Changes in the VOC of Fruits at Different Refrigeration Stages of ‘Ruixue’ and the Participation of Carboxylesterase MdCXE20 in the Catabolism of Volatile Esters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Determination of Fruit Physiological Characteristics
2.2.1. Firmness Measurement by Texture Analyzer
2.2.2. Determination of Lipoxygenase (LOX)
2.2.3. Determination of Hydroperoxide Lyase (HPL)
2.2.4. The Activities of Keto Acid Decarboxylase (PDC) and Aldehyde Dehydrogenase (ADH) Were Determined
2.3. VOC Extraction and GC-MS Analysis
2.4. RNA Extraction, Gene Expression Analysis by RT-qPCR
2.5. Bioinformatic Sequence Analysis
2.6. Transient Injection of the MdCXE20 in Apple Fruit
2.7. Overexpression of ‘Wanglin’ Callus Transgenic
2.8. Subcellular Localization Analysis
2.9. Statistical Analysis and Data Processing
3. Results
3.1. Changes of Fruit Firmness and Brittleness of ‘Ruixue’ at Different Cold Storage Stages
3.2. Changes of Aroma Synthase Enzyme Activity in ‘Ruixue’ at Different Cold Storage Stages
3.3. VOC in Apple Fruits during Different Cold Storage Periods
3.4. Bioinformatics and Phylogenetic Analysis of MdCXE Gene Family Proteins
3.5. MdCXE Gene Expression Analysis
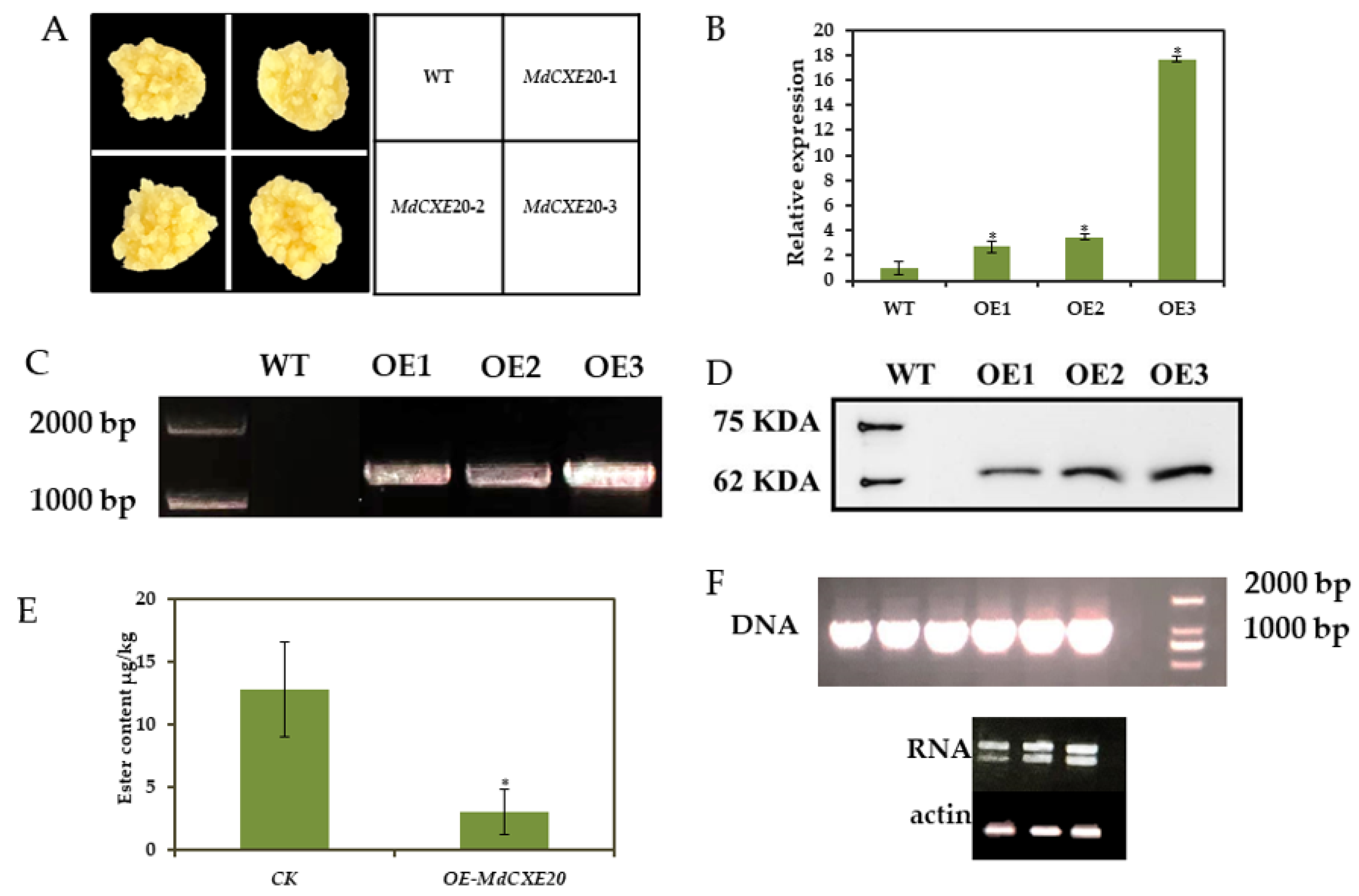
3.6. Transient Overexpression and Silencing of MdCXE20 Changed the Content of Esters in Apple Fruit
3.7. MdCXE20 Transgenic Reduced the Content of Ester Volatile Substances in ‘Wanglin’ Callus
3.8. Subcellular Localization Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.D.; Meng, X.X.; Zhao, R.X.; Dou, Y.T.; Xian, G.J.; Xu, Q. A preliminary exploration on the construction of a scientific data resource system for apple industry in China. J. Fruit Sci. 2016, 33, 719–726. [Google Scholar]
- Kondo, S.; Setha, S.; Rudell, D.R.; Buchanan, D.A.; Mattheis, J.P. Aroma volatile biosynthesis in apples affected by 1-MCP and methyl jasmonate. Postharvest Biol. Technol. 2005, 36, 61–68. [Google Scholar] [CrossRef]
- Echeverría, G.; Graell, J.; López, M.L.; Lara, I. Volatile production, quality and aroma-related enzyme activities during maturation of ‘Fuji’ apples. Postharvest Biol. Technol. 2004, 31, 217–227. [Google Scholar] [CrossRef]
- Young, H.; Gilbert, J.M.; Murray, S.H.; Ball, R.D. Causal Effects of Aroma Compounds on Royal Gala Apple Flavours. J. Sci. Food Agric. 1996, 71, 329–336. [Google Scholar] [CrossRef]
- Young, H.; Stec, M.; Paterson, V.J.; McMath, K.; Ball, R. Volatile Compounds Affecting Kiwifruit Flavor. Am. Chem. Soc. 1995, 596, 59–67. [Google Scholar]
