Biotechnological Production of Sustainable Microbial Proteins from Agro-Industrial Residues and By-Products
Abstract
:1. Introduction
2. Current Situation of Microbial Protein Production
3. Biotechnological Production of Single-Cell Proteins
3.1. SCP Producing Microorganisms
3.1.1. Fungi
3.1.2. Yeast
3.1.3. Algae
3.1.4. Bacteria
3.1.5. Mixed Cultures of Microorganisms
3.2. Substrates for SCP Production
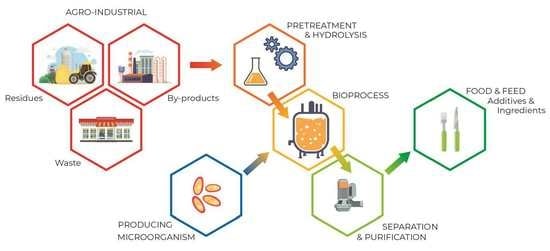
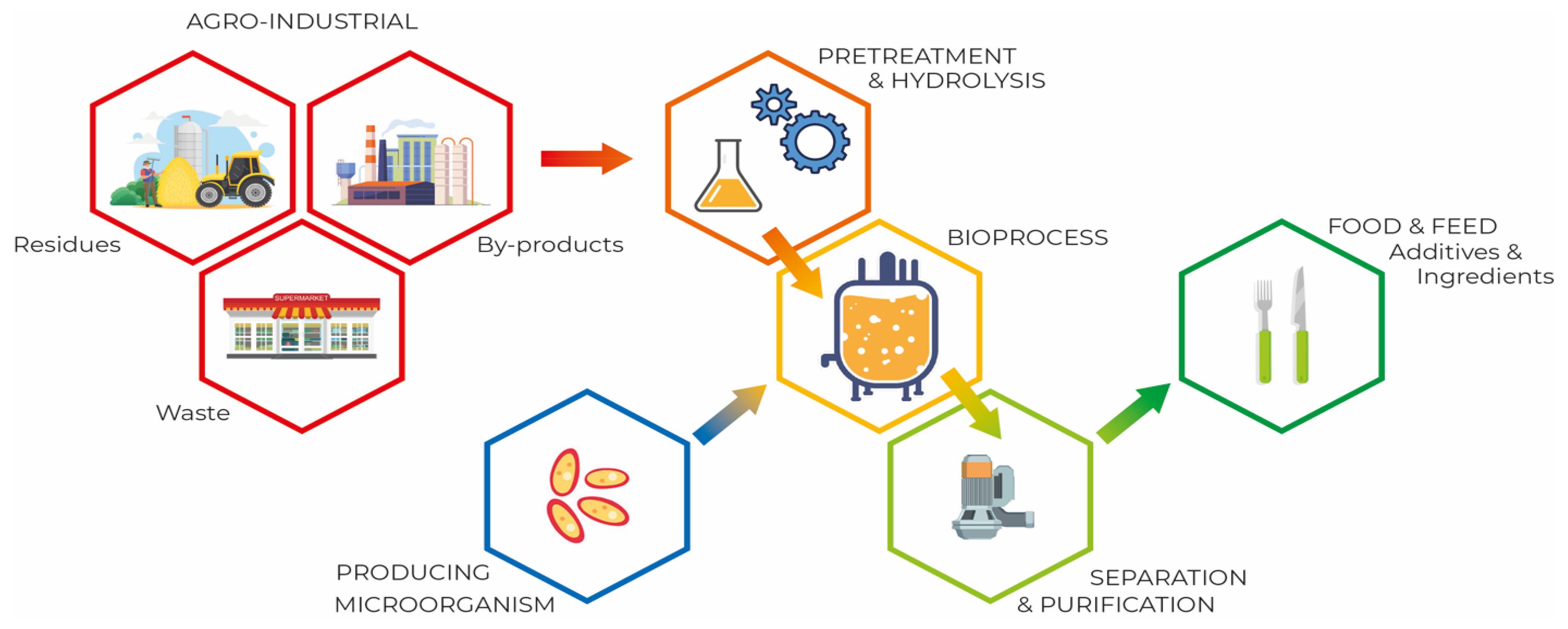
3.3. SCP Production Process
- Preparation of an adequate medium with a suitable carbon source,
- Prevention of contamination of the chosen fermentation medium and the bioreactor,
- Production of the desired microorganisms,
- Separation of microbial biomass and its processing.
4. Future Perspectives and Outcomes of Microbial Protein Production
- Agriculture—by using fewer land resources for crop and animal farming, as well as valorization of agro-industrial residues,
- Food production—in a faster and more cost-effective way to ensure food security for a growing world population,
- Feed production—in larger quantities with fewer resources,
- Environmental protection (circularity and sustainability)—by cutting deforestation and biodiversity loss, reducing greenhouse gas emissions (reversing climate change), and enhancing better air and water quality,
- Human health—by decreasing malnutrition, providing healthier and sustainable diets and diversifying the offer of proteins,
- Science and economy—by enhancing research, engaging young scientists, cooperating with stakeholders and industry, fostering competitiveness, triggering innovation, business models, value-added products, goods, services, and jobs,
- Society—by changing consumer habits, breaking down barriers to dietary transition, and educating and raising awareness about healthier and more sustainable choices.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khoshnevisan, B.; Tsapekos, P.; Zhang, Y.; Valverde-Pérez, B.; Angelidaki, I. Urban biowaste valorization by coupling anaerobic digestion and single cell protein production. Bioresour. Technol. 2019, 290, 121743. [Google Scholar] [CrossRef] [PubMed]
- Rischer, H.; Szilvay, G.R.; Oksman-Caldentey, K. Cellular agriculture—Industrial biotechnology for food and materials. Curr. Opin. Biotech. 2020, 61, 128–134. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Sustainable and circular bioeconomy for food systems transformation. Available online: https://www.fao.org/in-action/sustainable-and-circular-bioeconomy/resources/news/details/en/c/1459357/ (accessed on 20 September 2022).
