Jabuticaba (Plinia sp.) Peel as a Source of Pectin: Characterization and Effect of Different Extraction Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
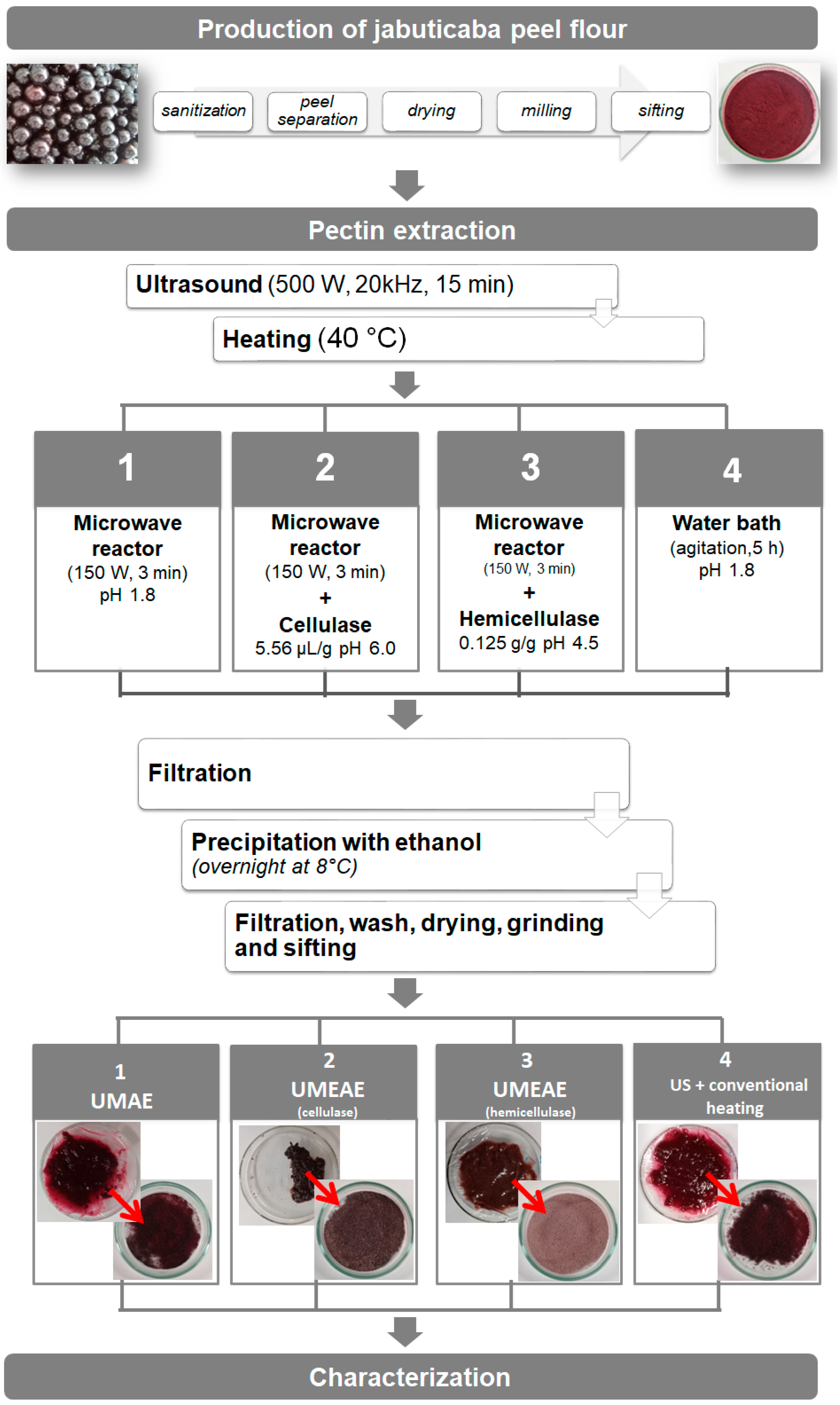
2.2. Pectin Extraction
2.3. Pectin Characterization
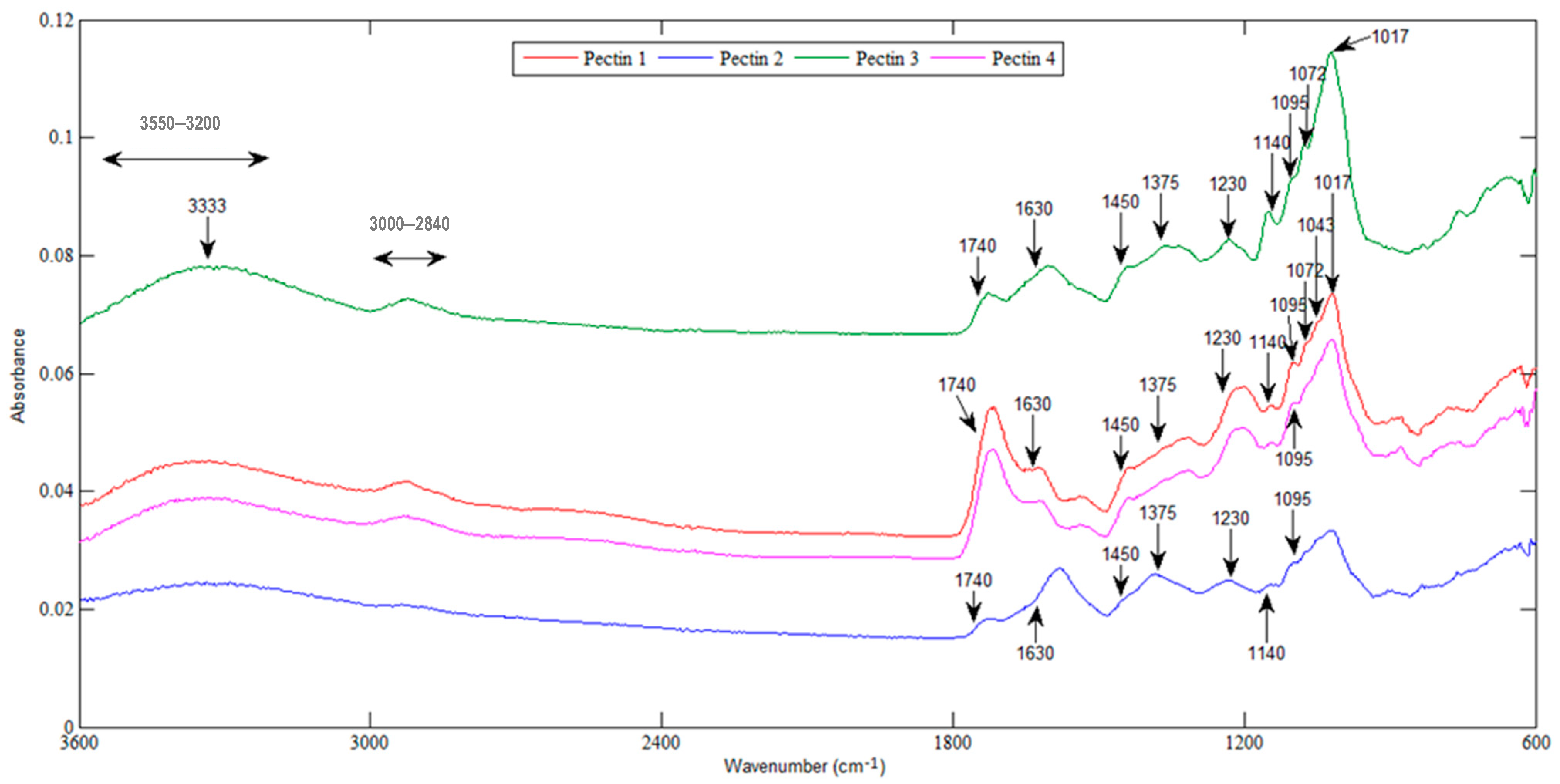
2.3.1. FTIR Analysis and DM
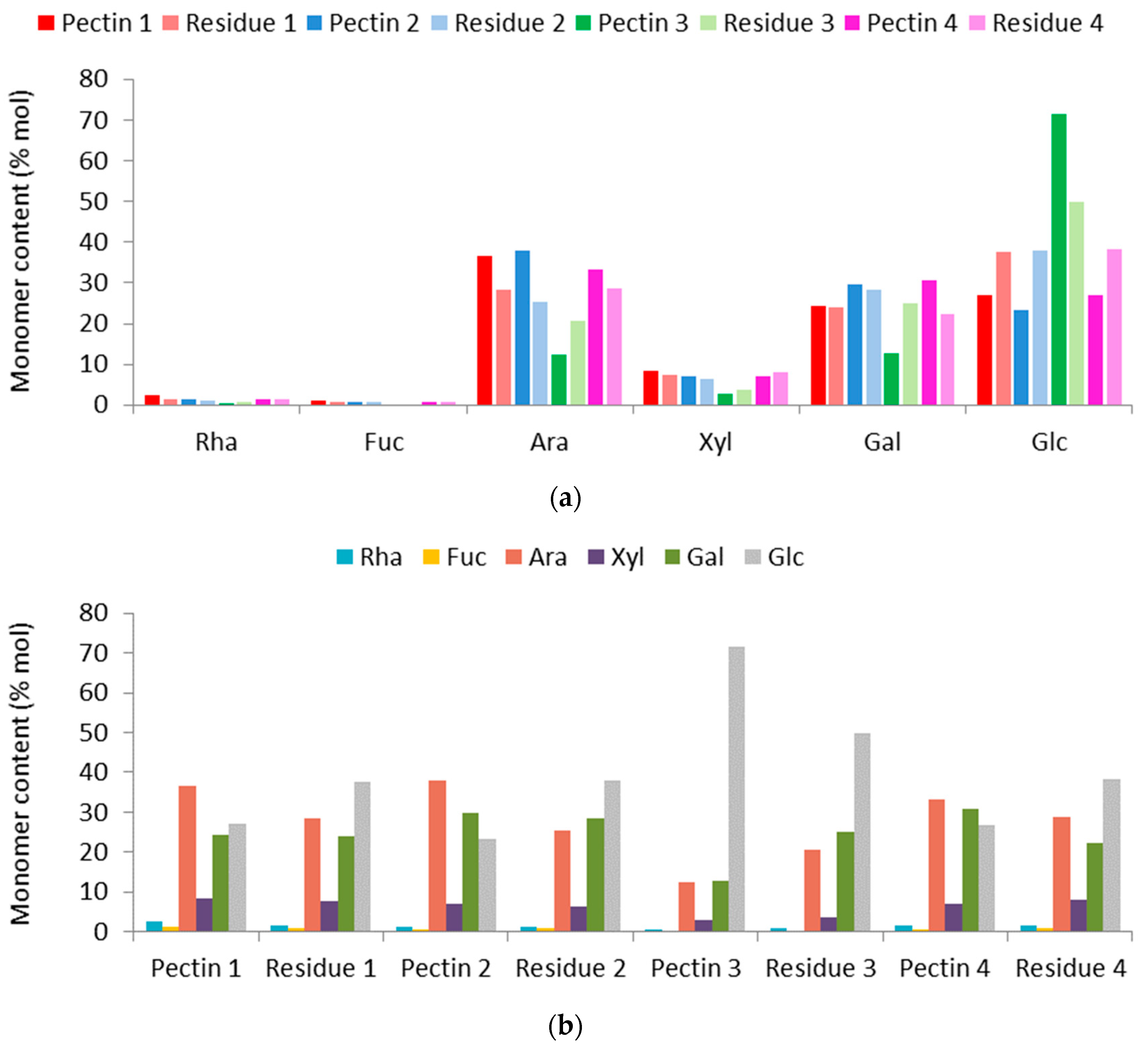
2.3.2. Determination of Neutral Monosaccharides
2.3.3. Phenolic Compounds
2.3.4. Technological Properties
2.4. Statistical Analysis
3. Results and Discussion
3.1. Pectin Extraction
3.2. Pectin Characterization
3.2.1. FTIR Analysis and DM
3.2.2. Neutral Monosaccharides
3.2.3. Phenolic Compounds
3.2.4. Technological Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salomão, L.C.C.; Siqueira, D.L.; Aquino, C.F.; Lins, L.C.R. Jabuticaba—Myrciaria Spp. In Exotic Fruits Reference Guide; Rodrigues, S., Silva, E., de Brito, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 237–244. [Google Scholar]
- Resende, L.M.; Oliveira, L.S.; Franca, A.S. Characterization of Jabuticaba (Plinia cauliflora) Peel Flours and Prediction of Compounds by FTIR Analysis. LWT 2020, 133, 110135. [Google Scholar] [CrossRef]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a Rheology Modifier: Origin, Structure, Commercial Production and Rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef] [PubMed]
- Dranca, F.; Oroian, M. Extraction, Puri Fi Cation and Characterization of Pectin from Alternative Sources with Potential Technological Applications. Food Res. Int. 2018, 113, 327–350. [Google Scholar] [CrossRef] [PubMed]
- Maric, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brncic, M.; Brncic, S.R. An Overview of the Traditional and Innovative Approaches for Pectin Extraction from Plant Food Wastes and By-Products: Ultrasound-, Microwaves-, and Enzyme-Assisted Extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Alexandri, M.; Kachrimanidou, V.; Papapostolou, H.; Papadaki, A.; Kopsahelis, N. Sustainable Food Systems: The Case of Functional Compounds towards the Development of Clean Label Food Products. Foods 2022, 11, 11182796. [Google Scholar] [CrossRef]
