Dissipation Kinetics and Risk Assessment of Diniconazole, Dinotefuran, Metconazole, and Tebuconazole in Raphanus sativus L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Standards
2.2. Greenhouse Experiments
2.3. Standard Solution
2.4. Method Validation
2.5. LC-MS/MS Analytical Conditions
2.6. Storage Stability and Daily Residue Levels
2.7. Statistical Analysis
2.8. Dietary Risk Assessment
3. Results and Discussion
3.1. Method Validation
3.2. Daily Residue Levels
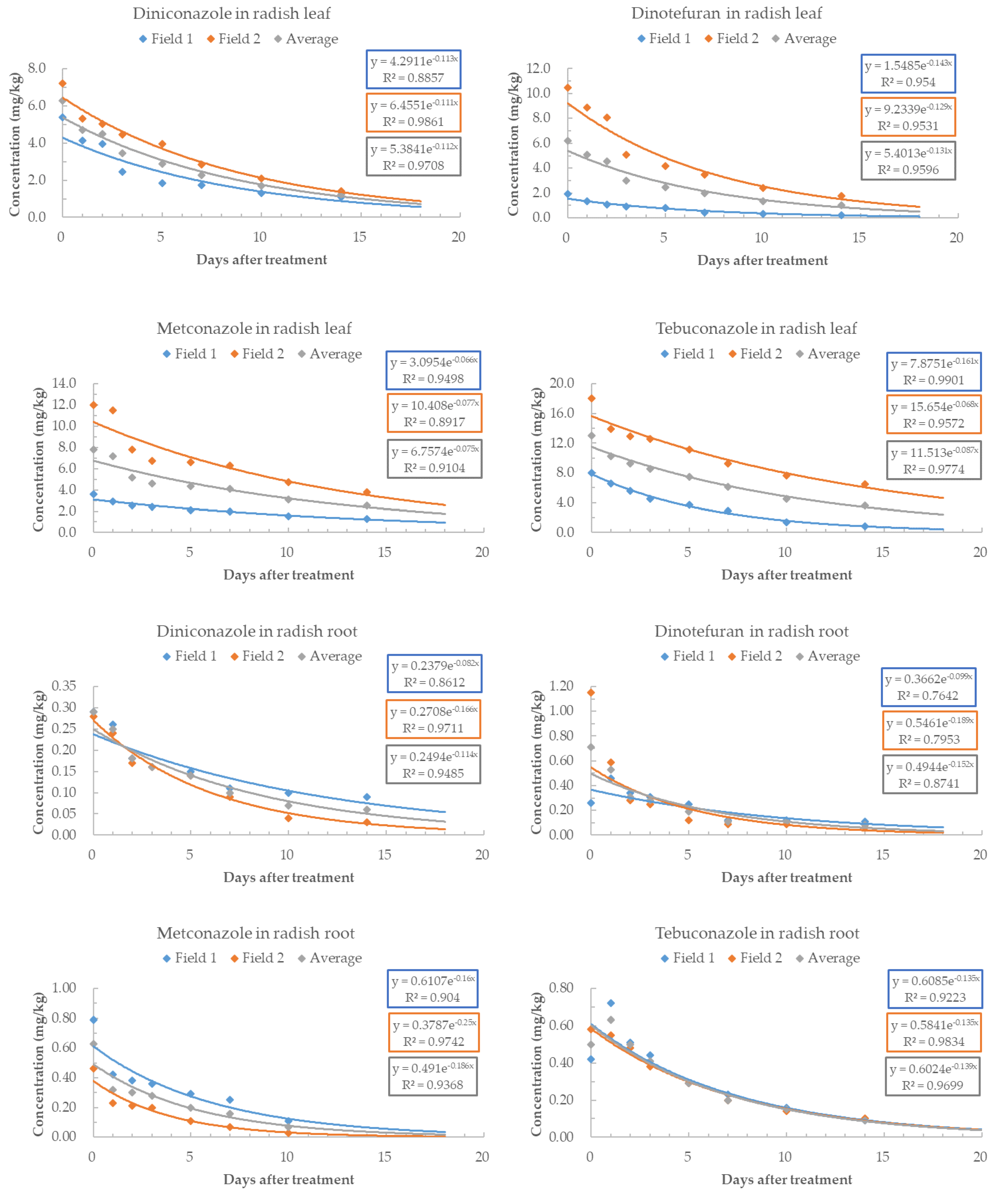
3.3. Residual Characteristics of Pesticides in Radish Leaf and Root
3.4. Calculation of Maximum Residue Limits during the Harvest Stage of Radish
3.5. Dietary Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Krol, W.J.; Arsenault, T.L.; Pylypiw, H.M.; Incorvia Mattina, M.J. Reduction of Pesticide Residues on Produce by Rinsing. J. Agric. Food Chem. 2000, 48, 4666–4670. [Google Scholar] [CrossRef] [PubMed]
- Fenoll, J.; Hellín, P.; Camacho, M.d.M.; López, J.; González, A.; Lacasa, A.; Flores, P. Dissipation Rates of Procymidone and Azoxystrobin in Greenhouse Grown Lettuce and under Cold Storage Conditions. Int. J. Environ. Anal. Chem. 2008, 88, 737–746. [Google Scholar] [CrossRef]
- Torres, C.M.; Picó, Y.; Mañes, J. Determination of Pesticide Residues in Fruit and Vegetables. J. Chromatogr. A 1996, 754, 301–331. [Google Scholar] [CrossRef]
- Kim, Y.E.; Yoon, J.H.; Lim, D.J.; Kim, S.W.; Cho, H.; Shin, B.G.; Kim, H.Y.; Kim, I.S. Plant Back Interval of Fluopyram Based on Primary Crop-Derived Soil and Bare Soil Residues for Rotational Cultivation of Radish. Korean J. Environ. Agric. 2021, 40, 99–107. [Google Scholar] [CrossRef]