- Kurek, M.A.; Onopiuk, A.; Pogorzelska-Nowicka, E.; Szpicer, A.; Zalewska, M.; Półtorak, A. Novel Protein Sources for Applications in Meat-Alternative Products—Insight and Challenges. Foods 2022, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Nutritional value of proteins from different food sources: A review. J. Agric. Food Chem. 1996, 44, 6–29. [Google Scholar] [CrossRef]
- Hadi, J.; Brightwell, G. Safety of Alternative Proteins: Technological, Environmental and Regulatory Aspects of Cultured Meat, Plant-Based Meat, Insect Protein and Single-Cell Protein. Foods 2021, 10, 1226. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, I. Single Cell Protein; Springer: Berlin, Germany, 1985; p. 3. [Google Scholar]
- Anupama; Ravindra, P. Value-added food: Single cell protein. Biotechnol. Adv. 2000, 18, 459–479. [Google Scholar] [CrossRef]
- Spalvins, K.; Zihare, L.; Blumberga, D. Single cell protein production from waste biomass: Comparison of various industrial by-products. Energy Procedia. 2018, 147, 409–418. [Google Scholar] [CrossRef]
- Zepka, L.Q.; Jacob-Lopez, E.; Goldbeck, R.; Souza-Soares, L.A.; Queiroz, M.I. Nutritional evaluation of single-cell protein produced by Aphanothece Microsc. Nägeli. Bioresour. Technol. 2010, 101, 7107–7111. [Google Scholar] [CrossRef]
- Molfetta, M.; Morais, E.G.; Barreira, L.; Bruno, G.L.; Porcelli, F.; Dugat-Bony, E.; Bonnarme, P.; Minervini, F. Protein Sources Alternative to Meat: State of the Art and Involvement of Fermentation. Foods 2022, 11, 2065. [Google Scholar] [CrossRef]
- Suman, G.; Nupur, M.; Anuradha, S.; Pradeep, B. Single Cell Protein Production: A Review. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 251–262. [Google Scholar]
- Mensah, J.K.M.; Twumasi, P. Use of pineapple waste for single cell protein (SCP) production and the effect of substrate concentration on the yield. J. Food Biochem. 2016, 40, e12478. [Google Scholar] [CrossRef]
- Nyyssölä, A.; Suhonen, A.; Ritala, A.; Oksman-Caldentey, K. The role of single cell protein in cellular agriculture. Curr. Opin. Biotechnol. 2022, 75, 102686. [Google Scholar] [CrossRef] [PubMed]
- Sharif, M.; Zafar, M.H.; Aqib, A.I.; Saeed, M.; Farag, M.R.; Alagawany, M. Single cell protein: Sources, mechanism of production, nutritional value and its uses in aquaculture nutrition. Aquaculture 2021, 531, 735885. [Google Scholar] [CrossRef]
- Wongputtisin, P.; Khanongnuch, C.; Khongbantad, W.; Niamsup, P.; Lumyong, S. Screening and selection of Bacillus spp. for fermented corticate soybean meal production. J. Appl. Microbiol. 2012, 113, 798–806. [Google Scholar] [CrossRef]
- Jones, S.W.; Karpol, A.; Friedman, S.; Maru, B.T.; Tracy, B.P. Recent advances in single cell protein use as a feed ingredient in aquaculture. Curr. Opin. Biotech. 2020, 61, 189–197. [Google Scholar] [CrossRef]
- Voutilainen, E.; Pihlajaniemi, V.; Parviainen, T. Economic comparison of food protein production with single-cell organisms from lignocellulose side-streams. Bioresour. Technol. Rep. 2021, 14, 100683. [Google Scholar] [CrossRef]
- Carranza-Méndez, R.C.; Chávez-González, M.L.; Sepúlveda-Torre, L.; Aquilar, N.C.; Govea-Salas, M.; Ramos-González, R. Production of single cell protein from orange peel residues by Candida utilis. Biocatal. Agric. Biotechnol. 2022, 40, 102298. [Google Scholar] [CrossRef]
- Nigam, P.S.; Singh, A. Single Cell Protein. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: London, UK, 2014; Volume 3, pp. 415–438. [Google Scholar] [CrossRef]
- Yadav, J.S.S.; Yan, S.; Pilli, S.; Kumar, L.; Tyagi, R.D.; Surampalli, R.Y. Cheese whey: A potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol. Adv. 2015, 33, 756–774. [Google Scholar] [CrossRef]
- Matassa, S.; Boon, N.; Pikaar, I.; Verstraete, W. Microbial protein: Future sustainable food supply route with low environmental foorprint. Microb. Biotechnol. 2016, 9, 568–575. [Google Scholar] [CrossRef]
- Shahbandeh, M. Meat Consumption Worldwide from 1990 to 2021, by Meat Type. Available online: https://www.statista.com/statistics/274522/global-per-capita-consumption-of-meat/#statisticContainer (accessed on 2 November 2022).
- Ritchie, H.; Rosado, P.; Roser, M. Meat and Dairy Production. 2017. Available online: https://ourworldindata.org/meat-production (accessed on 2 November 2022).
- FAO. Food Outlook: Biannual Report on Global Food Markets. In Food Outlook; FAO: Italy, Rome, 2020. [Google Scholar] [CrossRef]
- Bodnár, K.; Schuler, T. The surge in euro area food inflation and the impact of the Russia-Ukraine war. Econ. Bull. Boxes 2022, 4, 74–80. Available online: https://www.ecb.europa.eu/pub/pdf/ecbu/eb202204.en.pdf (accessed on 20 October 2022).
- European Neighbourhood Policy and Enlargement Negotiations. Commission Acts for Global Food Security and for Supporting EU Farmers and Consumers. 2022. Available online: https://neighbourhood-enlargement.ec.europa.eu/news/commission-acts-global-food-security-and-supporting-eu-farmers-and-consumers-2022-03-23_en (accessed on 10 October 2022).
- Santos, N.; di Sitizano, J.M.T.; Pedersen, E.; Borgomeo, E. Investing in Carbon Neutrality—Utopia or the New Green Wave?: Challenges and Opportunities for Agrifood Systems; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Hannah Ritchie. Half of the World’s Habitable Land Is Used for Agriculture. 2019. Available online: https://ourworldindata.org/global-land-for-agriculture (accessed on 2 November 2022).