- Müller-Maatsch, J.; Bencivenni, M.; Caligiani, A.; Tedeschi, T.; Bruggeman, G.; Bosch, M.; Petrusan, J.; Van Droogenbroeck, B.; Elst, K.; Sforza, S. Pectin Content and Composition from Different Food Waste Streams. Food Chem. 2016, 201, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Miranda, B.M.; Di-Medeiros, M.C.B.; Batista, K.A.; Carbonero, E.R.; Fernandes, K.F.; Silva, F.A. A Galactose-Rich Heteropolysaccharide Extracted from “Jaboticaba” (Plinia cauliflora) Peels. Carbohydr. Polym. 2020, 249, 116821. [Google Scholar] [CrossRef]
- Benvenutti, L.; Sanchez-Camargo, A. del P.; Zielinski, A.A.F.; Ferreira, S.R.S. NADES as Potential Solvents for Anthocyanin and Pectin Extraction from Myrciaria Cauli Fl Ora Fruit By-Product: In Silico and Experimental Approaches for Solvent Selection. J. Mol. Liq. 2020, 315, 113761. [Google Scholar] [CrossRef]
- Nascimento, R.S.; Pedrosa, L.F.; Diethelm, L.T.H.; Souza, T.; Shiga, T.M.; Fabi, J.P. The Purification of Pectin from Commercial Fruit Flours Results in a Jaboticaba Fraction That Inhibits Galectin-3 and Colon Cancer Cell Growth. Food Res. Int. J. 2020, 137, 109747. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, H.; Zhi, Z.; Zhang, H.; Linhardt, R.J.; Zhang, F.; Chen, S.; Ye, X. Extraction Temperature Is a Decisive Factor for the Properties of Pectin. Food Hydrocoll. 2021, 112, 106160. [Google Scholar] [CrossRef]
- Liew, S.Q.; Ngoh, G.C.; Yusoff, R.; Teoh, W.H. Sequential Ultrasound-Microwave Assisted Acid Extraction (UMAE) of Pectin from Pomelo Peels. Int. J. Biol. Macromol. 2016, 93, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Tomar, M.; Saurabh, V.; Mahajan, T.; Punia, S.; Contreras, M. del M.; Rudra, S.G.; Kaur, C.; Kennedy, J.F. Emerging Trends in Pectin Extraction and Its Anti-Microbial Functionalization Using Natural Bioactives for Application in Food Packaging. Trends Food Sci. Technol. 2020, 105, 223–237. [Google Scholar] [CrossRef]