- Kim, J.Y.; Woo, M.J.; Hur, K.J.; Manoharan, S.; Kwon, C.-H.; Hur, J.H. Establishment of Pre-Harvest Residue Limit for Pyrimethanil and Methoxyfenozide during Cultivation of Grape. Korean J. Pestic. Sci. 2015, 19, 81–87. [Google Scholar] [CrossRef]
- Gamba, M.; Asllanaj, E.; Raguindin, P.F.; Glisic, M.; Franco, O.H.; Minder, B.; Bussler, W.; Metzger, B.; Kern, H.; Muka, T. Nutritional and Phytochemical Characterization of Radish (Raphanus Sativus): A Systematic Review. Trends Food Sci. Technol. 2021, 113, 205–218. [Google Scholar] [CrossRef]
- Wakita, T.; Kinoshita, K.; Yamada, E.; Yasui, N.; Kawahara, N.; Naoi, A.; Nakaya, M.; Ebihara, K.; Matsuno, H.; Kodaka, K. The Discovery of Dinotefuran: A Novel Neonicotinoid. Pest Manag. Sci. 2003, 59, 1016–1022. [Google Scholar] [CrossRef]
- Watanabe, E.; Baba, K.; Miyake, S. Analytical Evaluation of Enzyme-Linked Immunosorbent Assay for Neonicotinoid Dinotefuran for Potential Application to Quick and Simple Screening Method in Rice Samples. Talanta 2011, 84, 1107–1111. [Google Scholar] [CrossRef]
- Price, C.L.; Parker, J.E.; Warrilow, A.G.; Kelly, D.E.; Kelly, S.L. Azole Fungicides—Understanding Resistance Mechanisms in Agricultural Fungal Pathogens. Pest Manag. Sci. 2015, 71, 1054–1058. [Google Scholar] [CrossRef]
- MFDS Pesticides and Veterinary Drugs Information. Available online: https://www.foodsafetykorea.go.kr/residue/main.do (accessed on 30 September 2022).