- Goodenough, P.W.; Entwistle, T.G. The hydrodynamic properties and kinetic constants with natural substrates of the esterase from Malus pumila fruit. Eur. J. Biochem. 1982, 127, 145–149. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Li, L.N.; Liu, Q.; Ding, Q.N.; Li, K.; Liu, P. Composition, Influencing Factors and Regulation Strategies of Aroma Substances in Apple. North. Hortic. 2023, 520, 119–127. [Google Scholar]
- Liu, J.L. Analysis of Volatile Flavor Compounds and Their Genetic Characteristics in Malus Domestica ‘Ruixue’. Northwest AF Univ. 2019, 36, 590–602. [Google Scholar]
- Echeverría, G.; Graell, J.; López, M.L. Effect of Harvest Date and Storage Conditions on Quality and Aroma Production of ‘Fuji’ Apples. Food Sci. Technol. Int. 2002, 8, 351–360. [Google Scholar]
- Yang, X.; Song, J.; Du, L.; Charles, F.; Campbell-Palmer, L.; Fillmore, S.; Paul, W.; Zhang, Z.Q. Ethylene and 1-MCP regulate major volatile biosynthetic pathways in apple fruit. Food Chem. 2016, 194, 325–336. [Google Scholar] [CrossRef]
- Li, D.; Xu, Y.F.; Xu, G.M.; Gu, L.K.; Li, D.; Shu, H. Molecular cloning and expression of a gene encoding alcohol acyltransferase (MdAAT2) from apple (cv. Golden Delicious). Phytochemistry 2006, 67, 658–667. [Google Scholar] [CrossRef]
- Zhong, R.; Lee, C.; Ye, Z. Global analysis of direct targets of secondary wall NAC master switches in Arabidopsis. Mol. Plant 2010, 3, 1087–1103. [Google Scholar] [CrossRef]
- Cumplido-Laso, G.; Medina-Puche, L.; Moyano, E.; Hoffmann, T.; Sinz, Q.; Ring, L.; Studart-Wittkowski, C.; Caballero, J.L.; Schwab, W.; Munoz-Blaoco, J. The fruit ripening-related gene FaAAT2 encodes an acyl transferase involved in strawberry aroma biogenesis. J. Exp. Bot. 2012, 63, 4275–4290. [Google Scholar] [CrossRef]
- Williams, A.A.; Knee, M. The flavour of Cox’s Orange Pippin apples and its variation with storage. Ann. Appl. Biol. 1977, 87, 127–131. [Google Scholar] [CrossRef]
- Levisson, M.; Oost, J.V.D.; Kengen, S.W.M. Carboxylic ester hydrolases from hyperthermophiles. Extremophiles 2009, 13, 567–581. [Google Scholar] [CrossRef]
- Wang, X.; Shi, J.; Zhu, H.J. Functional Study of Carboxylesterase 1 Protein Isoforms. Proteomics 2019, 19, 800288. [Google Scholar] [CrossRef]
- Ollis, D.L.; Cheah, E.; Cygler, M.; Dijkstra, B.; Frolow, F.; Franken, S.M.; Harel, M.; Remington, S.J.; Silman, I.; Schrag, J. The alpha/beta hydrolase fold. Protein Eng. 1992, 5, 197–211. [Google Scholar] [CrossRef]
- Ileperuma, N.R.; Marshall, S.D.; Squire, C.J.; Baker, H.M.; Oakeshott, J.G.; Russell, R.J.; Plummer, K.M.; Newcomb, R.D.; Baker, E.N. High-resolution crystal structure of plant carboxylesterase AeCXE1, from Actinidia eriantha, and its complex with a high-affinity inhibitor paraoxon. Biochemistry 2007, 46, 1851–1859. [Google Scholar] [CrossRef]