- Encalada, A.M.I.; Pérez, C.D.; Flores, S.K.; Rossetti, L.; Fissore, E.N.; Rojas, A.M. Antioxidant Pectin Enriched Fractions Obtained from Discarded Carrots (Daucus carota L.) by Ultrasound-Enzyme Assisted Extraction. Food Chem. 2019, 289, 453–460. [Google Scholar] [CrossRef]
- Dominiak, M.; Søndergaard, K.M.; Wichmann, J.; Vidal-Melgosa, S.; Willats, W.G.T.; Meyer, A.S.; Mikkelsen, J.D. Application of Enzymes for Efficient Extraction, Modification, and Development of Functional Properties of Lime Pectin. Food Hydrocoll. 2014, 40, 273–282. [Google Scholar] [CrossRef]
- Leão, D.P.; Botelho, B.G.; Oliveira, L.S.; Franca, A.S. Potential of Pequi (Caryocar brasiliense Camb.) Peels as Sources of Highly Esterified Pectins Obtained by Microwave Assisted Extraction. LWT 2018, 87, 575–580. [Google Scholar] [CrossRef]
- Kyomugasho, C.; Christiaens, S.; Shpigelman, A.; Van Loey, A.M.; Hendrickx, M.E. FT-IR Spectroscopy, a Reliable Method for Routine Analysis of the Degree of Methylesterification of Pectin in Different Fruit- and Vegetable-Based Matrices. Food Chem. 2015, 176, 82–90. [Google Scholar] [CrossRef]
- Resende, L.M.; Franca, A.S.; Oliveira, L.S. Buriti (Mauritia flexuosa L. f.) Fruit by-Products Flours: Evaluation as Source of Dietary Fibers and Natural Antioxidants. Food Chem. 2019, 270, 53–60. [Google Scholar] [CrossRef]
- Barros, H.D.F.Q.; Baseggio, A.M.; Angolini, C.F.F.; Pastore, G.M.; Cazarin, C.B.B.; Marostica-Junior, M.R. Influence of Different Types of Acids and PH in the Recovery of Bioactive Compounds in Jabuticaba Peel (Plinia cauliflora). Food Res. Int. 2019, 124, 16–26. [Google Scholar] [CrossRef]
- Plaza, M.; Batista, Â.G.; Cazarin, C.B.B.; Sandahl, M.; Turner, C.; Östman, E.; Maróstica Júnior, M.R. Characterization of Antioxidant Polyphenols from Myrciaria Jaboticaba Peel and Their Effects on Glucose Metabolism and Antioxidant Status: A Pilot Clinical Study. Food Chem. 2016, 211, 185–197. [Google Scholar] [CrossRef]
