Development of Enriched Oil with Polyphenols Extracted from Olive Mill Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reagents
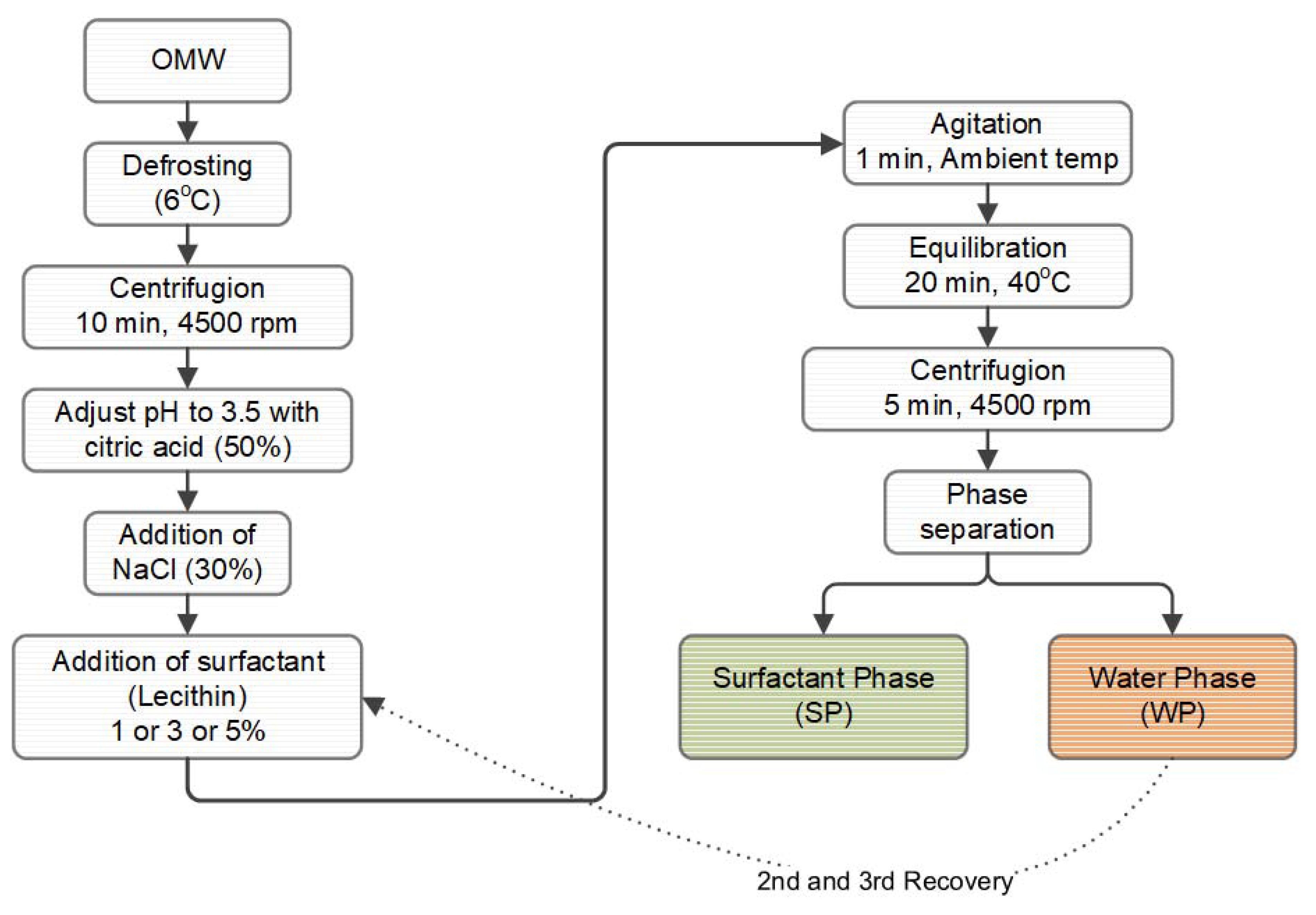
2.3. Extraction of Polyphenols from OMW
2.4. CPE Method Performance Determination
2.4.1. Total Polyphenol Content
2.4.2. Radical Scavenging Activity
2.5. Enrichment of Olive Oil with Micellar Dispersions
2.6. Enriched Olive Oil Quality Analysis
2.6.1. Acidity Value
2.6.2. Refractive Index
2.6.3. Colorimetry
2.6.4. Spectrophotometric Investigation in the Ultraviolet
2.6.5. Rancimat Method
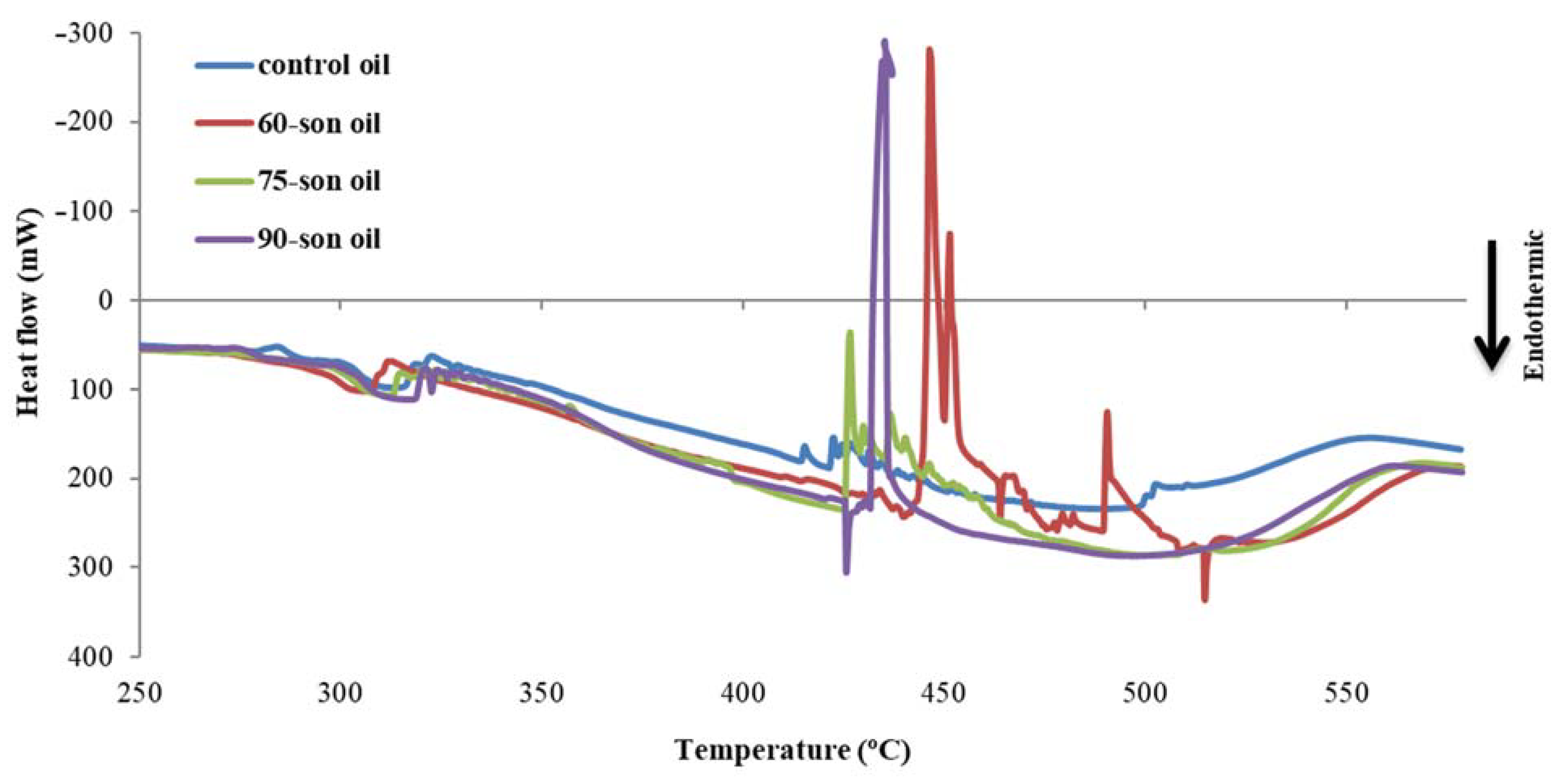
2.6.6. Differential Scanning Calorimetry
2.6.7. Extraction of Polyphenolic Compounds from the Enriched Olive Oils
2.6.8. Total Polyphenol Content
2.6.9. Radical-Scavenging Activity
2.7. Statistical Analysis
3. Results and Discussion
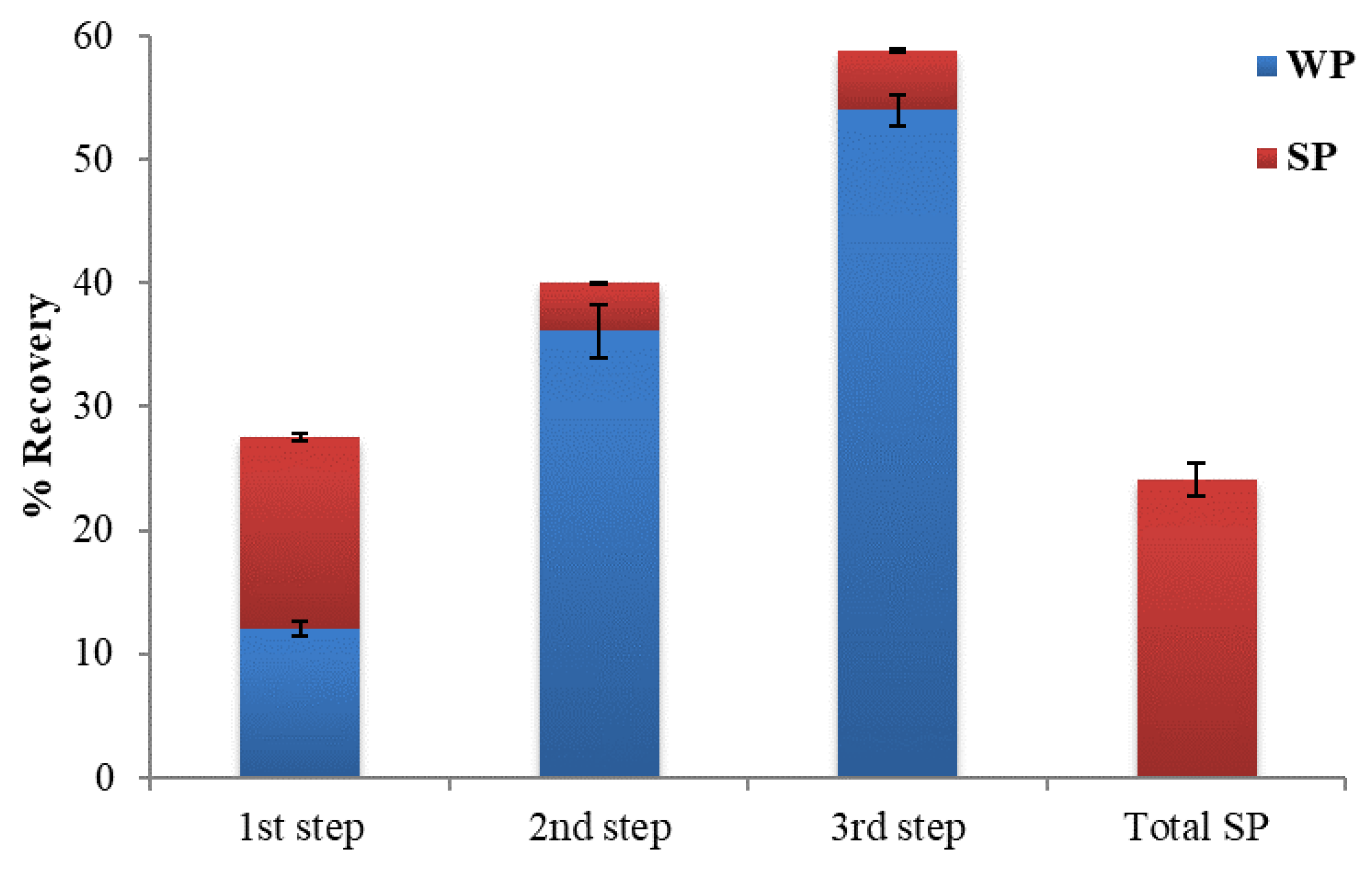
3.1. Recovery of Polyphenols