- Marshall, S.D.G.; Putterill, J.J.; Plummer, K.M.; Richard, D.; Newcomb, R.D. The carboxylesterase gene family from Arabidopsis thaliana. J. Mol. Evol. 2003, 57, 487–500. [Google Scholar]
- Schilmiller, A.L.; Gilgallon, K.; Ghosh, B.; Jones, A.D.; Last, R.L. Acylsugar Acylhydrolases: Carboxylesterase-Catalyzed Hydrolysis of Acylsugars in Tomato Trichomes. Plant Physiol. 2016, 170, 1331–1344. [Google Scholar] [CrossRef]
- Nomura, T.; Murase, T.; Ogita, S.; Kato, Y. Molecular identification of tuliposide B-converting enzyme: A lactone-forming carboxylesterase from the pollen of tulip. Plant J. 2015, 83, 252–262. [Google Scholar] [CrossRef]
- Lee, S.; Hwang, S.; Seo, Y.; Jeon, W. Molecular characterization of the AtCXE8 gene, which promotes resistance to Botrytis cinerea infection. Plant Biotechnol. Rep. 2013, 7, 109–119. [Google Scholar] [CrossRef]
- Pontier, D.; Godiard, L.; Marco, Y.; Roby, D. hsr203J, a tobacco gene whose activation is rapid, highly localized and specific for incompatible plant/pathogen interactions. Plant J. 1994, 5, 507–521. [Google Scholar] [CrossRef]
- Ko, M.; Cho, J.H.; Seo, H.H.; Lee, H.H.; Kang, H.Y.; Nguyen, T.S.; Soh, H.C.; Kim, Y.S.; Kim, J.I. Constitutive expression of a fungus-inducible carboxylesterase improves disease resistance in transgenic pepper plants. Planta 2016, 244, 379–392. [Google Scholar] [CrossRef]
- Souleyre, E.J.F.; Marshall, S.D.G.; Oakeshott, J.G.; Russell, R.J.; Plummer, K.M.; Newcomb, R.D. Show more Biochemical characterisation of MdCXE1, a carboxylesterase from apple that is expressed during fruit ripening. Phytochemistry 2011, 72, 564–571. [Google Scholar] [CrossRef]
- Goulet, C.; Mageroy, M.H.; Lam, N.B.; Floystad, A.; Tieman, D.M.; Klee, H.J. Role of an esterase in flavor volatile variation within the tomato clade. Proc. Natl. Acad. Sci. USA 2012, 109, 19009–19014. [Google Scholar] [CrossRef]
- Cao, X.; Xie, K.; Wenyi, D.; Zhu, Y. Peach Carboxylesterase PpCXE1 Is Associated with Catabolism of Volatile Esters. J. Agric. Food Chem. 2019, 67, 5189–5196. [Google Scholar] [CrossRef]
- Chen, K.S.; Xu, C.J.; Xu, W.P.; Wu, M.; Zhang, S.L. Establishment of a method for determining the activity of lipooxygenase in kiwi fruit and peach fruit. J. Fruit Sci. 2003, 20, 436–438. [Google Scholar]
- Zhang, X.M. Physiological Studies on Aroma Volatile Formation in Peach Fruit. Dep. Hortic. Zhejiang Univ. 2005. Available online: https://kns.cnki.net/kcms2/article/abstract?v=1UV6lUEUW658kDSswtdDD6HhzncyebpjLexctGdXWtVwW_7hfELsP1ddfk3Ny9ubzplLnUBQhLbOcjevA3xDQIcjlqRwPjPe9XW2kU1W0zrKH_YLWfayfyVa893Id2s9&uniplatform=NZKPT&language=CHS (accessed on 6 May 2023).