- Xu, K.; Martinez, M.M.; Yang, B.; Guo, M. Fine Structure, Physicochemical and Antioxidant Properties of LM-Pectins from Okra Pods Dried under Different Techniques. Carbohydr. Polym. 2020, 241, 116272. [Google Scholar] [CrossRef]
- Roman, L.; Guo, M.; Terekhov, A.; Grossutti, M.; Vidal, N.P.; Reuhs, B.L.; Martinez, M.M. Extraction and Isolation of Pectin Rich in Homogalacturonan Domains from Two Cultivars of Hawthorn Berry (Crataegus pinnatifida). Food Hydrocoll. 2021, 113, 106476. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Duan, X.; Yang, Z.; Yang, J.; Liu, F.; Xu, X.; Pan, S. Structural and Emulsifying Properties of Citric Acid Extracted Satsuma Mandarin Peel Pectin. Foods 2021, 10, 2459. [Google Scholar] [CrossRef] [PubMed]
- Valdivia-Rivera, S.; Herrera-Pool, I.E.; Ayora-Talavera, T.; Lizardi-Jiménez, M.A.; García-Cruz, U.; Cuevas-Bernardino, J.C.; Cervantes-Uc, J.M.; Pacheco, N. Kinetic, Thermodynamic, Physicochemical, and Economical Characterization of Pectin from Mangifera indica L. Cv. Haden Residues. Foods 2021, 10, 10092093. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, Z.; Khodaiyan, F.; Kazemi, M.; Sharifan, A. Optimization of Microwave-Assisted Extraction and Structural Characterization of Pectin from Sweet Lemon Peel. Int. J. Biol. Macromol. 2020, 147, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, G.; Negi, P. Isolation of Pectin from Kinnow Peels and Its Characterization. Food Bioprod. Process. 2020, 124, 342–353. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, Y.; Li, F.; Li, D.; Huang, Q. Pectin Extracted from Persimmon Peel: A Physicochemical Characterization and Emulsifying Properties Evaluation. Food Hydrocoll. 2020, 101, 105561. [Google Scholar] [CrossRef]
- Dranca, F.; Talón, E.; Vargas, M.; Oroian, M. Microwave vs. Conventional Extraction of Pectin from Malus Domestica ‘Fălticeni’ Pomace and Its Potential Use in Hydrocolloid-Based Films. Food Hydrocoll. 2021, 121, 107026. [Google Scholar] [CrossRef]
- Kurita, O.; Fujiwara, T.; Yamazaki, E. Characterization of the Pectin Extracted from Citrus Peel in the Presence of Citric Acid. Carbohydr. Polym. 2008, 74, 725–730. [Google Scholar] [CrossRef]