- EU Pesticides Database European Commission. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/mrls (accessed on 30 April 2023).
- Guo, W.; Chen, Y.; Jiao, H.; Hu, D.; Lu, P. Dissipation, Residues Analysis and Risk Assessment of Metconazole in Grapes under Field Conditions Using Gas Chromatography–Tandem Mass Spectrometry. Qual. Assur. Saf. Crops Foods 2021, 13, 84–97. [Google Scholar] [CrossRef]
- Farha, W.; Abd El-Aty, A.M.; Rahman, M.M.; Kabir, M.H.; Chung, H.S.; Lee, H.S.; Jeon, J.-S.; Wang, J.; Chang, B.-J.; Shin, H.-C.; et al. Dynamic Residual Pattern of Azoxystrobin in Swiss Chard with Contribution to Safety Evaluation. Biomed. Chromatogr. 2018, 32, e4092. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Chai, Y.; Liu, R.; Yuan, L. Dissipation, Residue, and Dietary Risk Assessment of Bifenthrin, Bifenazate, and Its Metabolite Bifenazate–Diazene in Apples Based on Deterministic and Probabilistic Methods. J. Agric. Food Chem. 2021, 69, 14302–14310. [Google Scholar] [CrossRef]
- Chai, Y.; Liu, R.; Du, X.; Yuan, L. Dissipation and Residue of Metalaxyl-M and Azoxystrobin in Scallions and Cumulative Risk Assessment of Dietary Exposure to Hepatotoxicity. Molecules 2022, 27, 5822. [Google Scholar] [CrossRef] [PubMed]
- NIFDS Guidelines for Calculating Food Intake and Standardizing Contamination Monitoring. Available online: https://www.mfds.go.kr/brd/b_1060/view.do?seq=14347&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=54 (accessed on 15 May 2019).
- de Castro, V.L.S.S.; Maia, A.H. Prenatal Epoxiconazole Exposure Effects on Rat Postnatal Development. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2012, 95, 123–129. [Google Scholar] [CrossRef]
- Nash, R.G. Comparative Volatilization and Dissipation Rates of Several Pesticides from Soil. J. Agric. Food Chem. 1983, 31, 210–217. [Google Scholar] [CrossRef]
- Zongmao, C.; Haibin, W. Factors Affecting Residues of Pesticides in Tea. Pestic. Sci. 1988, 23, 109–118. [Google Scholar] [CrossRef]
- Stenersen, J. Chemical Pesticides Mode of Action and Toxicology; CRC Press: Boca Raton, FL, USA, 2004; ISBN 9781135731809. [Google Scholar]
- Fantke, P.; Gillespie, B.W.; Juraske, R.; Jolliet, O. Estimating Half-Lives for Pesticide Dissipation from Plants. Environ. Sci. Technol. 2014, 48, 8588–8602. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhao, Y.; Cui, X.; Wang, X.; Shen, H.; Chen, Z.; Huang, C.; Meruva, N.; Zhou, L.; Wang, F.; et al. Application and Enantiomeric Residue Determination of Diniconazole in Tea and Grape and Apple by Supercritical Fluid Chromatography Coupled with Quadrupole-Time-of-Flight Mass Spectrometry. J. Chromatogr. A 2018, 1581–1582, 144–155. [Google Scholar] [CrossRef]