- Dopelt, K.; Radon, P.; Davidovitch, N. Environmental Effects of the Livestock Industry: The Relationship between Knowledge, Attitudes, and Behavior among Students in Israel. Int. J. Environ. Res. Public Health 2019, 16, 1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brack, D.; Glvoer, A.; Wellesley, L. Agricultural Commodity Supply Chains: Trade, Consumption and Deforestation; The Royal Institute of International Affairs, Chatham House: London, UK, 2016. [Google Scholar]
- Mancini, S.; Moruzzo, R.; Riccioli, F.; Paci, G. European consumers’ readiness to adopt insects as food: A review. Food Res. Int. 2019, 122, 661–678. [Google Scholar] [CrossRef] [PubMed]
- Humprnöder, F.; Bodirsky, B.L.; Weindl, I.; Lotze-Campen, H.; Linder, T.; Popp, A. Projected environmental benefits of replacing beef with microbial protein. Nature 2022, 605, 90–96. [Google Scholar] [CrossRef] [PubMed]
- GFI Company Database. 2022. Available online: https://gfi.org/resource/alternative-protein-company-database (accessed on 2 November 2022).
- The Good Food Institute. 2021 State of the Industry Report—Fermentation: Meat, Seafood, Eggs and Dairy. 2022. Available online: https://gfi.org/resource/fermentation-state-of-the-industry-report/ (accessed on 15 October 2022).
- Raziq, A.; Lateef, M.; Ullah, A.; Ullah, H.; Khan, M.W. Single cell protein (SCP) production and potential substrates: A comprehensive review. Pure Appl. Biol. 2020, 9, 1743–1754. [Google Scholar] [CrossRef]
- Ukaegbu-Obi, K.M. Single Cell Protein: A Resort to Global Protein Challenge and Waste Management. J. Microbiol. Microb. Technol. 2016, 1, 5. [Google Scholar]
- Bertasini, D.; Binati, R.L.; Bolzonella, D.; Battista, F. Single cell proteins production from food processing effluents and digestate. Chemosphere 2022, 296, 134076. [Google Scholar] [CrossRef]
- Koivurinta, J.; Kurkela, R.; Koivistoinen, P. Uses of Pekilo, a microfungus biomass from Paecilomyces varioti in sausage and meat balls. Int. J. Food Sci. Technol. 1979, 14, 561–570. [Google Scholar] [CrossRef]
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M.G. Single Cell Protein—State-of-the-art, Industrial Landscape and patents 2001–2016. Front. Microbiol. 2017, 8, 2009. [Google Scholar] [CrossRef] [Green Version]
- Ibarruri, J.; Cebrián, M.; Hernández, I. Valorisation of fruit and vegetable discards by fungal submerged and solid-state fermentation for alternative feed ingredients production. J. Environ. Manag. 2021, 281, 111901. [Google Scholar] [CrossRef]
- Bhalla, T.C.; Joshi, M. Protein enrichment of apple pomace by co-culture of cellulolytic moulds and yeasts. World J. Microbiol. Biotechnol. 1994, 10, 116–117. [Google Scholar] [CrossRef]
- Valentino, M.J.G.; Ganado, L.S.; Undan, J.R. Single cell protein potential of endophytic fungi associated with bamboo using rice bran as substrate. Adv. Appl. Sci. Res. 2016, 7, 69–72. [Google Scholar]
- Perincherry, L.; Lalak-Kańczugowska, J.; Stępień, Ł. Fusarium-Produced Mycotoxins in Plant-Pathogen Interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razzaq, Z.U.; Khan, M.K.I.; Maan, A.A.; ur Rahman, S. Characterization of single cell protein from Saccharomyces cerevisiae for nutritional, functional and antioxidant properties. J. Food Meas. Charact. 2020, 14, 2520–2528. [Google Scholar] [CrossRef]
- Karim, A.; Gerliani, N.; Aider, M. Kluyveromyces marxianus: An emerging yeast cell factory for applications in food and biotechnology. Int. J. Food Microbiol. 2020, 333, 108818. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, A.T.; Rasoul-Amini, S.; Morowvat, M.H.; Ghasemi, Y. Single Cell Protein: Production and Process. Am. J. Food Technol. 2011, 6, 103–116. [Google Scholar] [CrossRef]
- Nigam, J.N. Single cell protein from pineapple cannery effluent. World J. Microbiol. Biotechnol. 1998, 14, 693–696. [Google Scholar] [CrossRef]
- Yao, K.Y.; Zhang, T.Z.; Wang, H.F.; Liu, J.X. Upgrading of by-product from beverage industry through solid-state fermentation with Candida utilis and Bacillus subtilis. Lett. Appl. Microbiol. 2018, 67, 557–563. [Google Scholar] [CrossRef]
- Ouedraogo, N.; Savadogo, A.; Somba, M.K.; Tapsoba, F.; Zongo, C.; Traore, A.S. Effect of mineral salts and nitrogen source on yeast (Candida utilis NOY1) biomass production using tubers wastes. Afr. J. Biotechnol. 2017, 16, 359–365. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Yang, M.; Yang, Z. Biomass production of yeast isolate from salad oil manufacturing wastewater. Bioresour. Technol. 2005, 96, 1183–1187. [Google Scholar] [CrossRef]
- Magalhães, C.E.B.; Souza-Neto, M.S.; Astolfi-Filho, S.; Matos, I.T.S.R. Candida tropicalis able to produce yeast single cell protein using sugarcane bagasse hemicellulosic hydrolysate as carbon source. Biotechnol. Res. Innov. 2018, 2, 19–21. [Google Scholar] [CrossRef]
- Szabó, K.; Miskei, M.; Farkas, I.; Dombrádi, V. The phosphatome of opportunistic pathogen Candida species. Fingal Biol. Rev. 2021, 35, 40–51. [Google Scholar] [CrossRef]
