Effect of Thermosonication on Amino Acids, Phenolic Compounds, Sensory Properties and Microbial Quality in Freshly Squeezed Verjuice
Abstract
1. Introduction
2. Materials and Methods
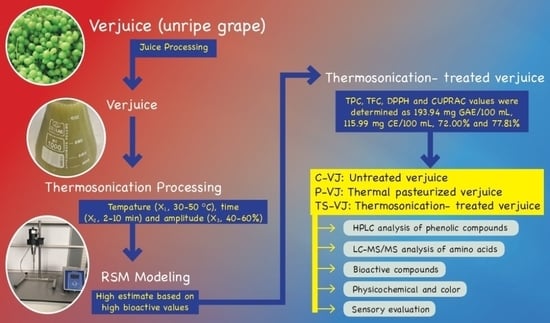
2.1. Thermal Pasteurization and Thermosonication Processing
2.2. Modelling Procedure for Response Surface Methodology
2.3. Determination of Bioactive Compounds
2.4. Determination of Phenolic Compounds
2.5. Determination of Amino Acids
2.6. Color and Physicochemical Analyses
2.7. Determination of Microbial Quality
2.8. Sensory Evaluation
2.9. Statistical Analysis
3. Results
3.1. Optimization of Bioactive Components
3.2. Amino Acids
3.3. Microbial Load
3.4. Phenolic Compounds
3.5. Physicochemical Properties and Color
3.6. Sensory Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayoglu, I.; Kola, O.; Kaya, C.; Özer, S.; Turkoglu, H. Chemical and sensory properties of verjuice, a traditional turkish non-fermented beverage from kabarcik and yediveren grapes. J. Food Process. Preserv. 2009, 33, 252–263. [Google Scholar] [CrossRef]
- Karapinar, M.; Sengun, I.Y. Antimicrobial effect of koruk (unripe grape—Vitis vinifera) juice against Salmonella typhimurium on salad vegetables. Food Control 2007, 18, 702–706. [Google Scholar] [CrossRef]
- Aminian, A.; Aminian, B.; Nekooian, A.A.; Hoseinali, F. Effect of unripe grape juice (verjuice) on plasma lipid levels in rabbits rendered hypercholesterolemic by feeding egg yolk. Acta Med. Iran. 2006, 230–234. [Google Scholar]
- Shojaee-Aliabadi, S.; Hosseini, S.M.; Tiwari, B.; Hashemi, M.; Fadavi, G.; Khaksar, R. Polyphenols content and antioxidant activity of Ghure (unripe grape) marc extract: Influence of extraction time, temperature and solvent type. Int. J. Food Sci. Technol. 2013, 48, 412–418. [Google Scholar] [CrossRef]
- Karabiyikli, S.; Oncul, N. Persistence and survival of some food borne pathogens in neutralized unripe grape products. Ukr. Food J. 2016, 5, 96–108. [Google Scholar]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Faisal Manzoor, M.; Ali, M.; Muhammad Aadil, R.; Ali, A.; Goksen, G.; Li, J.; Zeng, X.A.; Proestos, C. Sustainable emerging sonication processing: Impact on fungicide reduction and the overall quality characteristics of tomato juice. Ultrason. Sonochem. 2023, 94, 106313. [Google Scholar] [CrossRef]
- Putnik, P.; Kresoja, Ž.; Bosiljkov, T.; Režek Jambrak, A.; Barba, F.J.; Lorenzo, J.M.; Roohinejad, S.; Granato, D.; Žuntar, I.; Bursać Kovačević, D. Comparing the effects of thermal and non-thermal technologies on pomegranate juice quality: A review. Food Chem. 2019, 279, 150–161. [Google Scholar] [CrossRef]
- Chavan, P.; Sharma, P.; Sharma, S.R.; Mittal, T.C.; Jaiswal, A.K. Application of High-Intensity Ultrasound to Improve Food Processing Efficiency: A Review. Foods 2022, 11, 122. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Velázquez-Estrada, R.M.; Roig, A.X.; García-Galindo, H.S.; Sayago-Ayerdi, S.G.; Montalvo-González, E. Thermosonication: An alternative processing for fruit and vegetable juices. Trends Food Sci. Technol. 2017, 61, 26–37. [Google Scholar] [CrossRef]