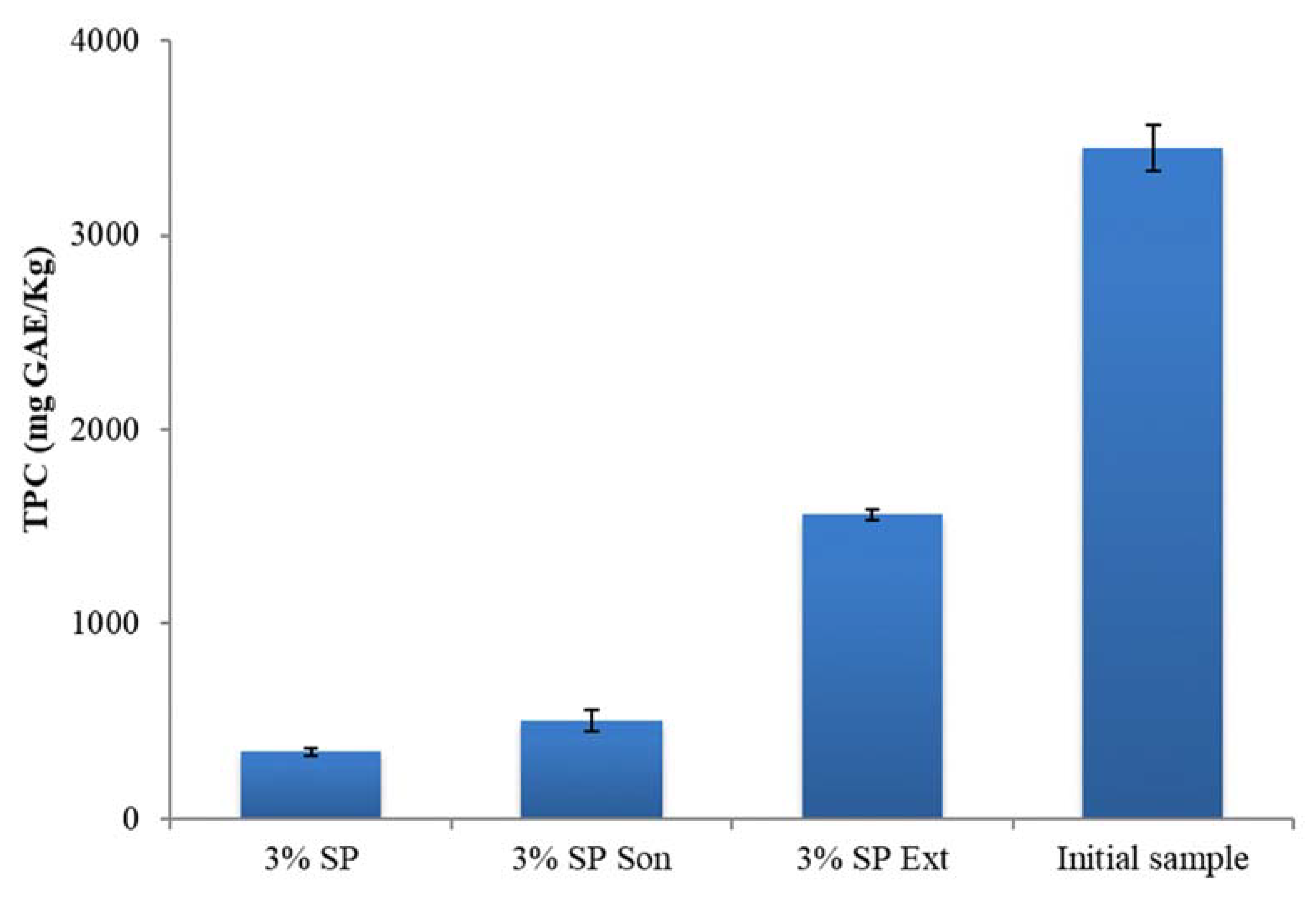
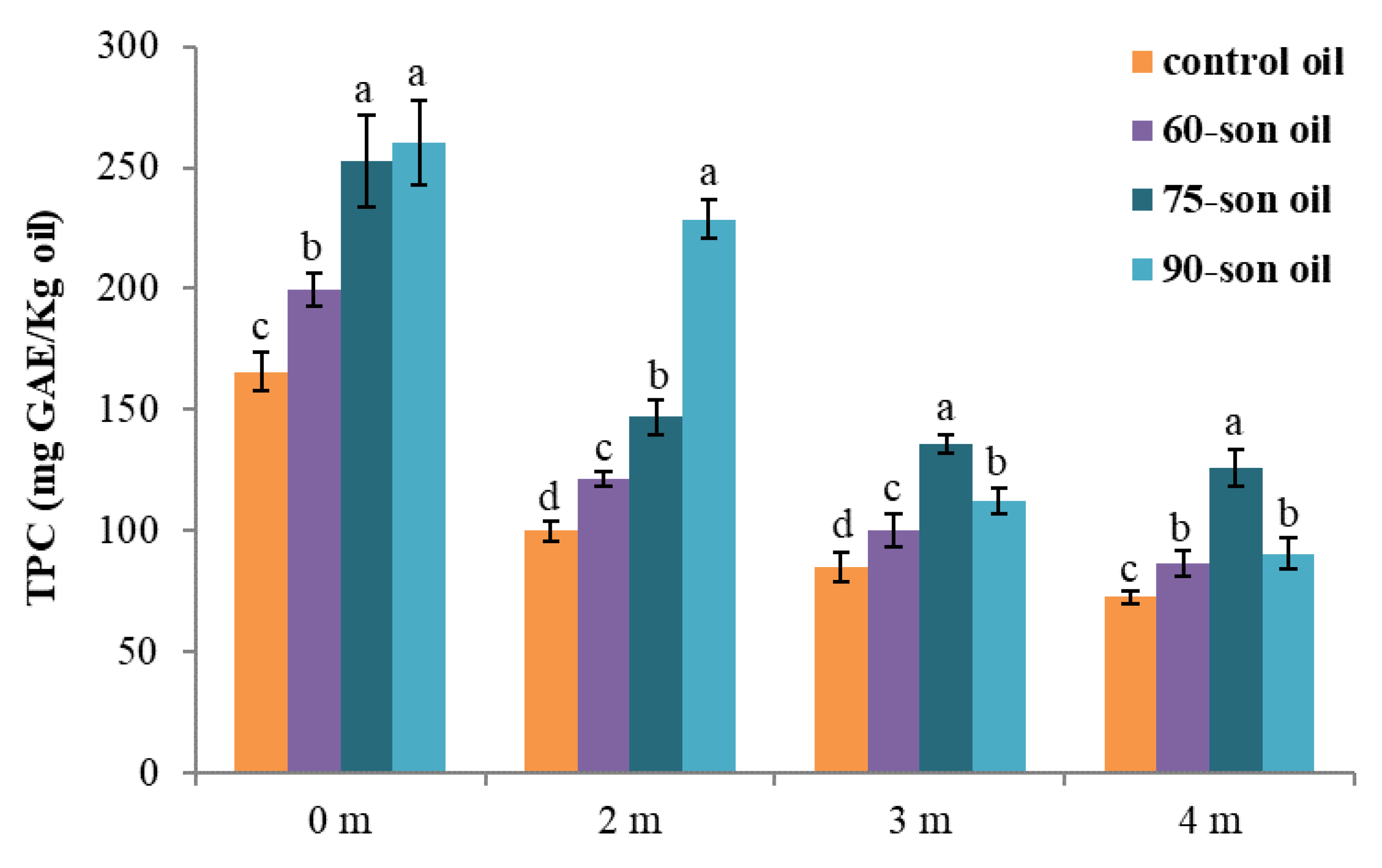
3.2. Enrichment of Olive Oil with Polyphenols from Olive Oil Wastewater
3.3. Quality Control of Enriched Olive Oils
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanifi, S.; Hadrami, I. El Olive Mill Wastewaters: Diversity of the Fatal Product in Olive Oil Industry and Its Valorisation as Agronomical Amendment of Poor Soils: A Review. J. Agron. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Jarboui, R.; Sellami, F.; Kharroubi, A.; Gharsallah, N.; Ammar, E. Olive Mill Wastewater Stabilization in Open-Air Ponds: Impact on Clay-Sandy Soil. Bioresour. Technol. 2008, 99, 7699–7708. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Spallarossa, A.; Comite, A.; Pagliero, M.; Guida, P.; Belotti, V.; Caviglia, D.; Schito, A.M. Valorization and Potential Antimicrobial Use of Olive Mill Wastewater (OMW) from Italian Olive Oil Production. Antioxidants 2022, 11, 903. [Google Scholar] [CrossRef] [PubMed]