- Yadav, J.S.S.; Bezawada, J.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Candida krusei: Biotechnological potentials and concerns about its safety. Can. J. Microbiol. 2012, 58, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Zieniuk, B.; Fabiszewska, A. Yarrowia lipolytica: A beneficious yeast in biotechnology as a rare opportunistic fungal pathogen: A minireview. World J. Microbiol. Biotechnol. 2019, 35, 10. [Google Scholar] [CrossRef] [Green Version]
- Karim, A.; Aider, M. Bioconversion of electro-activated lactose, whey and whey permeate to produce single cell protein, ethanol, aroma volatiles, organic acids and fat by Kluyveromyces marxianus. Int. Dairy J. 2022, 129, 105334. [Google Scholar] [CrossRef]
- John, R.P.; Anisha, G.S.; Nampoothiri, K.M.; Pandey, A. Micro and macroalgal biomass: A renewable source for bioethanol. Bioresour. Technol. 2011, 102, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.; Wijffels, R.H.; Barbosa, M.J. Microalgae based production of single-cell protein. Curr. Opin. Biotech. 2022, 75, 102705. [Google Scholar] [CrossRef] [PubMed]
- Safi, C.; Charton, M.; Ursu, A.V.; Laroche, C.; Zebib, B.; Pontalier, P.; Vaca-Garcia, C. Release of hydro-soluble microalgal proteins using mechanical and chemical treatments. Algal Res. 2014, 3, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Tibbetts, S.M.; Mann, J.; Dumas, A. Apparent digestibility of nutrients, energy, essential amino acids and fatty acids of juvenile Atlantic salmon (Salmo salar L.) diets containing whole-cell or cell-ruptured Chlorella vulgaris meals at five dietary inclusion levels. Aquaculture 2017, 481, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Petrus, M.; Culerrier, R.; Campistron, M.; Barre, A.; Rougé, P. First case report of anaphylaxis to spirulin: Identification of phycocyanin as responsible allergen. Allergy 2010, 65, 924–925. [Google Scholar] [CrossRef]
- Le, T.; Knulst, A.C.; Röckmann, H. Anaphylaxis to Spirulina confirmed by skin prick test with ingredients of Spirulina tablets. Food Chem. Toxicol. 2014, 74, 309–310. [Google Scholar] [CrossRef]
- Øverland, M.; Tauson, A.; Shearer, K.; Skrede, A. Evaluation of methane-utilising bacteria products as feed ingredients for monogastric animals. Arch. Anim. Nutr. 2010, 64, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Linder, T. Edible Microorganisms-An Overlooked Technology Option to Counteract Agricultural Expansion. Front. Sustain. Food Syst. 2019, 3, 32. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, Y.; Hu, W.; Zheng, X.; Chen, Y. Valorization of food waste fermentation liquid into single cell protein by photosynthetic bacteria via stimulating carbon metabolic pathway and environmental behaviour. Bioresour. Technol. 2022, 361, 127704. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.G.; Barashkov, V.A. Characteristics of Proteins Synthesized by Hydrogen-Oxidizing Microorganisms. Appl. Biochem. Microbiol. 2010, 46, 574–579. [Google Scholar] [CrossRef]
- Hülsen, T.; Sander, E.M.; Jensen, P.D.; Batstone, D.J. Application of purple phototrophic bacteria in a biofilm photobioreactor for single cell protein production: Biofilm vs suspended growth. Water Res. 2020, 181, 115909. [Google Scholar] [CrossRef]
- Cristiani-Urbina, E.; Netzahuatl-Muñoz, A.R.; Manriquez-Rojas, F.J.; Juárez-Ramírez, C.; Ruiz-Ordaz, N.; Galíndez-Mayer, J. Batch and fed-batch cultures for the treatment of whey with mixed yeast cultures. Process Biochem. 2000, 35, 649–657. [Google Scholar] [CrossRef]
- Yadav, J.S.S.; Bezawada, J.; Ajila, C.M.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Mixed culture of Kluyveromyces marxianus and Candida krusei for single-cell protein production and organic load removal from whey. Bioresour. Technol. 2014, 164, 119–127. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Y.; Guo, J.; Shen, Y.; Yan, P.; Yang, J. Recycling of orange waste for single cell protein production and the synergistic and antagonistic effects on production quality. J. Clean. Prod. 2019, 213, 384–392. [Google Scholar] [CrossRef]
- Myint, K.T.; Otsuka, M.; Okubo, A.; Mitsuhashi, R.; Oguro, A.; Maeda, H.; Shigeno, T.; Sato, K.; Nakajima-Kambe, T. Isolation and identification of flower yeasts for the development of mixed culture to produce single-cell protein from waste milk. Bioresour. Technol. Rep. 2020, 10, 100401. [Google Scholar] [CrossRef]
- Yadav, J.S.S.; Yan, S.; Ajila, C.M.; Bezawada, J.; Tyagi, R.D.; Surampalli, R.Y. Food-grade single-cell protein production, characterization and ultrafiltration recovery of residual fermented whey proteins from whey. Food Bioprod. Process. 2016, 99, 156–165. [Google Scholar] [CrossRef]
- Rajoka, M.I.; Ahmed, S.; Hashmi, A.S.; Athar, M. Production of microbial biomass protein from mixed substrates by sequential culture fermentation of Candida utilis and Brevibacterium lactofermentum. Ann. Microbiol. 2012, 62, 1173–1179. [Google Scholar] [CrossRef]