- Yıkmış, S.; Ozer, H.; Levent, O.; Çöl, B.G.; Erdal, B.; Yıkmış, S. Effect of thermosonication and thermal treatments on antidiabetic, antihypertensive, mineral elements and in vitro bioaccessibility of bioactive compounds in freshly squeezed pomegranate juice. J. Food Meas. Charact. 2022, 16, 3023–3041. [Google Scholar] [CrossRef]
- Xu, B.; Feng, M.; Chitrakar, B.; Cheng, J.; Wei, B.; Wang, B.; Zhou, C.; Ma, H. Multi-frequency power thermosonication treatments of clear strawberry juice: Impact on color, bioactive compounds, flavor volatiles, microbial and polyphenol oxidase inactivation. Innov. Food Sci. Emerg. Technol. 2023, 84, 103295. [Google Scholar] [CrossRef]
- Raju, S.; Deka, S.C. Influence of thermosonication treatments on bioactive compounds and sensory quality of fruit (Haematocarpus validus) juice. J. Food Process. Preserv. 2018, 42, e13701. [Google Scholar] [CrossRef]
- Abdulstar, A.R.; Altemimi, A.B.; Al-Hilphy, A.R. Exploring the Power of Thermosonication: A Comprehensive Review of Its Applications and Impact in the Food Industry. Foods 2023, 12, 1459. [Google Scholar] [CrossRef]
- Lafarga, T.; Ruiz-Aguirre, I.; Abadias, M.; Viñas, I.; Bobo, G.; Aguiló-Aguayo, I. Effect of Thermosonication on the Bioaccessibility of Antioxidant Compounds and the Microbiological, Physicochemical, and Nutritional Quality of an Anthocyanin-Enriched Tomato Juice. Food Bioprocess Technol. 2019, 12, 147–157. [Google Scholar] [CrossRef]
- Adiamo, O.Q.; Ghafoor, K.; Al-Juhaimi, F.; Mohamed Ahmed, I.A.; Babiker, E.E. Effects of thermosonication and orange by-products extracts on quality attributes of carrot (Daucus carota) juice during storage. Int. J. Food Sci. Technol. 2017, 52, 2115–2125. [Google Scholar] [CrossRef]
- Oliveira, G.A.R.; Guimarães, J.T.; Ramos, G.L.P.A.; Esmerino, E.A.; Pimentel, T.C.; Neto, R.P.C.; Tavares, M.I.B.; Sobral, L.A.; Souto, F.; Freitas, M.Q.; et al. Benefits of thermosonication in orange juice whey drink processing. Innov. Food Sci. Emerg. Technol. 2022, 75, 102876. [Google Scholar] [CrossRef]
- Bermúdez-Aguirre, D.; Barbosa-Cánovas, G.V. Inactivation of Saccharomyces cerevisiae in pineapple, grape and cranberry juices under pulsed and continuous thermo-sonication treatments. J. Food Eng. 2012, 108, 383–392. [Google Scholar] [CrossRef]
- Oladunjoye, A.O.; Adeboyejo, F.O.; Okekunbi, T.A.; Aderibigbe, O.R. Effect of thermosonication on quality attributes of hog plum (Spondias mombin L.) juice. Ultrason. Sonochem. 2021, 70, 105316. [Google Scholar] [CrossRef]
- Ramírez-Melo, L.M.; Cruz-Cansino, N.d.S.; Delgado-Olivares, L.; Ramírez-Moreno, E.; Zafra-Rojas, Q.Y.; Hernández-Traspeña, J.L.; Suárez-Jacobo, Á. Optimization of antioxidant activity properties of a thermosonicated beetroot (Beta vulgaris L.) juice and further in vitro bioaccessibility comparison with thermal treatments. LWT 2022, 154, 112780. [Google Scholar] [CrossRef]
- Abid, M.; Jabbar, S.; Hu, B.; Hashim, M.M.; Wu, T.; Lei, S.; Khan, M.A.; Zeng, X. Thermosonication as a potential quality enhancement technique of apple juice. Ultrason. Sonochem. 2014, 21, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Krishnan Kesavan, R.; Begum, S.; Das, P.; Nayak, P.K. Hurdle effect of thermosonication and non-thermal processing on the quality characteristics of fruit juices: An overview. J. Food Process Eng. 2023, 14310. [Google Scholar] [CrossRef]