- Saez, L.; Perez, J.; Martinez, J. Low Molecular Weight Phenolics Attenuation during Simulated Treatment of Wastewaters from Olive Oil Mills in Evaporation Ponds. Water Res. 1992, 26, 1261–1266. [Google Scholar] [CrossRef]
- Motikar, P.D.; More, P.R.; Arya, S.S. A Novel, Green Environment-Friendly Cloud Point Extraction of Polyphenols from Pomegranate Peels: A Comparative Assessment with Ultrasound and Microwave-Assisted Extraction. Sep. Sci. Technol. 2021, 56, 1014–1025. [Google Scholar] [CrossRef]
- Alibade, A.; Batra, G.; Bozinou, E.; Salakidou, C.; Lalas, S. Optimization of the Extraction of Antioxidants from Winery Wastes Using Cloud Point Extraction and a Surfactant of Natural Origin (Lecithin). Chem. Pap. 2020, 74, 4517–4524. [Google Scholar] [CrossRef]
- Śliwa, P.; Śliwa, K. Nanomicellar Extraction of Polyphenols—Methodology and Applications Review. Int. J. Mol. Sci. 2021, 22, 11392. [Google Scholar] [CrossRef]
- Leouifoudi, I.; Zyad, A.; Amechrouq, A.; Oukerrou, M.A.; Mouse, H.A.; Mbarki, M. Identification and Characterisation of Phenolic Compounds Extracted from Moroccan Olive Mill Wastewater. Food Sci. Technol. 2014, 34, 249–257. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G.F. Phenolic Compounds in Olive Oil: Antioxidant, Health and Organoleptic Activities According to Their Chemical Structure. Inflammopharmacology 2009, 17, 76–84. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A.; Galli, C. Antioxidant and Other Biological Activities of Phenols from Olives and Olive Oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef]
- Ben Saad, A.; Jerbi, A.; Khlif, I.; Ayedi, M.; Allouche, N. Stabilization of Refined Olive Oil with Phenolic Monomers Fraction and Purified Hydroxytyrosol from Olive Mill Wastewater. Chem. Afr. 2020, 3, 657–665. [Google Scholar] [CrossRef]