- Reihani, S.F.S.; Khosravi-Darani, K. Influencing factors on single-cell protein production by submerged fermentation: A review. Electron. J. Biotechnol. 2019, 37, 34–40. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Spalvins, K.; Ivanovs, K.; Blumberga, D. Single cell protein production from waste biomass: Review of various agricultural by-products. Agron. Res. 2018, 16, 1493–1508. [Google Scholar] [CrossRef]
- Patsios, S.I.; Dedousi, A.; Sossidou, E.N.; Zdragas, A. Sustainable Animal Feed Protein through the Cultivation of YARROWIA Lipolytica on the Agro-Industrial Wastes and By-Products. Sustainability 2020, 12, 1398. [Google Scholar] [CrossRef] [Green Version]
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Lonkila, A.; Yang, B. Alternative proteins and EU food law. Food Control. 2021, 130, 108336. [Google Scholar] [CrossRef]
- Oshoma, C.; Eguakun-owie, S. Conversion of food waste to Single Cell Protein using Aspergillus niger. J. Appl. Sci. Environ. Manage. 2018, 22, 350–355. [Google Scholar] [CrossRef] [Green Version]
- Vaccarino, C.; Lo Curto, R.; Tripodo, M.M.; Patané, R.; Schachter, S.L. SCP from Orange Peel by Fermentation with Fungi-Submerged and ‘Surface’ Fermentations. Biol. Wastes 1989, 29, 279–287. [Google Scholar] [CrossRef]
- Stoffel, F.; Santana, W.O.; Gregolon, J.G.N.; Kist, T.B.L.; Fontana, R.C.; Camassola, M. Production of edible mycoprotein using agroindustrial wastes: Influence on nutritional, chemical and biological properties. Innov. Food Sci. Emerg. Technol. 2019, 58, 102227. [Google Scholar] [CrossRef]
- Najari, Z.; Khodaiyan, F.; Yarmand, M.S.; Hosseini, S.S. Almond hulls waste valorization towards sustainable agricultural development: Production of pectin, phenolics, pullulan, and single cell protein. Waste Manag. 2022, 141, 208–219. [Google Scholar] [CrossRef]
- Rajoka, M.I.; Khan, S.H.; Jabbar, M.A.; Awan, M.S.; Hashmi, A.S. Kinetics of batch single cell protein production from rice polishings with Candida utilis in continuously aerated tank reactors. Bioresour. Technol. 2006, 97, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Rages, A.A.; Haider, M.M.; Aydin, M. Alkaline hydrolysis of olive fruits wastes for the production of single cell protein by Candida lipolytica. Biocatal. Agric. Biotechnol. 2021, 33, 101999. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, L.; Ding, H.; Gao, X.; Chen, Y.; Li, D. Optimization of culture conditions of screened Galactomyces candidum for the production of single cell protein from biogas slurry. Electron. J. Biotechnol. 2022, 55, 47–54. [Google Scholar] [CrossRef]
- Yang, R.; Chen, Z.; Hu, P.; Zhang, S.; Luo, G. Two-stage fermentation enhanced single-cell protein production by Yarrowia lipolytica from food waste. Bioresour. Technol. 2022, 361, 127677. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.I.; Asif, M.; Razzaq, Z.U.; Nazir, A.; Maan, A.A. Sustainable food industrial waste management through single cell protein production and characterization of protein enriched bread. Food Biosci. 2022, 46, 101406. [Google Scholar] [CrossRef]
- Putra, M.D.; Abasaeed, A.E.; Al-Zahrani, S.M. Prospective production of fructose and single cell protein from date palm waste. Electron. J. Biotechnol. 2020, 48, 46–52. [Google Scholar] [CrossRef]
- Patelski, P.; Berlowska, J.; Dziugan, P.; Pielech-Przybylska, K.; Balcerek, M.; Dziekonska, U.; Kalinowska, H. Utilisation of sugar beet bagasse for the biosynthesis of yeast SCP. J. Food Eng. 2015, 167, 32–37. [Google Scholar] [CrossRef]
- Yunus, F.; Nadeem, M.; Rashid, F. Single-cell protein production through microbial conversion of lignocellulosic residue (wheat bran) for animal feed. J. Inst. Brew. 2015, 121, 553–557. [Google Scholar] [CrossRef] [Green Version]
- Zepka, L.O.; Jacob-Lopes, E.; Goldbeck, R.; Queiroz, M.I. Production and biochemical profile of the microalgae Aphanothece microscopica Nägeli submitted to different drying conditions. Chem. Eng. Process. Process Intensif. 2008, 47, 1305–1310. [Google Scholar] [CrossRef]
- Liu, B.; Li, Y.; Song, J.; Zhang, L.; Dong, J.; Yang, Q. Production of single-cell protein with two-step fermentation for treatment of potato starch processing waste. Cellulose 2014, 21, 3637–3645. [Google Scholar] [CrossRef]
- Kurbanoglu, E.B.; Algur, O.F. Single-cell protein production from ram horn hydrolysate by bacteria. Bioresour. Technol. 2002, 85, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Wongputtisin, P.; Khanongnuch, C.; Kongbuntad, W.; Niamsup, P.; Lumyong, S.; Sarkar, P.K. Use of Bacillus subtilis isolates from Tuanao towards nutritional improvement of soya bean hull for monogastric feed application. Lett. Appl. Microbiol. 2014, 59, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Khumchai, J.; Wong, A.; On-uma, R.; Sabour, A.; Alshiekheid, M.; Narayanan, M.; Karuppusamy, I.; Pugazhendi, A.; Brindhadevi, K.; Thuy Lan Chi, N. A viable bioremediation strategy for treating paper and pulp industry effluents and assessing the prospect of resulted bacterial biomass as single cell protein (SCP) using indigenous bacterial species. Chemosphere 2022, 304, 135246. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, R.; Phukon, L.C.; Abedin, A.M.; Padhi, S.; Singh, S.P.; Rai, A.K. Whey valorization by microbial and enzymatic bioprocesses for the production of nutraceuticals and value-added products. Bioresour. Technol. Rep. 2022, 19, 101144. [Google Scholar] [CrossRef]