- Rosenthal, A.; Maciel Guedes, A.M.; dos Santos, K.M.O.; Deliza, R. Healthy food innovation in sustainable food system 4.0: Integration of entrepreneurship, research, and education. Curr. Opin. Food Sci. 2021, 42, 215–223. [Google Scholar] [CrossRef]
- Walkling-Ribeiro, M.; Noci, F.; Riener, J.; Cronin, D.A.; Lyng, J.G.; Morgan, D.J. The impact of thermosonication and pulsed electric fields on Staphylococcus aureus inactivation and selected quality parameters in orange juice. Food Bioprocess Technol. 2009, 2, 422–430. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Salas, E.; Barouh, N.; Baréa, B.; Panya, A.; Figueroa-Espinoza, M.C. Antioxidant activity of protocatechuates evaluated by DPPH, ORAC, and CAT methods. Food Chem. 2016, 194, 749–757. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Esin Karademir, S.; Erçağ, E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef]
- Portu, J.; López, R.; Santamaría, P.; Garde-Cerdán, T. Elicitation with methyl jasmonate supported by precursor feeding with phenylalanine: Effect on Garnacha grape phenolic content. Food Chem. 2017, 237, 416–422. [Google Scholar] [CrossRef]
- Bïlgïn, Ö.; Çarli, U.; Erdoğan, S.; Emrah Maviş, M.; Göksu Gürsu, G.; Yilmaz, M. Karadeniz’de (Sinop Yarımadası Civarı) Avlanan İzmarit Balığı, Spicara smaris (Linnaeus, 1758), Etinin LC-MS/MS Kullanarak Amino Asit İçeriğinin Tespiti ve Ağırlık-Boy İlişkisi. Türk Tarım ve Doğa Bilim. Derg. 2019, 6, 130–136. [Google Scholar] [CrossRef]
- Boghossian, M.; Brassesco, M.E.; Miller, F.A.; Silva, C.L.M.; Brandão, T.R.S. Thermosonication Applied to Kiwi Peel: Impact on Nutritional and Microbiological Indicators. Foods 2023, 12, 622. [Google Scholar] [CrossRef]
- Basumatary, B.; Nayak, M.; Nayak, P.K.; Kesavan, R. krishnan Assessment of quality changes of tangor fruit juice after pasteurization and thermosonication treatments. J. Food Process Eng. 2022, 45, e14170. [Google Scholar] [CrossRef]
- Sobolev, A.P.; Mannina, L.; Proietti, N.; Carradori, S.; Daglia, M.; Giusti, A.M.; Antiochia, R.; Capitani, D. Untargeted NMR-Based Methodology in the Study of Fruit Metabolites. Molecules 2015, 20, 4088–4108. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ariza, J.L.; Villegas-Portero, M.J.; Bernal-Daza, V. Characterization and analysis of amino acids in orange juice by HPLC–MS/MS for authenticity assessment. Anal. Chim. Acta 2005, 540, 221–230. [Google Scholar] [CrossRef]
- Onuegbu, N.C.; Adedokun, I.I.; Kabuo, N.O.; Nwosu, J.N. Amino acid profile and micronutrient composition of the African pear (Dacryodes edulis) pulp. Pakistan J. Nutr. 2011, 10, 555–557. [Google Scholar] [CrossRef]
- Erdal, B.; Yıkmış, S.; Demirok, N.T.; Bozgeyik, E.; Levent, O. Effects of Non-Thermal Treatment on Gilaburu Vinegar (Viburnum opulus L.): Polyphenols, Amino Acid, Antimicrobial, and Anticancer Properties. Biology 2022, 11, 926. [Google Scholar] [CrossRef] [PubMed]
- Tokatlı Demirok, N. Sonication processing of mallow vinegar: Effects on the bioactive compounds, amino acids, organic acid, sugar, mineral and microstructure. Food Sci. Technol. 2022, 42. [Google Scholar] [CrossRef]