- Bravi, E.; Perretti, G.; Falconi, C.; Marconi, O.; Fantozzi, P. Antioxidant Effects of Supercritical Fluid Garlic Extracts in Sunflower Oil. J. Sci. Food Agric. 2017, 97, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Hannachi, H.; Elfalleh, W. Enrichment of Olive Oil with Polyphenols from Oleaster Leaves Using Central Composite Design for the Experimental Measurements. Anal. Lett. 2021, 54, 590–607. [Google Scholar] [CrossRef]
- Chedea, V.S.; Tomoiagǎ, L.L.; Macovei, Ş.O.; Mǎgureanu, D.C.; Iliescu, M.L.; Bocsan, I.C.; Buzoianu, A.D.; Voşloban, C.M.; Pop, R.M. Antioxidant/Pro-Oxidant Actions of Polyphenols from Grapevine and Wine By-Products-Base for Complementary Therapy in Ischemic Heart Diseases. Front. Cardiovasc. Med. 2021, 8, 1522. [Google Scholar] [CrossRef]
- Chatzilazarou, A.; Katsoyannos, E.; Gortzi, O.; Lalas, S.; Paraskevopoulos, Y.; Dourtoglou, E.; Tsaknis, J. Removal of Polyphenols from Wine Sludge Using Cloud Point Extraction. J. Air Waste Manag. Assoc. 2010, 60, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Katsoyannos, E.; Gortzi, O.; Chatzilazarou, A.; Athanasiadis, V.; Tsaknis, J.; Lalas, S. Evaluation of the Suitability of Low Hazard Surfactants for the Separation of Phenols and Carotenoids from Red-Flesh Orange Juice and Olive Mill Wastewater Using Cloud Point Extraction. J. Sep. Sci. 2012, 35, 2665–2670. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EEC) No 2568/91 of 11 July 1991 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. Off. J. Eur. Communities 1991, L248, 27–28.
- Commission Implementing Regulation (EU) No 299/2013 of 26 March 2013 Amending Regulation (EEC) No 2568/91 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. Off. J. Eur. Union 2013, L90, 55–56.