- Yadav, J.S.S.; Bezawada, J.; Elharche, S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Simultaneous single-cell protein production and COD removal with characterization of residual protein and intermediate metabolites during whey fermentation by K. marxianus. Bioprocess. Biosyst. Eng. 2014, 37, 1017–1029. [Google Scholar] [CrossRef]
- Matassa, S.; Pegalli, V.; Papirio, S.; Zamalloa, C.; Verstraete, W.; Esposito, G.; Pirozzi, F. Direct nitrogen stripping and upcycling from anaerobic digestate during conversion of chesse whey into single cell protein. Bioresour. Technol. 2022, 358, 127308. [Google Scholar] [CrossRef]
- Thompson, J.C.; He, B.B. Characterization of crude glycerol from biodiesel production from multiple feedstocks. Appl. Eng. Agric. 2006, 22, 261–265. [Google Scholar] [CrossRef]
- Juszczyk, P.; Tomaszewska, L.; Kita, A.; Rymowicz, W. Biomass production by novel strains of Yarrowia lipolytica using raw glycerol, derived from biodiesel production. Bioresour. Technol. 2013, 137, 124–131. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, X.; Zeng, D.; Su, Y.; Zhang, Y. Microbial conversion of syngas to single cell protein: The role of carbon monoxide. Chem. Eng. J. 2022, 450, 138041. [Google Scholar] [CrossRef]
- Adedayo, M.R.; Ajiboye, E.A.; Akintunde, J.K.; Odaibo, A. Single Cell Proteins: As Nutritional Enchancer. Adv. Appl. Sci. Res. 2011, 2, 396–409. [Google Scholar]
- Zeng, D.; Jiang, Y.; Su, Y.; Zhang, Y. Upcycling waste organic acids and nitrogen into single cell protein via brewer’s yeast. J. Clean. Prod. 2022, 369, 133279. [Google Scholar] [CrossRef]
- Bratosin, B.C.; Darjan, S.; Vodnar, D.C. Single Cell Protein: A Potential Substitute in Human and Animal Nutrition. Sustainability 2021, 13, 9284. [Google Scholar] [CrossRef]
- Subramaniyam, R.; Vimala, R. Solid state and submerged fermentation for the production of bioactive substances: A comparative study. Int. J. Sci. Nat. 2012, 3, 480–486. [Google Scholar]
- Gibbs, P.A.; Seviour, R.J.; Schmid, F. Growth of Filamentous Fungi in Submerged Culture: Problems and Possible Solutions. Crit. Rev. Biotechnol. 2000, 20, 17–48. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Mitchell, D. New developments in solid state fermentation: I-bioprocesses and products. Process Biochem. 2000, 35, 11153–11169. [Google Scholar] [CrossRef]
- Lizardi-Jiménez, M.A.; Hernández-Martínez, R. Solid state fermentation (SSF): Diversity of applications to valorize waste and biomass. 3 Biotech 2017, 7, 44. [Google Scholar] [CrossRef]
- Gervais, P.; Molin, P. The role of water in solid-state fermentation. Biochem. Eng. J. 2003, 13, 85–101. [Google Scholar] [CrossRef]
- Krishna, C. Solid-State Fermentation Systems—An Overview. Crit. Rev. Biotechnol. 2005, 25, 1–30. [Google Scholar] [CrossRef]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Bhargav, S.; Panda, B.P.; Ali, M.; Javed, S. Solid-state Fermentation: An Overview. Chem. Biochem. Eng. 2008, 22, 49–70. [Google Scholar]
- Singhania, R.R.; Patel, A.K.; Soccol, C.R.; Pandey, A. Recent advances in solid-state fermentation. Biochem. Eng. J. 2009, 44, 13–18. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Kapilan, R.; Merah, O.; Madhujith, T. Single Cell Protein Production Using Different Fruit Waste: A Review. Separations 2022, 9, 178. [Google Scholar] [CrossRef]
- Oliveira, S.D.; Padilha, C.E.A.; Asevedo, E.A.; Pimentel, V.C.; Araújo, F.R.; Macedo, G.R.; Santos, E.S. Utilization of agroindustrial residues for producing cellulases by Aspergillus fumigatus on Semi-Solid fermentation. J. Environ. Chem. Eng. 2018, 6, 937–944. [Google Scholar] [CrossRef]
- Zhang, W.; Zou, H.; Jiang, L.; Yao, J.; Liang, J.; Wang, Q. Semi-solid State Fermentation of Food Waste for Production of Bacillus thuringiensis Biopesticide. Biotechnol. Bioprocess Eng. 2015, 20, 1123–1132. [Google Scholar] [CrossRef]
- Rani, K.Y.; Rao, V.S.R. Control of fermenters—A review. Bioprocess Eng. 1999, 21, 77–88. [Google Scholar] [CrossRef]
- Bekatorou, A.; Psarianos, C.; Koutinas, A.A. Production of Food Grade Yeasts. Food Technol. Biotechnol. 2006, 44, 407–415. [Google Scholar]
- Alvarez, R.; Enriquez, A. Nucleic acid reduction in yeast. Appl. Microbiol. Biotechnol. 1988, 29, 208–210. [Google Scholar] [CrossRef]
- Parajó, J.C.; Santoz, V.; Domínguez, H.; Vazquez, M. NH4OH-Based Pretreatment for Improving the Nutritional Quality of Single-Cell protein (SCP). Appl. Biochem. Biotechn. 1995, 55, 133–149. [Google Scholar] [CrossRef]