- Mohanty, B.; Mahanty, A.; Ganguly, S.; Sankar, T.V.; Chakraborty, K.; Rangasamy, A.; Paul, B.; Sarma, D.; Mathew, S.; Asha, K.K.; et al. Amino Acid Compositions of 27 Food Fishes and Their Importance in Clinical Nutrition. J. Amino Acids 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Hendriks, W.H. Amino Acid Availability in Heat-Damaged Ingredients. J. Anim. Sci. 2018, 96, 25. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145. [Google Scholar] [CrossRef]
- Ahmed, Z.; Manzoor, M.F.; Begum, N.; Khan, A.; Shah, I.; Farooq, U.; Siddique, R.; Zeng, X.A.; Rahaman, A.; Siddeeg, A. Thermo-Ultrasound-Based Sterilization Approach for the Quality Improvement of Wheat Plantlets Juice. Processes 2019, 7, 518. [Google Scholar] [CrossRef]
- Ampofo, J.O.; Ngadi, M. Ultrasonic assisted phenolic elicitation and antioxidant potential of common bean (Phaseolus vulgaris) sprouts. Ultrason. Sonochem. 2020, 64, 104974. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Mason, T.J.; Lelas, V.; Paniwnyk, L.; Herceg, Z. Effect of ultrasound treatment on particle size and molecular weight of whey proteins. J. Food Eng. 2014, 121, 15–23. [Google Scholar] [CrossRef]
- Kaya, Z.; Unluturk, S.; Martin-Belloso, O.; Soliva-Fortuny, R. Effectiveness of pulsed light treatments assisted by mild heat on Saccharomyces cerevisiae inactivation in verjuice and evaluation of its quality during storage. Innov. Food Sci. Emerg. Technol. 2020, 66, 102517. [Google Scholar] [CrossRef]
- Kaya, Z.; Unluturk, S. Pasteurization of verjuice by UV-C irradiation and mild heat treatment. Food Process Eng. 2019, 42, 13131. [Google Scholar] [CrossRef]
- Öncül, N.; Karabiyikli, Ş. Factors Affecting the Quality Attributes of Unripe Grape Functional Food Products. J. Food Biochem. 2015, 39, 689–695. [Google Scholar] [CrossRef]
- Karabiyikli, Ş.; Öncül, N. Inhibitory Effect of Unripe Grape Products on Foodborne Pathogens. J. Food Process. Preserv. 2016, 40, 1459–1465. [Google Scholar] [CrossRef]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; de Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef]
- de la Rosa, L.; Moreno-Escamilla, J.; Rodrigo-García, J.; Alvarez-Parrilla, E. Içinde Phenolic compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Woodhead Publishing: Sawston, UK, 2019; pp. 253–271. ISBN 9780128132791. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Sabir, A.; Kafkas, E.; Tangolar, S. Distribution of major sugars, acids, and total phenols in juice of five grapevine (Vitis spp.) cultivars at different stages of berry development. Spanish J. Agric. Res. 2010, 8, 425–433. [Google Scholar] [CrossRef]
- Pour Nikfardjam, M.S. General and polyphenolic composition of unripe grape juice (verjus/verjuice) from various producers. Mitteulingen Klosterneubg. 2008, 58, 28–31. [Google Scholar]
- Güler, A.; Aşiklar, F.B.; Özaltin, K.E.; Candemir, A. Koruk Suyu Üretiminde Prosesin Kaliteye Etkilerinin Belirlenmesi. Turkish J. Agric. Nat. Sci. 2022, 9, 535–546. [Google Scholar] [CrossRef]