- Ford, K.A.; Casida, J.E. Comparative Metabolism and Pharmacokinetics of Seven Neonicotinoid Insecticides in Spinach. J. Agric. Food Chem. 2008, 56, 10168–10175. [Google Scholar] [CrossRef]
- Bai, A.; Chen, A.; Chen, W.; Liu, S.; Luo, X.; Liu, Y.; Zhang, D. Residue Behavior, Transfer and Risk Assessment of Tolfenpyrad, Dinotefuran and Its Metabolites during Tea Growing and Tea Brewing. J. Sci. Food Agric. 2021, 101, 5992–6000. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kwak, S.-Y.; Hwang, J.-I.; Kim, H.-J.; Kim, T.-H.; Kim, J.-E. Correlation between Physicochemical Properties and Biological Half-Life of Triazole Fungicides in Perilla Leaf. J. Appl. Biol. Chem. 2019, 62, 407–415. [Google Scholar] [CrossRef]
- Zhao, J.; Tan, Z.; Wen, Y.; Fan, S.; Gao, Y.; Liu, C. Residues, Dissipation and Risk Assessment of Triazole Fungicide Tebuconazole in Green Onion (Allium Fistulosum L.). Int. J. Environ. Anal. Chem. 2022, 102, 3833–3840. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Gao, J.; Liu, C.; Cui, L.; Li, A. Dissipation, Residues, and Safety Evaluation of Trifloxystrobin and Tebuconazole on Ginseng and Soil. Environ. Monit. Assess. 2015, 187, 344. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Takkar, R.; Bhardwaj, U.; Kumar, R.; Battu, R.S.; Singh, B. Dissipation Kinetics of Trifloxystrobin and Tebuconazole on Wheat Leaves and Their Harvest Time Residues in Wheat Grains and Soil. Bull. Environ. Contam. Toxicol. 2012, 89, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, O.I.; Alrasheed, A.M.; Al-Mundarij, A.A.; Omar, A.F.; Alhewairini, S.S.; Al-Jamhan, K.A. Levels of Residues and Dietary Risk Assessment of the Fungicides Myclobutanil, Penconazole, Tebuconazole, and Triadimenol in Squash. Biomed. Chromatogr. 2021, 35, e5126. [Google Scholar] [CrossRef] [PubMed]
- Yogendraiah Matadha, N.; Mohapatra, S.; Siddamallaiah, L.; Udupi, V.R.; Gadigeppa, S.; Raja, D.P.; Donagar, S.P.; Hebbar, S.S. Persistence and Dissipation of Fluopyram and Tebuconazole on Bell Pepper and Soil under Different Environmental Conditions. Int. J. Environ. Anal. Chem. 2021, 101, 2408–2427. [Google Scholar] [CrossRef]
- An, D.; Ko, R.; Kim, J.; Lee, K.; Lee, J.-H. Fungicide Dissipation Kinetics and Dietary Exposure Risk Assessment in Squash Fruit and Leaf. Foods 2023, 12, 1291. [Google Scholar] [CrossRef]
- KHIDI National Nutrition Statistics. Available online: https://www.khidi.or.kr/kps/dhraStat/result1?MenuId=MENU01652&year=2020 (accessed on 28 February 2021).