- Jach, M.E.; Serefko, A. Nutritional Yeast Biomass: Characterization and Application. In Handbook of Food Bioengineering, Diet, Microbiome and Health; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: London, UK, 2018; pp. 237–270. [Google Scholar] [CrossRef]
- Balagurunathan, B.; Ling, H.; Choi, W.J.; Chang, M.W. Potential use of microbial engineering in single-cell protein production. Curr. Opin. Biotechnol. 2022, 76, 102740. [Google Scholar] [CrossRef]
- FOOD 2030. Pathways for Action - alternative proteins and dieatary shift; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Szepe, K.J.; Dyer, P.S.; Johnson, R.I.; Salter, A.M.; Avery, S.V. Influence of environmental and genetic factors on food protein quality: Current knowledge and future directions. Curr. Opin. Food Sci. 2021, 40, 94–101. [Google Scholar] [CrossRef]
- Fletcher, E.; Baetz, K. Multi-Faceted Systems Biology Approaches Present a Cellular Landscape of Phenolic Compound Inhibition in Saccharomyces cerevisiae. Front. Bioeng. Biotechnol. 2020, 8, 539902. [Google Scholar] [CrossRef] [PubMed]
- Lo, T.; Teo, W.S.; Ling, H.; Chen, B.; Kang, A.; Chang, M.W. Microbial engineering strategies to improve cell viability for biochemical production. Biotechnol. Adv. 2013, 31, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhao, D.; Qui, H.; Fan, F.; Man, S.; Bi, C.; Zhang, X. The CRISPR/Cas9-facilitated multiplex pathway optimization (CFPO) technique and its application to improve the Escherichia coli xylose utilization pathway. Metab. Eng. 2017, 43, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, L.F.; Marx, C.J. Methylotrophs for Aquaculture and Animal Feed. U.S. Patent 10920230B2, 16 February 2021. [Google Scholar]
- Sillman, J.; Nygren, L.; Kahiluoto, H.; Ruuskanen, V.; Tamminen, A.; Bajamundi, C.; Nappa, M.; Wuokko, M.; Lindh, T.; Vainikka, P.; et al. Bacterial protein for food and feed generated via renewable energy and direct air capture of CO2: Can it reduce land and water use? Glob. Food Secur. 2019, 22, 25–32. [Google Scholar] [CrossRef]
- Schaerer, L.G.; Wu, R.; Putman, L.I.; Pearce, J.M.; Lu, T.; Shonnard, D.R.; Ong, R.G.; Techtmann, S.M. Killing two birds with one stone: Chemical and biological upcycling of polyethylene terephthalate plastics into food. Trends Biotechnol. 2022. [Google Scholar] [CrossRef]
- Marcellin, E.; Angenent, L.T.; Nielsen, L.K.; Molitor, B. Recycling carbon for sustainable protein production using gas fermentation. Curr. Opin. Biotechnol. 2022, 76, 102723. [Google Scholar] [CrossRef]
- Hu, X.; Kerckhof, F.M.; Ghesquiere, J.; Bernaerts, K.; Boeckx, P.; Clauwaert, P.; Boon, N. Microbial Protein out of Thin Air: Fixation of Nitrogen Gas by an Autotrophic Hydrogen-Oxidizing Bacterial Enrichment. Environ. Sci. Technol. 2020, 54, 3609–3617. [Google Scholar] [CrossRef]
- Bedoya, M.G.; Montoya, D.R.; Tabilo-Munizaga, G.; Perez-Won, M.; Lemus-Mondaca, R. Promising perspectives on novel protein food sources combining artificial intelligence and 3D food printing for food industry. Trends Food Sci. Technol. 2022, 128, 38–52. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Cao, X.; Huang, C.; Liu, E.; Qian, S.; Liu, X.; Wu, Y.; Dong, F.; Qiu, C.W.; et al. Artificial intelligence: A powerful paradigm for scientific research. Innovation 2021, 2, 100179. [Google Scholar] [CrossRef]
- Mavani, N.R.; Ali, J.M.; Othman, S.; Hussain, M.A.; Hashim, H.; Rahman, N.A. Application of Artificial Intelligence in Food Industry—A Guideline. Food Eng. Rev. 2022, 14, 134–175. [Google Scholar] [CrossRef]
- Samad, S.; Ahmed, F.; Naher, S.; Kabir, M.A.; Das, A.; Amin, S.; Islam, S.M.S. Smartphone apps for tracking food consumption and recommendations: Evaluating artificial intelligence-based functionalities, features and quality of current apps. Intell. Syst. Appl. 2022, 15, 200103. [Google Scholar] [CrossRef]
- FDA. Regulatory Framework for Substances Intended for Use in Human Food or Animal Food on the Basis of the Generally Recognized as Safe (GRAS) Provision of the Federal Food, Drug, and Cosmetic Act: Guidance for Industry. Available online: https://www.fda.gov/media/109117/download (accessed on 8 October 2022).
- EU Regulation on Novel Foods 2015/2283. Available online: http://data.europa.eu/eli/reg/2015/2283/oj (accessed on 12 October 2022).
- The Good Food Institute. Alternative Proteins: 2021 State of Global Policy Report. Available online: https://gfi.org/resource/alternative-proteins-state-of-global-policy/ (accessed on 15 November 2022).
- ING Research. Growth of Meat and Dairy Alternatives Is Stirring Up the European Food Industry. 2020. Available online: https://think.ing.com/uploads/reports/ING_report_-_Growth_of_meat_and_dairy_alternatives_is_stirring_up_the_European_food_industry.pdf (accessed on 30 October 2022).
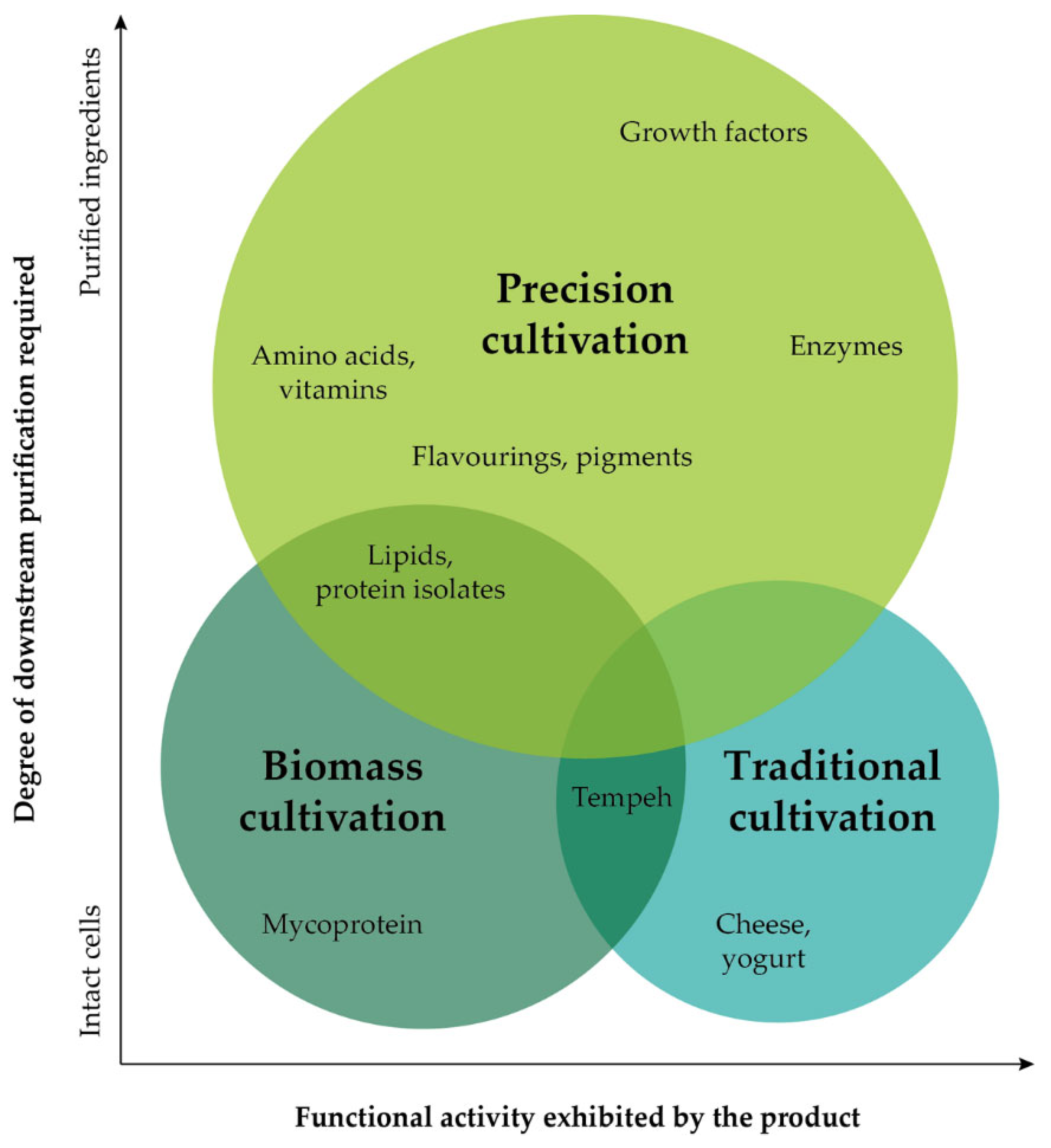
| Cultivation Approach | Product Description | Company | Country | Year Founded |
|---|---|---|---|---|