- Hotchkiss, A.T., Jr.; Chau, H.K.; Strahan, G.D.; Nuñez, A.; Simon, S.; White, A.K.; Dieng, S.; Heuberger, E.R.; Yadav, M.P.; Hirsch, J. Structure and Composition of Blueberry Fiber Pectin and Xyloglucan That Bind Anthocyanins during Fruit Puree Processing. Food Hydrocoll. 2021, 116, 106572. [Google Scholar] [CrossRef]
- Jakobek, L.; Matić, P. Non-Covalent Dietary Fiber–Polyphenol Interactions and Their Influence on Polyphenol Bioaccessibility. Trends Food Sci. Technol. 2019, 83, 235–247. [Google Scholar] [CrossRef]
- Inada, K.O.P.; Silva, T.B.R.; Lobo, L.A.; Domingues, R.M.C.P.; Perrone, D.; Monteiro, M. Bioaccessibility of Phenolic Compounds of Jaboticaba (Plinia Jaboticaba) Peel and Seed after Simulated Gastrointestinal Digestion and Gut Microbiota Fermentation. J. Funct. Foods 2020, 67, 103851. [Google Scholar] [CrossRef]
- Monteiro, G.C.; Minatel, I.O.; Junior, A.P.; Gomez-Gomez, H.A.; de Camargo, J.P.C.; Diamante, M.S.; Pereira Basílio, L.S.; Tecchio, M.A.; Pereira Lima, G.P. Bioactive Compounds and Antioxidant Capacity of Grape Pomace Flours. LWT 2021, 135, 110053. [Google Scholar] [CrossRef]
- Fernandes, A.; Oliveira, J.; Fonseca, F.; Ferreira-da-Silva, F.; Mateus, N.; Vincken, J.P.; de Freitas, V. Molecular Binding between Anthocyanins and Pectic Polysaccharides–Unveiling the Role of Pectic Polysaccharides Structure. Food Hydrocoll. 2020, 102, 105625. [Google Scholar] [CrossRef]
- Fracassetti, D.; Costa, C.; Moulay, L.; Tomás-Barberán, F.A. Ellagic Acid Derivatives, Ellagitannins, Proanthocyanidins and Other Phenolics, Vitamin C and Antioxidant Capacity of Two Powder Products from Camu-Camu Fruit (Myrciaria dubia). Food Chem. 2013, 139, 578–588. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, G.; Wang, X.; Luo, J.; Qian, X.; Ling, W. Cyanidin-3-O-β-Glucoside Polarizes LPS-Induced M1 into M2 Macrophage in J774 Cells via PPARγ-Mediated NF-ΚB and STAT6 Signaling Pathway. J. Funct. Foods 2021, 77, 104314. [Google Scholar] [CrossRef]