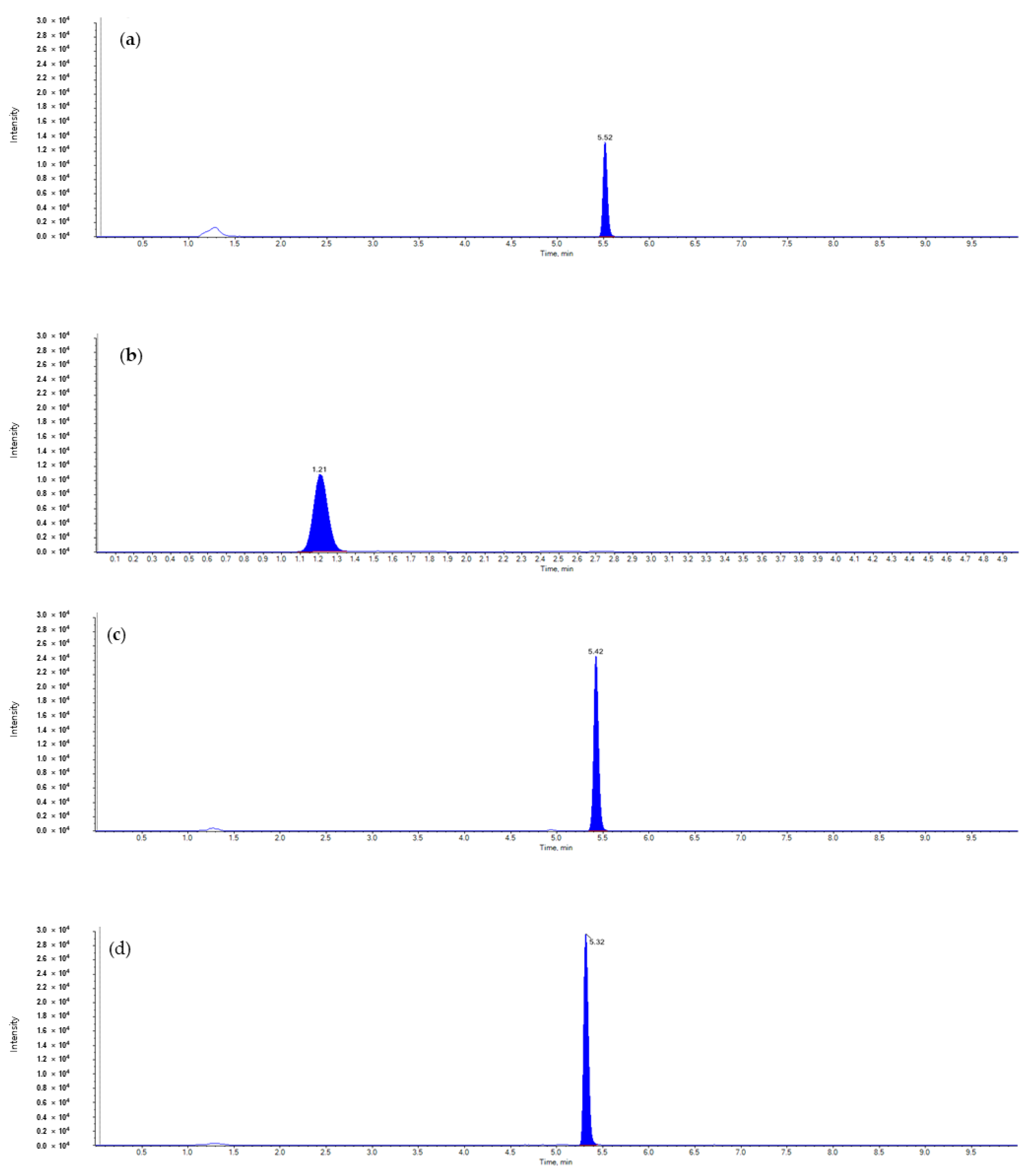
| Pesticide | Formulation | Application | PHI (Days) | ||||
|---|---|---|---|---|---|---|---|
| Type | AI | Dilution Rate | Spray No. | Interval (Days) | TSA | ||
| Diniconazole | WP | 5 | 2000 | 3 | 7 | 0.2475 | 7 |
| Dinotefuran | WP | 10 | 1000 | 3 | 7 | 0.99 | 14 |
| Metconazole | SC | 20 | 3000 | 1 | 7 | 0.22 | 14 |
| Tebuconazole | SC | 20 | 3000 | 2 | 7 | 0.44 | 7 |
| Analytes | Ionization | Precursor Ion (m/z) | Product Ion (m/z) | Retention Time (min) | |
|---|---|---|---|---|---|
| Quantitation (Collision Energy, eV) | Qualification (Collision Energy, eV) | ||||
| Diniconazole | [M + H]+ | 326.1 | 70.2 (42) | 159.1 (40) | 5.5 |
| Dinotefuran | [M + H]+ | 203.1 | 129.2 (17) | 114.2 (17) | 1.2 |
| Metconazole | [M + H]+ | 320.0 | 70.0 (58) | 125.0 (56) | 5.4 |
| Tebuconazole | [M + H]+ | 308.0 | 70.0 (51) | 125.0 (49) | 5.3 |
| Pesticide | Linear Range (mg/L) | Radish Leaf | Radish Root | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Field 1 | Field 2 | Field 1 | Field 2 | ||||||
| Linear Equation | R2 | Linear Equation | R2 | Linear Equation | R2 | Linear Equation | R2 | ||
| DNC | 0.005–0.25 | y = 240376.5527x + 356.7611 | 0.9903 | y = 242721.8089x − 83.5911 | 0.9908 | y = 248804.1197x + 321.0432 | 0.9969 | y = 244659.5860x + 591.6900 | 0.9950 |
| DNT | y = 285107.3727x + 1330.1962 | 1.0000 | y = 261336.3591x + 10356.9996 | 0.9993 | y = 254921.7192x + 1574.7351 | 0.9988 | y = 227456.5412x + 1764.5123 | 0.9966 | |
| MTC | y = 919210.5027x + 1209.6831 | 0.9997 | y = 916146.4301x + 637.5441 | 0.9999 | y = 837243.8481x + 871.9971 | 0.9997 | y = 828347.2924x + 818.4145 | 0.9999 | |
