ± 0.63 | 17.67 ± 0.90 bcd | 7.89 ± 0.40 | 2.69 ± 0.13 |
| Spatoglossum schroederi | 7.86 ± 0.25 b | 30.44 ± 1.19 c | nd | 1.16 ± 0.25 | 3.54 ± 0.00 | 0.19 ± 0.16 | 18.26 ± 0.86 ab | 3.93 ± 0.18 | 2.01 ± 0.06 |
| Zonaria tournefortii | 14.01 ± 0.77 a | 22.49 ± 1.17 d | 0.44 ± 0.75 | 1.69 ± 0.09 | nd | nd | 15.58 ± 1.24 abc | 5.40 ± 0.39 | nd |
| Chlorophyta (green algae) | |||||||||
| Codium isthmocladum | 2.20 ± 0.18 g | 28.03 ± 2.67 cd | nd | 1.50 ± 0.12 | nd | 6.09 ± 0.69 | 13.66 ± 1.35 abcd | 2.36 ± 0.18 | 2.10 ± 0.19 |
| Species | pH 2 | pH 4 | pH 6 | pH 8 | pH 10 | pH 12 | pH 13 | Total Proteins |
|---|---|---|---|---|---|---|---|---|
| Rhodophyta (red algae) | ||||||||
| Agardhiella ramosissima | 27.3 ± 2.5 c | 22.4 ± 2.5 cde | 34.7 ± 0.1 b | 40.9 ± 8.7 a | 33.5 ± 1.3 cd | 29.8 ± 2.5 d | 51.0 ± 1.2 a | 7.3 ± 0.1 i |
| Alsidium seaforthii | 27.4 ± 4.0 c | 39.5 ± 0.9 a | 40.4 ± 0.9 a | 38.6 ± 0.9 a | 40.8 ± 2.2 a | 42.2 ± 0.9 b | 54.8 ± 1.8 a | 19.7 ± 0.1 b |
| Alsidium triquetrum | 23.7 ± 0.01 cd | 25.9 ± 2.1 cd | 25.9 ± 0.7 c | 27.3 ± 0.7 b | 29.3 ± 0.1 cd | 35.6 ± 0.6 c | nd | 12.8 ± 0.2 f |
| Gracilaria domingensis | 32.8 ± 0.5 b | 33.9 ± 0.5 a | 37.4 ± 0.1 ab | 35.9 ± 0.5 a | 38.0 ± 1.6 cd | 38.5 ± 0.1 bc | 52.5 ± 1.5 a | 16.8 ± 0.1 c |
| Halymenia brasiliana | 18.2 ± 0.1 e | 19.4 ± 1.1 de | 22.7 ± 0.1 cd | 19.3 ± 1.1 cd | 20.5 ± 2.3 e | 21.6 ± 1.1 e | 34.0 ± 2.3 bc | 8.2 ± 0.4 h |
| Osmundaria obtusiloba | 39.8 ± 1.6 a | 37.2 ± 2.1 a | 35.0 ± 3.2 b | 39.9 ± 0.5 a | 32.4 ± 2.7 cd | 33.4 ± 3.7 cd | 34.0 ± 0.1 bc | 14.6 ± 0.2 d |
| Spyridia clavata | 27.2 ± 0.7 c | 33.2 ± 0.1 ab | 42.0 ± 3.3 a | 37.2 ± 1.3 a | 35.2 ± 3.3 abc | 48.6 ± 2.0 a | 26.0 ± 0.8 c | 13.6 ± 0.2 e |
| Phaeophyceae (brown algae) | ||||||||
| Dictyopteris jolyana | 3.6 ± 0.7 fg | 4.3 ± 0.1 f | 6.5 ± 0.7 ef | 7.3 ± 0.1 ef | 6.5 ± 0.7 f | 7.2 ± 0.1 f | 25.3 ± 0.8 c | 12.5 ± 0.2 f |
| Spatoglossum schroederi | 0.8 ± 0.1 g | nd | 2.9 ± 2.1 f | 4.2 ± 0.1 ef | 5.8 ± 0.8 f | 6.3 ± 0.4 f | 26.3 ± 1.3 c | 21.5 ± 0.2 a |
| Zonaria tournefortii | 6.7 ± 0.1 f | 15.9 ± 5.9 e | 9.2 ± 0.8 e | 11.7 ± 1.7 de | 24.2 ± 2.5 de | 29.2 ± 2.5 d | 40.0 ± 6.7 b | 10.9 ± 0.4 g |
| Chlorophyta (green algae) | ||||||||
| Codium isthmocladum | nd | 1.8 ± 1.8 f | 3.5 ± 0.1 f | 1.8 ± 1.8 f | 3.5 ± 0.1 f | 7.0 ± 0.1 f | nd | 5.1 ± 0.1 j |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandalka, A.; Cavalcanti, M.I.L.G.; Harb, T.B.; Toyota Fujii, M.; Eisner, P.; Schweiggert-Weisz, U.; Chow, F. Nutritional Composition of Beach-Cast Marine Algae from the Brazilian Coast: Added Value for Algal Biomass Considered as Waste. Foods 2022, 11, 1201. https://doi.org/10.3390/foods11091201
Mandalka A, Cavalcanti MILG, Harb TB, Toyota Fujii M, Eisner P, Schweiggert-Weisz U, Chow F. Nutritional Composition of Beach-Cast Marine Algae from the Brazilian Coast: Added Value for Algal Biomass Considered as Waste. Foods. 2022; 11(9):1201. https://doi.org/10.3390/foods11091201
Chicago/Turabian StyleMandalka, Andrea, Maria Irisvalda Leal Gondim Cavalcanti, Talissa Barroco Harb, Mutue Toyota Fujii, Peter Eisner, Ute Schweiggert-Weisz, and Fungyi Chow. 2022. "Nutritional Composition of Beach-Cast Marine Algae from the Brazilian Coast: Added Value for Algal Biomass Considered as Waste" Foods 11, no. 9: 1201. https://doi.org/10.3390/foods11091201
APA StyleMandalka, A., Cavalcanti, M. I. L. G., Harb, T. B., Toyota Fujii, M., Eisner, P., Schweiggert-Weisz, U., & Chow, F. (2022). Nutritional Composition of Beach-Cast Marine Algae from the Brazilian Coast: Added Value for Algal Biomass Considered as Waste. Foods, 11(9), 1201. https://doi.org/10.3390/foods11091201