- Lin, Z.; Lin, C.; Fu, C.; Lu, H.; Jin, H.; Chen, Q.; Pan, J. The Protective Effect of Ellagic Acid (EA) in Osteoarthritis: An in Vitro and in Vivo Study. Biomed. Pharmacother. 2020, 125, 109845. [Google Scholar] [CrossRef]
- Matsukawa, T.; Inaguma, T.; Han, J.; Villareal, M.O.; Isoda, H. Cyanidin-3-Glucoside Derived from Black Soybeans Ameliorate Type 2 Diabetes through the Induction of Differentiation of Preadipocytes into Smaller and Insulin-Sensitive Adipocytes. J. Nutr. Biochem. 2015, 26, 860–867. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Nishii, S.; Zaima, N.; Moriyama, T.; Kawamura, Y. Ellagic Acid Improves Hepatic Steatosis and Serum Lipid Composition through Reduction of Serum Resistin Levels and Transcriptional Activation of Hepatic Ppara in Obese, Diabetic KK-Ay Mice. Biochem. Biophys. Res. Commun. 2013, 434, 486–491. [Google Scholar] [CrossRef]
- Huang, J.Y.; Liao, J.S.; Qi, J.R.; Jiang, W.X.; Yang, X.Q. Structural and Physicochemical Properties of Pectin-Rich Dietary Fiber Prepared from Citrus Peel. Food Hydrocoll. 2021, 110, 106140. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Rodríguez-Gutiérrez, G.; Lama-Muñoz, A.; García, A.; Fernández-Bolaños, J. Novel Pectin Present in New Olive Mill Wastewater with Similar Emulsifying and Better Biological Properties than Citrus Pectin. Food Hydrocoll. 2015, 50, 237–246. [Google Scholar] [CrossRef]
- Li, X.; He, X.; Lv, Y.; He, Q. Extraction and Functional Properties of Water-Soluble Dietary Fiber from Apple Pomace. J. Food Process Eng. 2014, 37, 293–298. [Google Scholar] [CrossRef]
- Shuai, X.; Chen, J.; Liu, Q.; Dong, H.; Dai, T.; Li, Z.; Liu, C.; Wang, R. The Effects of Pectin Structure on Emulsifying, Rheological, and In Vitro Digestion Properties of Emulsion. Foods 2022, 11, 3444. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Yu, K.S.; Hwang, K.T. Effect of Ellagic Acid Incorporation on the Oxidative Stability of Sodium Caseinate-Polysaccharide-Stabilized Emulsions. LWT 2017, 84, 601–607. [Google Scholar] [CrossRef]