- Najwa, R.; Azrina, A. Comparison of vitamin C content in citrus fruits by titration and high performance liquid chromatography (HPLC) methods. Int. Food Res. J. 2017, 24, 726. [Google Scholar]
- Rekha, C.; Poornima, G.; Manasa, M.; Abhipsa, V.; Devi, J.P.; Kumar, H.T.V.; Kekuda, T.R.P. Ascorbic Acid, Total Phenol Content and Antioxidant Activity of Fresh Juices of Four Ripe and Unripe Citrus Fruits. Chem. Sci. Trans. 2012, 1, 303–310. [Google Scholar] [CrossRef]
- Shakir, B.K.; Rashid, R.M.S. Physicochemical and phytochemical profile of unripe black grape juice (verjuice). Ann. Trop. Med. Public Heal. 2019, 22, 48–61. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.-A.; Han, Z.; Sun, D.-W. Effects of ultrasound treatments on quality of grapefruit juice. Food Chem. 2013, 141, 3201–3206. [Google Scholar] [CrossRef]
- Nayak, P.K.; Basumatary, B.; Chandrasekar, C.M.; Seth, D.; Kesavan, R.K. Impact of thermosonication and pasteurization on total phenolic contents, total flavonoid contents, antioxidant activity, and vitamin C levels of elephant apple (Dillenia indica) juice. J. Food Process Eng. 2020, 43, 13447. [Google Scholar] [CrossRef]
- Santhirasegaram, V.; Razali, Z.; Somasundram, C. Effects of thermal treatment and sonication on quality attributes of Chokanan mango (Mangifera indica L.) juice. Ultrason. Sonochem. 2013, 20, 1276–1282. [Google Scholar] [CrossRef]
- Abid, M.; Jabbar, S.; Wu, T.; Hashim, M.M.; Hu, B.; Lei, S.; Zeng, X. Sonication enhances polyphenolic compounds, sugars, carotenoids and mineral elements of apple juice. Ultrason. Sonochem. 2014, 21, 93–97. [Google Scholar] [CrossRef]
- Yıkmış, S. Sensory, physicochemical, microbiological and bioactive properties of red watermelon juice and yellow watermelon juice after ultrasound treatment. J. Food Meas. Charact. 2020, 14, 1417–1426. [Google Scholar] [CrossRef]
- Bhat, R.; Kamaruddin, N.; Min-Tze, L.; Karim, A. Sonication improves kasturi lime (Citrus microcarpa) juice quality. Ultrason. Sonochem. 2011, 18, 1295–1300. [Google Scholar] [CrossRef]
- Ergezer, H.; Gökçe, R.; Akcan, T. Koruk Sularının Bazı Kalite Karakteristikleri Üzerine Pastörizasyon ve Potasyum Sorbat İlavesinin Etkisi. Akad. Gıda 2018, 16, 287–292. [Google Scholar] [CrossRef]
- Cruz-Cansino, N.d.S.; Ramírez-Moreno, E.; León-Rivera, J.E.; Delgado-Olivares, L.; Alanís-García, E.; Ariza-Ortega, J.A.; Manríquez-Torres, J.d.J.; Jaramillo-Bustos, D.P. Shelf life, physicochemical, microbiological and antioxidant properties of purple cactus pear (Opuntia ficus indica) juice after thermoultrasound treatment. Ultrason. Sonochem. 2015, 27, 277–286. [Google Scholar] [CrossRef]
- Jabbar, S.; Abid, M.; Hu, B.; Hashim, M.M.; Lei, S.; Wu, T.; Zeng, X. Exploring the potential of thermosonication in carrot juice processing. J. Food Sci. Technol. 2015, 52, 7002–7013. [Google Scholar] [CrossRef]
- Dinçer, C.; Topuz, A. Inactivation of Escherichia coli and Quality Changes in Black Mulberry Juice Under Pulsed Sonication and Continuous Thermosonication Treatments. J. Food Process. Preserv. 2015, 39, 1744–1753. [Google Scholar] [CrossRef]
- Deli, M.G.E.P.; Kirit, B.D.; Ağçam, E.; Cinkir, N.I.; Akyildiz, A. Changes in cashew apple juice treated with optimum thermosonication during storage. Food Chem. Adv. 2022, 1, 100120. [Google Scholar] [CrossRef]