| TBC | y = 1007501x + 1291.8580 | 0.9999 | y = 1007846.6112x + 1287.1053 | 0.9997 | y = 962329.3438x + 775.5557 | 0.9999 | y = 1012285.6426x − 468.7282 | 0.9996 | |
| Pesticide | Spiking Level (mg/kg) | Recovery (%) | MLOQ (mg/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Radish Leaf | Radish Root | ||||||||||
| Field 1 | Field 2 | Field 1 | Field 2 | ||||||||
| Average ± SD | CV | Average ± SD | CV | Average ± SD | CV | Average ± SD | CV | ||||
| DNC | Recovery | 0.01 | 81.6 ± 2.8 | 3.5 | 87.3 ± 2.9 | 3.4 | 84.7 ± 2.5 | 2.9 | 73.5 ± 1.8 | 2.4 | 0.01 |
| 0.10 | 87.5 ± 2.5 | 2.9 | 80.7 ± 1.9 | 2.4 | 96.3 ±. 1.3 | 1.4 | 84.3 ± 1.7 | 2.1 | |||
| Highest | 87.9 ± 2.8 (2) | 3.2 | 85.1 ± 4.0 (2) | 4.8 | 106.7 ± 2.1 (1) | 2.0 | 84.3 ± 1.7 (1) | 7.3 | |||
| Storage | 0.10 | 87.5 ± 1.2 | 1.4 | 81.1 ± 3.1 | 3.8 | 97.7 ± 1.4 | 1.4 | 85.8 ± 2.4 | 2.8 | ||
| DNT | Recovery | 0.01 | 86.0 ± 11.8 | 13.8 | 83.2 ± 9.7 | 11.7 | 88.0 ± 12.5 | 14.2 | 83.7 ± 2.8 | 3.4 | 0.01 |
| 0.10 | 103.7 ± 1.4 | 1.3 | 93.6 ± 1.5 | 1.6 | 114.0 ±. 4.4 | 3.8 | 115.8 ± 4.0 | 3.7 | |||
| Highest | 99.0 ± 7.7 (1) | 7.8 | 107.7 ± 4.0 (1) | 3.7 | 87.9 ± 1.9 (1) | 2.2 | 108.7 ± 4.0 (1) | 7.3 | |||
| Storage | 0.10 | 102.8 ± 4.6 | 1.4 | 99.2 ± 5.9 | 5.9 | 109.1 ± 2.9 | 2.7 | 100.5 ± 1.5 | 1.5 | ||
| MTC | Recovery | 0.01 | 93.4 ± 1.1 | 1.2 | 91.3 ± 2.1 | 1.2 | 98.5 ± 1.9 | 2.9 | 97.4 ± 2.0 | 2.1 | 0.01 |
| 0.10 | 98.7 ± 0.8 | 0.8 | 101.7 ± 0.9 | 0.8 | 101.6 ± 1.3 | 1.4 | 106.0 ± 3.6 | 3.4 | |||