- Ke, D.; Zhou, L.; Kader, A.A. Mode of Oxygen and Carbon Dioxide Action on Strawberry Ester Biosynthesis. J. Am. Soc. Hortic. Sci. 1994, 119, 971–975. [Google Scholar]
- Yang, S.; Li, D.M.; Li, S.S.; Yang, H.J.; Zhao, Z.Y. GC-MS Metabolite and Transcriptome Analyses Reveal the Differences of Volatile Synthesis and Gene Expression Profiling between Two Apple Varieties. Int. J. Mol. Sci. 2022, 23, 2939. [Google Scholar] [CrossRef]
- Meng, Z.P. Study on Genetic Characteristics of Volatile Compounds in Apple Fruit. Northwest AF Univ. 2022. [Google Scholar] [CrossRef]
- Gambino, G.; Perrone, I.; Gribaudo, I. A Rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. Phytochem. Anal. 2008, 19, 520–525. [Google Scholar] [CrossRef]
- Wu, D.; Hao, J.F.; Zhang, L.Q.; Zhao, A.Q.; Ha, A. Analysis of Function of a-mannosidase Gene in Promoting Melon Fruit Ripening by Transient Expression. Acta Hortic. Sin. 2014, 41, 1601–1608. [Google Scholar]
- Liu, X.; Li, D.M.; Li, Y.J.; Li, S.S.; Zhao, Z.Y. Brassinosteroids are involved in volatile compounds biosynthesis related to MdBZR1 in ‘Ruixue’ (Malus × domestica Borkh.) fruit. Postharvest Biol. Technol. 2022, 189, 111931. [Google Scholar] [CrossRef]
- He, Y.; Liu, H.; Li, H.; Jin, M.; Wang, X.L.; Yin, X.R.; Zhu, Q.J.; Rao, J.P. Transcription factors DkBZR1/2 regulate cell wall degradation genes and ethylene biosynthesis genes during persimmon fruit ripening. J. Exp. Bot. 2021, 72, 6437–6446. [Google Scholar] [CrossRef]
- Jia, C.S.; Sun, S.M.; Bao, A.M.; Wang, Z.H. Comparison of postharvest physiology and storage tolerance of four characteristic small apples. Trans. Chin. Soc. Agric. Eng. 2022, 38, 308–316. [Google Scholar]
- Liu, Y.W.; Song, Z.B.; Chen, G.J.; Wang, B.J.; Yang, G.H.; Yuan, D.H. Chengde City develops cold apple industry to help rural revitalization. Mod. Rural. Sci. Technol. 2022, 11, 7–8. [Google Scholar]
- Zhang, F.; Gong, X.M.; Ma, S.S.; Zhang, J.F.; Guan, J.F. Effect of 1-MCP storage and temperature on the quality and postharvest physiology of Qinyang Apple. J. Northwest AF Univ. (Nat. Sci. Ed.) 2009, 37, 115–119. [Google Scholar]
- Fan, X.G. Cytological Studies on Texture Differ Ences in Fruit Development of ‘Ruiyang’, ‘Ruixue’ and Theie Parents. Northwest AF Univ. 2017. Available online: https://kns.cnki.net/kcms2/article/abstract?v=1UV6lUEUW65E8nc7eaVi9OMyDQAEe86Wgwy9lccUR9HLIdat8wPLUhPbELp4qg_QlSJqbwYfm8uoStjf_afRqYyIF9_Do3xpH2CIN5c7TBq9A27_V3nb4SaupScZTm4gtrwxjBV2u3Q=&uniplatform=NZKPT&language=CHS (accessed on 6 May 2023).