- Lalas, S.; Athanasiadis, V.; Gortzi, O.; Bounitsi, M.; Giovanoudis, I.; Tsaknis, J.; Bogiatzis, F. Enrichment of Table Olives with Polyphenols Extracted from Olive Leaves. Food Chem. 2011, 127, 1521–1525. [Google Scholar] [CrossRef]
- Pardauil, J.J.R.; Souza, L.K.C.; Molfetta, F.A.; Zamian, J.R.; Rocha Filho, G.N.; Da Costa, C.E.F. Determination of the Oxidative Stability by DSC of Vegetable Oils from the Amazonian Area. Bioresour. Technol. 2011, 102, 5873–5877. [Google Scholar] [CrossRef]
- Kalantzakis, G.; Blekas, G.; Pegklidou, K.; Boskou, D. Stability and Radical-Scavenging Activity of Heated Olive Oil and Other Vegetable Oils. Eur. J. Lipid Sci. Technol. 2006, 108, 329–335. [Google Scholar] [CrossRef]
- Víctor-Ortega, M.D.; Martins, R.C.; Gando-Ferreira, L.M.; Quinta-Ferreira, R.M. Recovery of Phenolic Compounds from Wastewaters through Micellar Enhanced Ultrafiltration. Colloids Surf. A Physicochem. Eng. Asp. 2017, 531, 18–24. [Google Scholar] [CrossRef]
- Sliwa, K.; Sliwa, P. The Accumulated Effect of the Number of Ethylene Oxide Units and/or Carbon Chain Length in Surfactants Structure on the Nano-Micellar Extraction of Flavonoids. J. Funct. Biomater. 2020, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Katsoyannos, E.; Chatzilazarou, A.; Gortzi, O.; Lalas, S.; Konteles, S.; Tataridis, P. Application of Cloud Point Extraction Using Surfactants in the Isolation of Physical Antioxidants (Phenols) from Olive Mill Wastewater. Fresenius Environ. Bull. 2006, 15, 1122–1125. [Google Scholar]
- Gortzi, O.; Lalas, S.; Chatzilazarou, A.; Katsoyannos, E.; Papaconstandinou, S.; Dourtoglou, E. Recovery of Natural Antioxidants from Olive Mill Wastewater Using Genapol-X080. J. Am. Oil Chem. Soc. 2008, 85, 133–140. [Google Scholar] [CrossRef]
- Śliwa, P.; Śliwa, K.; Sikora, E.; Ogonowski, J.; Oszmiański, J.; Nowicka, P. Incorporation of Bioflavonoids from Bidens Tripartite into Micelles of Non-Ionic Surfactants—Experimental and Theoretical Studies. Colloids Surf. B Biointerfaces 2019, 184, 110553. [Google Scholar] [CrossRef]
- Xenakis, A.; Papadimitriou, V.; Sotiroudis, T.G. Colloidal Structures in Natural Oils. Curr. Opin. Colloid Interface Sci. 2010, 15, 55–60. [Google Scholar] [CrossRef]
- Li, F.; Raza, A.; Wang, Y.-W.; Xu, X.-Q.; Chen, G.-H. Optimization of Surfactant-Mediated, Ultrasonic-Assisted Extraction of Antioxidant Polyphenols from Rattan Tea (Ampelopsis Grossedentata) Using Response Surface Methodology. Pharmacogn. Mag. 2017, 13, 446–453. [Google Scholar] [CrossRef]
- Noipa, T.; Srijaranai, S.; Tuntulani, T.; Ngeontae, W. New Approach for Evaluation of the Antioxidant Capacity Based on Scavenging DPPH Free Radical in Micelle Systems. Food Res. Int. 2011, 44, 798–806. [Google Scholar] [CrossRef]
- Karadag, A.; Kayacan Cakmakoglu, S.; Metin Yildirim, R.; Karasu, S.; Avci, E.; Ozer, H.; Sagdic, O. Enrichment of Lecithin with Phenolics from Olive Mill Wastewater by Cloud Point Extraction and Its Application in Vegan Salad Dressing. J. Food Process. Preserv. 2022, 46, e16645. [Google Scholar] [CrossRef]
- Yangui, A.; Abderrabba, M. Towards a High Yield Recovery of Polyphenols from Olive Mill Wastewater on Activated Carbon Coated with Milk Proteins: Experimental Design and Antioxidant Activity. Food Chem. 2018, 262, 102–109. [Google Scholar] [CrossRef]
- Roig, A.; Cayuela, M.L.; Sánchez-Monedero, M.A. An Overview on Olive Mill Wastes and Their Valorisation Methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Kotsou, M.; Mari, I.; Lasaridi, K.; Chatzipavlidis, I.; Balis, C.; Kyriacou, A. The Effect of Olive Oil Mill Wastewater (OMW) on Soil Microbial Communities and Suppressiveness against Rhizoctonia Solani. Appl. Soil Ecol. 2004, 26, 113–121. [Google Scholar] [CrossRef]