| Traditional cultivation | Fermented plant-based food products (conventional cheese analogues) | Väcka | Spain | 2019 |
| Minimally processed whole-cut meat and fish alternatives grown naturally from fungal mycelium | Bosque Foods | USA | 2020 | |
| Uses raw materials of the Mediterranean Diet: grains, legumes, nuts, and seeds to drive fermentation | The Mediterranean Food Lab | Israel | 2017 | |
| Fermented plant-based yogurt optimized for gut health | Wellme | China | 2021 | |
| Pea and rice proteins fermented by shiitake mycelium | MycoTechnology | USA | 2013 | |
| Biomass cultivation | Mycoprotein-based meat substitutes | Quorn | UK | 1985 |
| Microalgae-based plant-based foods including egg, seafood, meat, and dairy replacements | Algama | France | 2013 | |
| Beef production via a high protein yeast blend. | More Foods | Israel | 2019 | |
| Mycelium-based whole cut meats, including bacon under the brand “MyBacon” | MyForest Foods | USA | 2019 | |
| Algae-based protein | Sophie’s BioNutrients | Singapore | 2010 | |
| Precision cultivation | Fermentation based non-GM functional proteins for the food industry, starting with vegan ovalbumin and related proteins | Eggmented Reality | Israel | 2022 |
| Animal-origin-free dairy proteins and fats | Maya Milk | Turkey | 2021 | |
| Plant-based meat products, under the brand “BUDS,” and dairy products, under the brand “MilkCELL,” using precision fermentation | All G Foods | Australia | 2020 | |
| Milk protein using microbial fermentation | Zero Cow Factory | India | 2020 | |
| Meat and fish proteins through precision fermentation | Paleo | Belgium | 2020 | |
| Fermentation of dairy triglycerides and synthetic polymers | Circe | USA | 2020 |
| Producing Microorganism | Substrate | References |
|---|---|---|
| Fungi | ||
| Aspergillus niger | Banana, cucumber, orange, pineapple, and watermelon food wastes | [79] |
| Aspergillus (Aspergillus niger, Aspergillus flavus and Aspergillus ochraceus), Fusarium (Fusarium semitectum, Fusarium sp1, Fusarium sp 2), Monascus ruber, Penecillium citrinum and Cladosporium cladosporioides | Rice bran | [43] |
| Trichoderma viride and Geotrichum candidum | Orange peel | [80] |
| Agaricus blazei, Auricularia fuscosuccinea and Pleurotus albidus | Brewer-spent grain and grape bagasse | [81] |
| Aureobasidium pullulans | Almond hulls waste | [82] |
| Rhizopus oryzae | Fruit and vegetable discards | [41] |
| Yeast | ||
| Candida utilis | Orange peel residues | [19] |
| Candida utilis | Rice polishings | [83] |
| Candida utilis | Pineapple cannery effluent | [48] |
| Candida utilis | Salad oil manufacturing wastewater | [51] |
| Candida tropicalis | Sugarcane bagasse hemicellulosic hydrolysate | [52] |
| Candida lipolytica | Olive fruit wastes | [84] |
| Candida tropicalis, Aspergillus oryzae and Trichoderma koningii | Orange waste | [70] |
| Galactomyces candidum | Biogas slurry | [85] |
| Yarrowia lipolytica | Food waste from the feed of anaerobic digestion reactor | [86] |
| Saccharomyces cerevisiae | Fruits and vegetables wastes (banana peel, citrus peel, potato peel, and carrot pomace) | [87] |
| Saccharomyces cerevisiae | Candy Production Effluent | [38] |
| Saccharomyces cerevisiae | Date palm waste | [88] |
| Trichosporon cutaneum, Candida tropicalis Pichia stipitis, Candida guilliermondii and Saccharomyces cerevisiae | Sugar beet pulp | [89] |
| Kluyveromyces marxianus and Candida krusei | Whey | [69] |
| Rhizopus oligosporus and Candida utilis | Wheat bran | [90] |
| Algae | ||
| Aphanothece microscopica Nägeli | Parboiled rice effluent | [91] |
| Bacteria | ||
| Rhodobacter capsulatus | Carbohydrate-rich food waste | [65] |
| Bacillus licheniformis | Potato starch processing waste | [92] |
| Bacillus cereus, Bacillus subtilis, Escherichia coli | Ram horn hydrolysate | [93] |
| Bacillus subtilis | Soya bean hull | [94] |
| Streptomyces tuirus | Pulp and paper mill effluent | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajić, B.; Vučurović, D.; Vasić, Đ.; Jevtić-Mučibabić, R.; Dodić, S. Biotechnological Production of Sustainable Microbial Proteins from Agro-Industrial Residues and By-Products. Foods 2023, 12, 107. https://doi.org/10.3390/foods12010107
Bajić B, Vučurović D, Vasić Đ, Jevtić-Mučibabić R, Dodić S. Biotechnological Production of Sustainable Microbial Proteins from Agro-Industrial Residues and By-Products. Foods. 2023; 12(1):107. https://doi.org/10.3390/foods12010107
Chicago/Turabian StyleBajić, Bojana, Damjan Vučurović, Đurđina Vasić, Rada Jevtić-Mučibabić, and Siniša Dodić. 2023. "Biotechnological Production of Sustainable Microbial Proteins from Agro-Industrial Residues and By-Products" Foods 12, no. 1: 107. https://doi.org/10.3390/foods12010107
APA StyleBajić, B., Vučurović, D., Vasić, Đ., Jevtić-Mučibabić, R., & Dodić, S. (2023). Biotechnological Production of Sustainable Microbial Proteins from Agro-Industrial Residues and By-Products. Foods, 12(1), 107. https://doi.org/10.3390/foods12010107