- Lewinsohn, E.; Schalechet, F.; Wilkinson, J.; Matsui, K.; Tadmor, Y.; Nam, K.H.; Amar, O.; Lastochkin, E.; Larkov, O.; Ravid, U.; et al. Enhanced Levels of the Aroma and Flavor Compound S-Linalool by Metabolic Engineering of the Terpenoid Pathway in Tomato Fruits. Plant Physiol. 2001, 127, 1256–1265. [Google Scholar] [CrossRef]
- Dixon, J.; Hewett, E.W. Factors affecting apple aroma/flavour volatile concentration: A Review. N. Z. J. Crop Hortic. Sci. 2000, 28, 155–173. [Google Scholar] [CrossRef]
- El-Sharkawy, I.; Manriquez, D.; Flores, F.B.; Regad, F.; Bouzayen, M.; Latche, A.; Pech, J.C. Functional characterization of a melon alcohol acyl-transferase gene family involved in the biosynthesis of ester volatiles. Identification of the crucial role of a threonine residue for enzyme activity. Plant Mol. Biol. 2005, 59, 345–362. [Google Scholar]
- Tian, L.P.; Zhang, Q.; Li, R.; Ren, X.L. Effects of n-Butanol Treatment on Volatile Compounds of ‘Pink Lady’ Apple during Storage. Sci. Technol. Food Ind. 2022, 43, 337–345. [Google Scholar]
- Defilippi, B.G.; Kader, A.A.; Dandekar, A.M. Apple aroma: Alcohol acyltransferase, a rate limiting step for ester biosynthesis, is regulated by ethylene. Plant Sci. 2005, 168, 1199–1210. [Google Scholar] [CrossRef]
- Yan, D.; Shi, J.; Ren, X.L.; Tao, Y.S.; Ma, F.W.; Li, R.; Liu, X.R.; Liu, C.H. Insights into the aroma profiles and characteristic aroma of ‘Honeycrisp’ apple (Malus × domestica). Food Chem. 2020, 327, 127074. [Google Scholar] [CrossRef]
- Rowan, D.D.; Allen, J.M.; Fielder, S.; Hunt, M.B. Biosynthesis of straight-chain ester volatiles in red delicious and granny smith apples using deuterium-labeled precursors. J. Agric. Food Chem. 1999, 47, 2553–2562. [Google Scholar] [CrossRef]
- Fellman, J.K.; Rudell, D.R.; Mattinson, D.S.; Mattheis, J.P. Relationship of harvest maturity to flavor regeneration after CA storage of ‘Delicious’ apples. Postharvest Biol. Technol. 2003, 27, 39–51. [Google Scholar] [CrossRef]
- Beekwilder, J.; Alvarez-Huerta, M.; Neef, E.; Verstappen, F.W.A.; Bouwmeester, H.J.; Aharoni, A. Functional Characterization of Enzymes Forming Volatile Esters from Strawberry and Banana. Plant Physiol. 2004, 135, 1865–1878. [Google Scholar] [CrossRef]
- Aharoni, A.; Keizer, L.C.; Bouwmeester, H.J.; Sun, Z.; Alvarez-Huerta, M.; Verhoeven, H.A.; Blaas, J.; van Houwelingen, A.M.M.L.; De Vos, R.C.H.; Voet, H.V.D.; et al. Identification of the SAAT Gene Involved in Strawberry Flavor Biogenesis by Use of DNA Microarrays. Plant Cell 2000, 12, 647–661. [Google Scholar] [CrossRef]
- Li, G.; Jia, H.J.; Li, J.H.; Wang, Q.; Zhang, M.J.; Teng, Y.W. Emission of volatile esters and transcription of ethylene- and aroma-related genes during ripening of ‘Pingxiangli’ pear fruit (Pyrus ussuriensis Maxim). Sci. Hortic. 2014, 170, 17–23. [Google Scholar] [CrossRef]
- Goulet, C.; Kamiyoshihara, Y.; Lam, N.B.; Richard, T.; Taylor, M.G.; Tieman, D.M.; Klee, H.J. Divergence in the enzy-matic activities of a tomato and Solanum pennellii alcohol acyltransferase impacts fruit volatile ester composition. Mol. Plant 2015, 8, 153–162. [Google Scholar] [CrossRef]
- Li, D.P. Expression Characterization of the MdAAT2 gene and Its Regulation Mechanism of Volatile Ester Biosynthesis in Apple (cv. Golden Delicious). Shandong Agric. Univ. 2005. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=2006100793.nh&DbName=CDFD2006 (accessed on 6 May 2023).