- Herceg, Z.; Lelas, V.; Jambrak, A.R.; Vukušić, T.; Levaj, B. Influence of thermo-sonication on microbiological safety, color and anthocyanins content of strawberry juice | Request PDF. J. Hyg. Eng. Des. 2013, 4, 26–37. [Google Scholar]
- Tomadoni, B.; Cassani, L.; Viacava, G.; Moreira, M.D.R.; Ponce, A. Effect of ultrasound and storage time on quality attributes of strawberry juice. J. Food Process Eng. 2017, 40, 12533. [Google Scholar] [CrossRef]
- Yıkmış, S.; Aksu, H.; Çöl, B.G.; Alpaslan, M. Thermosonication processing of quince (Cydonia Oblonga) juice: Effects on total phenolics, ascorbic acid, antioxidant capacity, color and sensory properties. Ciência e Agrotecnologia 2019, 43, 1–15. [Google Scholar] [CrossRef]


| Sample | Encoded Independent Variables | Dependent Variables | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temputure (X1) | Time (X2) | Amplitude (X3) | TPC (mg GAE/100 mL) | TFC (mg CE/100 mL) | DPPH (% inhibition) | CUPRAC (% inhibition) | |||||
| Experimental Data | RSM Predicted | Experimental Data | RSM Predicted | Experimental Data | RSM Predicted | Experimental Data | RSM Predicted | ||||
| 1 | 50 | 6 | 50 | 173.66 ± 2.67 | 172.44 | 81.57 ± 1.16 | 81.44 | 50.83 ± 0.86 | 50.89 | 54.65 ± 0.61 | 54.61 |
| 2 | 45 | 4 | 55 | 169.95 ± 2.14 | 170.26 | 79.29 ± 1.47 | 79.86 | 50.12 ± 0.76 | 50.21 | 53.13 ± 1.09 | 53.53 |
| 3 | 35 | 8 | 55 | 178.27 ± 1.58 | 177.57 | 100.88 ± 0.33 | 101.47 | 62.86 ± 0.23 | 63.16 | 67.59 ± 0.16 | 68.03 |
| 4 | 35 | 4 | 45 | 190.20 ± 2.80 | 189.45 | 99.87 ± 1.41 | 100.69 | 62.23 ± 0.92 | 62.66 | 66.91 ± 0.18 | 67.49 |
| 5 | 45 | 8 | 45 | 170.70 ± 1.05 | 171.05 | 88.91 ± 1.26 | 89.27 | 55.40 ± 1.03 | 55.55 | 59.57 ± 0.62 | 59.86 |
| 6 | 45 | 8 | 55 | 190.50 ± 0.40 | 190.96 | 99.81 ± 0.89 | 99.81 | 62.19 ± 0.48 | 62.20 | 66.88 ± 0.49 | 66.91 |
| 7 | 45 | 4 | 45 | 174.11 ± 2.64 | 174.52 | 91.86 ± 1.12 | 92.08 | 57.24 ± 0.76 | 57.38 | 61.55 ± 0.33 | 61.72 |
| 8 | 40 | 6 | 50 | 182.94 ± 0.71 | 184.83 | 96.80 ± 0.52 | 97.91 | 60.32 ± 0.64 | 61.04 | 64.86 ± 0.13 | 65.57 |
| 9 | 35 | 8 | 45 | 168.42 ± 1.24 | 167.82 | 84.99 ± 0.78 | 85.23 | 52.95 ± 0.18 | 53.31 | 56.94 ± 1.16 | 57.16 |
| 10 | 35 | 4 | 55 | 175.68 ± 0.81 | 175.03 | 93.72 ± 0.54 | 94.17 | 58.39 ± 0.58 | 58.69 | 62.79 ± 0.62 | 63.12 |
| 11 | 40 | 6 | 50 | 183.75 ± 0.75 | 184.83 | 98.69 ± 0.21 | 97.91 | 61.50 ± 0.07 | 61.04 | 66.12 ± 0.61 | 65.57 |
| 12 | 40 | 6 | 50 | 184.79 ± 0.51 | 184.83 | 98.62 ± 0.76 | 97.91 | 61.45 ± 0.95 | 61.04 | 66.08 ± 0.48 | 65.57 |
| 13 | 40 | 6 | 50 | 185.48 ± 1.67 | 184.83 | 98.35 ± 0.30 | 97.91 | 61.28 ± 0.23 | 61.04 | 65.90 ± 1.07 | 65.57 |
| 14 | 40 | 6 | 40 | 172.98 ± 1.15 | 172.89 | 93.58 ± 0.91 | 93.14 | 58.31 ± 0.49 | 58.01 | 62.70 ± 0.31 | 62.47 |
| 15 | 40 | 2 | 50 | 175.20 ± 1.06 | 175.13 | 90.01 ± 1.12 | 89.41 | 56.09 ± 0.55 | 55.86 | 60.31 ± 0.24 | 59.94 |
| 16 | 40 | 6 | 60 | 178.52 ± 1.09 | 178.37 | 97.55 ± 0.24 | 97.16 | 60.78 ± 1.24 | 60.69 | 65.36 ± 1.13 | 65.15 |
| 17 | 30 | 6 | 50 | 172.99 ± 0.27 | 173.98 | 92.38 ± 1.31 | 91.70 | 57.56 ± 0.62 | 57.12 | 61.89 ± 1.09 | 61.50 |