| Method* | Yield (%) | DM (%) | Phenolic Compounds | |
|---|---|---|---|---|
| Cyanidin-3-O-Glucoside (mg/100g) | Ellagic Acid (mg/100g) | |||
| 1 | 17.79 ± 0.63b | 52.93± 0.71a | 176.77 ± 5.97a | 33.74 ± 2.85a |
| 2 | 21.80 ± 2.25a | 44.65 ± 2.02c | 104.31 ± 17.57b | 40.31 ± 9.05a |
| 3 | 22.06 ± 0.42a | 48.58 ± 0.21b | 115.94 ± 18.13b | 24.85 ± 5.17a |
| 4 | 18.38 ± 0.35b | 53.01 ± 0.87a | 169.73 ± 13.93a | 30.80 ± 4.55a |
| Method** | 1 L* (%) | h | c* |
|---|---|---|---|
| 1 | 13.08 ± 0.26a | 4.04 ± 2.33b | 4.89 ± 0.77a |
| 2 | 16.73 ± 1.71a | 35.62 ± 9.20a | 3.14 ± 0.23b |
| 3 | 20.59 ± 2.28a | 25.51 ± 5.01a | 4.67 ± 0.31a |
| 4 | 13.18 ± 0.33a | 6.20 ± 0.67b | 5.52 ± 0.25a |
| Method* | Technological Properties | Emulsifying Properties | ||||
|---|---|---|---|---|---|---|
| ORC (g/g) | WRC (g/g) | 1 SC (mL/g) | WSI (g/100g) | 1 EA (%) | ES (%) | |
| 1 | 1.02 ± 0.00a | 1.03 ± 0.01a | 7.00 ± 0.33b | 2.22 ± 0.66ab | 20.88 ± 3.76ab | 49.99 ± 6.20ab |
| 2 | 1.02 ± 0.00a | 1.03 ± 0.02a | 9.78 ± 0.77ab | 3.21 ± 0.49ab | 48.77 ± 6.73a | 55.05 ± 1.52a |
| 3 | 1.04 ± 0.01a | 1.02 ± 0.01a | 15.11 ± 1.39a | 3.74 ± 0.40a | 10.58 ± 3.29ab | 43.30 ± 3.26ab |
| 4 | 1.03 ± 0.01a | 1.02 ± 0.03a | 7.33 ± 0.67ab | 2.27 ± 0.72ab | 13.41 ± 4.27ab | 46.88 ± 5.41ab |
| CCP** | 1.02 ± 0.01a | 1.04 ± 0.03a | - | 2.01 ± 0.54b | 5.57 ± 0.31b | 40.63 ± 4.27b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resende, L.M.; Franca, A.S. Jabuticaba (Plinia sp.) Peel as a Source of Pectin: Characterization and Effect of Different Extraction Methods. Foods 2023, 12, 117. https://doi.org/10.3390/foods12010117
Resende LM, Franca AS. Jabuticaba (Plinia sp.) Peel as a Source of Pectin: Characterization and Effect of Different Extraction Methods. Foods. 2023; 12(1):117. https://doi.org/10.3390/foods12010117
Chicago/Turabian StyleResende, Laís M., and Adriana S. Franca. 2023. "Jabuticaba (Plinia sp.) Peel as a Source of Pectin: Characterization and Effect of Different Extraction Methods" Foods 12, no. 1: 117. https://doi.org/10.3390/foods12010117