| Name | Apple ID | Primers (F) | Primer (R) |
|---|---|---|---|
| MdCXE3 | MD02G1275900 | TGATGGCTCTGTCGACCGG | AAATATATTCGGACACGATGG |
| MdCXE5 | MD02G1276600 | TGGATTCGTTCGATCAGT | TAAAGACGGTGCTCTGGA |
| MdCXE6 | MD03G1273300 | CTCGTTTGATCACGTCGACAC | CTGCTCAAGATCCGAAATGCC |
| MdCXE9 | MD05G1076400 | AAGAACCAGCATTGTCCGT | AATGCAGTTTCAACCACGAA |
| MdCXE10 | MD05G1078900 | TGCCTACAATCTCCATACTCG | ATGAAACGCTCAATTGTACCA |
| MdCXE12 | MD05G1191100 | CAACCCGAAACCGGAGTCC | GTGGGGGAGGAAGCGCTTTC |
| MdCXE17 | MD08G1226300 | ATCATTCGAGTTCCCGACCCT | CTTTGGATTGGACCCCGGTT |
| MdCXE20 | MD10G1068500 | GAGGAGGTACTACCAGTGGTTGAAG | TCACACGGAAGCAATGAAATCTTTG |
| MdCXE23 | MD10G1091200 | ATGGATTCAGCCTTGAGCAAC | ACATTCGACGCCTGTTTGTG |
| MdCXE25 | MD10G1091900 | ATGACAAACGAAGTAGCCCAT | CCTTGGATTGGACACCGATT |
| MdActin | MD01G1001600 | GATATCTCCACTGACGTAAGGGATG | AGGGTCAGCTTGCCGTAGGTGGCA |
| Gene | Vector | Primers Sequences |
|---|---|---|
| MdCXE20 | pCAMBIA2300-OE-Primers (F) | acgggggacgagctcggtaccATGACGACGTCGTTGGACTCC |
| pCAMBIA2300-OE-Primer (R) | ggtgtcgactctagaggatccCACGGAAGCAATGAAATCTT | |
| MdCXE20 | TRV2-Primers (F) | gtgagtaaggttaccgaattcTTTTTCGGGGGAGAGGAGC |
| TRV2-Primer (R) | gagacgcgtgagctcggtaccCACGGAAGCAATGAAATCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Guo, J.; Ma, H.; Pei, L.; Liu, X.; Wang, H.; Chen, R.; Zhao, Z.; Gao, H. Changes in the VOC of Fruits at Different Refrigeration Stages of ‘Ruixue’ and the Participation of Carboxylesterase MdCXE20 in the Catabolism of Volatile Esters. Foods 2023, 12, 1977. https://doi.org/10.3390/foods12101977
Li D, Guo J, Ma H, Pei L, Liu X, Wang H, Chen R, Zhao Z, Gao H. Changes in the VOC of Fruits at Different Refrigeration Stages of ‘Ruixue’ and the Participation of Carboxylesterase MdCXE20 in the Catabolism of Volatile Esters. Foods. 2023; 12(10):1977. https://doi.org/10.3390/foods12101977
Chicago/Turabian StyleLi, Dongmei, Jianhua Guo, Hai Ma, Linna Pei, Xiaojie Liu, Hui Wang, Rongxin Chen, Zhengyang Zhao, and Hua Gao. 2023. "Changes in the VOC of Fruits at Different Refrigeration Stages of ‘Ruixue’ and the Participation of Carboxylesterase MdCXE20 in the Catabolism of Volatile Esters" Foods 12, no. 10: 1977. https://doi.org/10.3390/foods12101977
APA StyleLi, D., Guo, J., Ma, H., Pei, L., Liu, X., Wang, H., Chen, R., Zhao, Z., & Gao, H. (2023). Changes in the VOC of Fruits at Different Refrigeration Stages of ‘Ruixue’ and the Participation of Carboxylesterase MdCXE20 in the Catabolism of Volatile Esters. Foods, 12(10), 1977. https://doi.org/10.3390/foods12101977