| 18 | 40 | 6 | 50 | 186.40 ± 0.75 | 184.83 | 98.14 ± 0.97 | 97.91 | 61.15 ± 0.72 | 61.04 | 65.26 ± 0.57 | 65.57 |
| 19 | 40 | 6 | 50 | 186.41 ± 0.40 | 184.83 | 97.69 ± 0.71 | 97.91 | 60.87 ± 0.45 | 61.04 | 65.48 ± 1.32 | 65.57 |
| 20 | 40 | 10 | 50 | 174.36 ± 1.16 | 174.19 | 94.12 ± 0.61 | 93.90 | 58.65 ± 0.30 | 58.50 | 63.06 ± 0.88 | 62.99 |
| TS-VJ | 41 | 10 | 60 | 190.44 ± 0.93 | 193.94 | 113.77 ± 0.91 | 115.99 | 70.77 ± 0.44 | 72.00 | 76.17 ± 1.34 | 77.81 |
| C-VJ | 172.44 ± 0.31 | 106.22 ± 0.52 | 63.25 ± 0.52 | 69.48 ± 0.49 | |||||||
| P-VJ | 164.33 ± 0.47 | 97.33 ± 0.47 | 62.11 ± 0.54 | 65.33 ± 0.3 | |||||||
| Source | DF | TPC (mg GAE/100 mL) | TFC (mg CE/100 mL) | DPPH (% Inhibition) | CUPRAC (% Inhibition) | ||||
|---|---|---|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | ||
| Model | 9 | 68.07 | 0.0000 | 142.6 | 0.0000 | 157.71 | 0.0000 | 138.58 | 0.0000 |
| Linear | 3 | 7.6 | 0.0060 | 84.12 | 0.0000 | 93.19 | 0.0000 | 82.4 | 0.0000 |
| X1 | 1 | 1.52 | 0.2460 | 187.82 | 0.0000 | 205.22 | 0.0000 | 183.98 | 0.0000 |
| X2 | 1 | 0.59 | 0.4590 | 36.13 | 0.0000 | 36.65 | 0.0000 | 35.39 | 0.0000 |
| X3 | 1 | 20.7 | 0.0010 | 28.41 | 0.0000 | 37.68 | 0.0000 | 27.83 | 0.0000 |
| Square | 3 | 81.27 | 0.0000 | 133.86 | 0.0000 | 153.13 | 0.0000 | 127.84 | 0.0000 |
| X12 | 1 | 144.5 | 0.0000 | 358.51 | 0.0000 | 410.59 | 0.0000 | 343.32 | 0.0000 |
| X22 | 1 | 110.64 | 0.0000 | 109.01 | 0.0000 | 123.91 | 0.0000 | 102.47 | 0.0000 |
| X32 | 1 | 90.56 | 0.0000 | 21.24 | 0.0010 | 23.64 | 0.0010 | 18.93 | 0.0010 |
| 2-Way Interaction | 3 | 115.33 | 0.0000 | 209.81 | 0.0000 | 226.82 | 0.0000 | 205.51 | 0.0000 |
| X1 * X2 | 1 | 112.22 | 0.0000 | 141.63 | 0.0000 | 149.25 | 0.0000 | 138.72 | 0.0000 |
| X1 * X3 | 1 | 35.11 | 0.0000 | 28.82 | 0.0000 | 26.98 | 0.0000 | 28.23 | 0.0000 |
| X2 * X3 | 1 | 198.65 | 0.0000 | 458.98 | 0.0000 | 504.22 | 0.0000 | 449.58 | 0.0000 |
| Error | 10 | ||||||||
| Lack-of-Fit | 5 | 0.47 | 0.7860 | 1.23 | 0.4130 | 0.93 | 0.5310 | 1.03 | 0.4870 |
| Pure Error | 5 | ||||||||
| Total | 19 | ||||||||
| R2 | 98.39% | 99.23% | 99.30% | 99.20% | |||||
| Adj R2 | 96.95% | 98.53% | 98.67% | 98.49% | |||||
| Pred. R2 | 94.15% | 96.19% | 96.78% | 96.27% | |||||
| Analyses | Samples | |||
|---|---|---|---|---|
| C-VJ | P-VJ | TS-VJ | ||
| Amino acid content (mg/100 mL) | Alanine | 1.11 ± 0.00 a | 0.46 ± 0.00 b | 1.10 ± 0.00 a |
| Arginine | 1.98 ± 0.00 b | 1.04 ± 0.01 c | 2.11 ± 0.01 a | |
| Aspartic Acid | 1.20 ± 0.00 a | 0.76 ± 0.01 c | 0.92 ± 0.00 b | |
| Cystine | n.d | n.d | n.d | |
| Glutamic Acid | 1.10 ± 0.00 a | 0.66 ± 0.00 c | 0.93 ± 0.00 b | |
| Glycine | n.d | n.d | n.d | |
| Histidine | 0.57 ± 0.00 b | 0.32 ± 0.00 c | 0.60 ± 0.01 a | |
| Isoleucine | 0.33 ± 0.00 a | 0.25 ± 0.00 c | 0.30 ± 0.01 b | |
| Leucine | 0.81 ± 0.00 a | 0.52 ± 0.03 b | 0.79 ± 0.01 a | |
| Lysine | 0.35 ± 0.00 b | 0.13 ± 0.00 c | 0.39 ± 0.00 a | |
| Methionine | 0.06 ± 0.00 a | 0.05 ± 0.00 a | 0.06 ± 0.00 a | |
| Ornitine | 0.31 ± 0.00 b | 0.25 ± 0.00 c | 0.39 ± 0.01 a | |
| Phenylalanine | 0.54 ± 0.00 a | 0.37 ± 0.00 b | 0.54 ± 0.00 a | |
| Proline | 0.38 ± 0.01 a | 0.13 ± 0.00 b | 0.39 ± 0.00 a | |