- Zoidou, E.; Melliou, E.; Gikas, E.; Tsarbopoulos, A.; Magiatis, P.; Skaltsounis, A.L. Identification of Throuba Thassos, a Traditional Greek Table Olive Variety, as a Nutritional Rich Source of Oleuropein. J. Agric. Food Chem. 2010, 58, 46–50. [Google Scholar] [CrossRef]
- Rodis, P.S.; Karathanos, V.T.; Mantzavinou, A. Partitioning of Olive Oil Antioxidants between Oil and Water Phases. J. Agric. Food Chem. 2002, 50, 596–601. [Google Scholar] [CrossRef]
- Soler-Rivas, C.; Espín, J.C.; Wichers, H.J. Oleuropein and Related Compounds. J. Sci. Food Agric. 2000, 80, 1013–1023. [Google Scholar] [CrossRef]
- Garrido-Fernandez, A.; Fernandez-Diez, M.J.; Adams, M.R. Table Olives: Production and Processing; Chapman & Hall: London, UK, 1997; ISBN 0412718103. [Google Scholar]
- Bouaziz, M.; Feki, I.; Ayadi, M.; Jemai, H.; Sayadi, S. Stability of Refined Olive Oil and Olive-pomace Oil Added by Phenolic Compounds from Olive Leaves. Eur. J. Lipid Sci. Technol. 2010, 112, 894–905. [Google Scholar] [CrossRef]


| Samples | IC50 |
|---|---|
| 60-son | 938.9 ± 33.8 a,* |
| 75-son | 945.6 ± 45.4 a |
| 90-son | 991.3 ± 69.4 a |
| Total SP | 1008.8 ± 25.2 a |
| Samples | Acidity | Ref. Index | Colorimetry | Conjugated Diene and Triene | Rancimat | DSC | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FFA (%) | nD20 | L* | a* | b* | K232 | K270 | ΔΚ | IT (h) | PF | Tmax (°C) | |
| control oil | 0.790 ± 0.028 a,* | 1.4684 ± 0.0009 a | 71.4 ± 0.5 b | −6.7 ± 0.3 b | 67.1 ± 0.4 a | 3.94 ± 0.25 a | 0.59 ± 0.04 b | 0.042 ± 0.002 a | 34.5 ± 2.1 a | - | 423 ± 27 a |
| 60-son oil | 0.790 ± 0.017 a | 1.4683 ± 0.0006 a | 71.4 ± 0.4 b | −5.9 ± 0.2 a | 66.3 ± 0.4 b | 3.78 ± 0.19 a | 0.66 ± 0.03 a,b | 0.021 ± 0.001 b | 33 ± 1.6 a | 0.96 ± 0.05 a | 455 ± 8 a |
| 75-son oil | 0.733 ± 0.018 b | 1.4684 ± 0.0009 a | 72.5 ± 0.2 a | −5.9 ± 0.2a | 60.3 ± 0.1 c | 3.70 ± 0.14 a | 0.68 ± 0.05 a | 0.043 ± 0.003 a | 29.7 ± 1.7 b | 0.86 ± 0.03 b | 425 ± 10 a |
| 90-son oil | 0.675 ± 0.022 c | 1.4682 ± 0.0010 a | 72.9 ± 0.5 a | −5.9 ± 0.2a | 60.0 ± 0.2 c | 3.89 ± 0.13 a | 0.64 ± 0.04 a,b | 0.044 ± 0.003 a | 29.8 ± 0.7 b | 0.86 ± 0.02 b | 435 ± 18 a |
| Samples | IC50 |
|---|---|
| control oil | 205,400 ± 12,529 b,* |
| 60-son oil | 219,050 ± 8,105 b |
| 75-son oil | 154,650 ± 11,599 c |
| 90-son oil | 359,000 ± 20,104 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athanasiadis, V.; Voulgaris, A.; Katsoulis, K.; Lalas, S.I.; Roussis, I.G.; Gortzi, O. Development of Enriched Oil with Polyphenols Extracted from Olive Mill Wastewater. Foods 2023, 12, 497. https://doi.org/10.3390/foods12030497
Athanasiadis V, Voulgaris A, Katsoulis K, Lalas SI, Roussis IG, Gortzi O. Development of Enriched Oil with Polyphenols Extracted from Olive Mill Wastewater. Foods. 2023; 12(3):497. https://doi.org/10.3390/foods12030497
Chicago/Turabian StyleAthanasiadis, Vassilis, Andreas Voulgaris, Konstantinos Katsoulis, Stavros I. Lalas, Ioannis G. Roussis, and Olga Gortzi. 2023. "Development of Enriched Oil with Polyphenols Extracted from Olive Mill Wastewater" Foods 12, no. 3: 497. https://doi.org/10.3390/foods12030497
APA StyleAthanasiadis, V., Voulgaris, A., Katsoulis, K., Lalas, S. I., Roussis, I. G., & Gortzi, O. (2023). Development of Enriched Oil with Polyphenols Extracted from Olive Mill Wastewater. Foods, 12(3), 497. https://doi.org/10.3390/foods12030497