| Serine | 0.79 ± 0.00 b | 0.50 ± 0.00 c | 1.04 ± 0.00 a | |
| Threonine | 0.60 ± 0.01 a | 0.13 ± 0.00 c | 0.36 ± 0.00 b | |
| Tyrosine | 0.29 ± 0.00 a | 0.16 ± 0.00 c | 0.28 ± 0.00 b | |
| Valine | 0.49 ± 0.00 a | 0.18 ± 0.00 c | 0.48 ± 0.00 b | |
| Taurine | n.d | n.d | n.d | |
| Compounds | Formula | Samples (μg/mL) | ||
|---|---|---|---|---|
| C-VJ | P-VJ | TS-VJ | ||
| Ascorbic acid |  | 10.14 ± 0.16 a | 10.24 ± 0.31 a | 10.66 ± 0.97 a |
| Gallic acid |  | 11.38 ± 0.41 a | 11.64 ± 0.44 a | 13.25 ± 1.42 a |
| Protocatechuic acid |  | 10.03 ± 0.30 a | 8.99 ± 0.64 a | 8.20 ± 0.81 a |
| Catechin |  | 39.14 ± 1.55 a | 35.42 ± 1.76 a | 55.54 ± 3.27 b |
| Hydroxybenzoic acid |  | 18.66 ± 0.62 a | 18.28 ± 1.03 a | 24.73 ± 1.32 b |
| Vanillic acid |  | 0.57 ± 0.11 a | 0.47 ± 0.04 a | 0.47 ± 0.05 a |
| Ferulic acid |  | n.d | n.d | 0.06 ± 0.01 |
| Naringin |  | n.d | n.d | 1.32 ± 0.26 |
| o-coumaric acid |  | n.d | 0.09 ± 0.01 | n.d |
| Neohesperidin |  | 0.05 ± 0.03 a | n.d | 0.08 ± 0.02 b |
| Coumarin |  | 0.09 ± 0.00 a | 0.10 ± 0.02 a | 0.05 ± 0.02 a |
| Quercetin |  | n.d | n.d | 1.11 ± 0.17 |
| Trans-cinnamic acid |  | n.d | 0.17 ± 0.03 | n.d |
| Analyzes | Samples | |||
|---|---|---|---|---|
| C-VJ | P-VJ | TS-VJ | ||
| Physicochemical parameters | pH | 2.74 ± 0.01 a | 2.71 ± 0.01 b | 2.74 ± 0.01 a |
| TSS (°Brix) | 3.07 ± 0.03 a | 2.43 ± 1.16 a | 3.08 ± 0.03 a | |
| TA (g TA/L) | 3.83 ± 0.03 a | 3.80 ± 0.00 a | 3.83 ± 0.03 a | |
| Color properties | L | 49.33 ± 1.27 a | 47.39 ± 1.92 a | 46.06 ± 0.49 a |
| a | 4.44 ± 0.29 a | 3.94 ± 0.30 a | 3.96 ± 0.04 a | |
| b | 15.89 ± 0.15 a | 15.37 ± 0.10 b | 15.56 ± 0.06 b | |
| Chroma (C) | 16.50 ± 0.09 a | 15.87 ± 0.04 b | 16.05 ± 0.07 c | |
| Hue angle (h°) | 74.40 ± 1.08 a | 75.62 ± 1.10 a | 75.71 ± 0.10 a | |
| BI | 44.83 ± 1.45 a | 44.67 ± 2.26 a | 46.79 ± 0.42 a | |
| ΔE | - | 2.14 ± 0.69 | 3.34 ± 1.09 | |
| Microbiological analyses (log CFU/mL) | TMAB | n.d | n.d | n.d |
| YM | n.d | n.d | n.d | |
| CB | n.d | n.d | n.d | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çöl, B.G.; Akhan, M.; Sancar, B.Ç.; Türkol, M.; Yıkmış, S.; Hecer, C. Effect of Thermosonication on Amino Acids, Phenolic Compounds, Sensory Properties and Microbial Quality in Freshly Squeezed Verjuice. Foods 2023, 12, 2167. https://doi.org/10.3390/foods12112167
Çöl BG, Akhan M, Sancar BÇ, Türkol M, Yıkmış S, Hecer C. Effect of Thermosonication on Amino Acids, Phenolic Compounds, Sensory Properties and Microbial Quality in Freshly Squeezed Verjuice. Foods. 2023; 12(11):2167. https://doi.org/10.3390/foods12112167
Chicago/Turabian StyleÇöl, Başak Gökçe, Meryem Akhan, Burcu Çakmak Sancar, Melikenur Türkol, Seydi Yıkmış, and Canan Hecer. 2023. "Effect of Thermosonication on Amino Acids, Phenolic Compounds, Sensory Properties and Microbial Quality in Freshly Squeezed Verjuice" Foods 12, no. 11: 2167. https://doi.org/10.3390/foods12112167
APA StyleÇöl, B. G., Akhan, M., Sancar, B. Ç., Türkol, M., Yıkmış, S., & Hecer, C. (2023). Effect of Thermosonication on Amino Acids, Phenolic Compounds, Sensory Properties and Microbial Quality in Freshly Squeezed Verjuice. Foods, 12(11), 2167. https://doi.org/10.3390/foods12112167