| Highest | 100.8 ± 6.9 (1) | 6.9 | 112.9 ± 5.7 (1) | 6.9 | 97.1 ± 2.1 (1) | 2.0 | 96.4 ± 12.3 (1) | 12.8 | |||
| Storage | 0.10 | 87.5 ± 1.2 | 2.4 | 101.7 ± 0.8 | 0.8 | 100.1 ± 1.1 | 1.1 | 104.2 ± 3.3 | 3.2 | ||
| TBC | Recovery | 0.01 | 87.5 ± 6.4 | 7.3 | 88.6 ± 8.3 | 9.3 | 95.9 ± 2.4 | 2.5 | 106.3 ± 2.8 | 2.6 | 0.01 |
| 0.10 | 106.3 ± 2.5 | 2.4 | 107.2 ± 2.3 | 2.1 | 107.2 ± 1.5 | 14 | 101.4 ± 2.3 | 2.3 | |||
| Highest | 100.2 ± 5.8 (2) | 5.8 | 105.0 ± 6.3 (2) | 6.0 | 106.4 ± 8.4 (1) | 7.9 | 102.7 ± 11.0 (1) | 10.7 | |||
| Storage | 0.10 | 104.0 ± 2.1 | 2.1 | 104.7 ± 1.6 | 1.6 | 109.4 ± 1.2 | 1.1 | 102.5 ± 1.6 | 1.5 | ||
| Pesticide | Harvest Time (after Spraying the Pesticides) | Radish Leaf | Radish Root | KOR MRL of Radish (mg/kg) | EU MRL of Radish (mg/kg) | ||
|---|---|---|---|---|---|---|---|
| Field 1 | Field 2 | Field 1 | Field 2 | ||||
| DNC | 0 | 5.39 ± 0.52 | 7.21 ± 0.61 | 0.29 ± 0.02 | 0.28 ± 0.01 | 2.0 (leaf) 0.05 (root) | - |
| 1 | 4.14 ± 0.09 | 5.31 ± 0.18 | 0.26 ± 0.02 | 0.24 ± 0.02 | |||
| 2 | 3.97 ± 0.10 | 5.03 ± 0.06 | 0.18 ± 0.01 | 0.17 ± 0.00 | |||
| 3 | 2.45 ± 0.08 | 4.47 ± 0.18 | 0.16 ± 0.00 | 0.16 ± 0.01 | |||
| 5 | 1.84 ± 0.28 | 3.96 ± 0.56 | 0.15 ± 0.00 | 0.14 ± 0.01 | |||
| 7 | 1.72 ± 0.26 | 2.85 ± 0.43 | 0.11 ± 0.02 | 0.09 ± 0.01 | |||
| 10 | 1.31 ± 0.13 | 2.08 ± 0.19 | 0.10 ± 0.00 | 0.04 ± 0.00 | |||
| 14 | 1.09 ± 0.07 | 1.40 ± 0.10 | 0.09 ± 0,00 | 0.03 ± 0.00 | |||
| DNT | 0 | 1.94 ± 0.01 | 10.48 ± 0.39 | 0.26 ± 0.01 | 1.15 ± 0.06 | 1.5 (leaf) 0.05 (root) | - |
| 1 | 1.34 ± 0.14 | 8.85 ± 0.28 | 0.46 ± 0.02 | 0.59 ± 0.03 | |||
| 2 | 1.07 ± 0.04 | 8.05 ± 0.69 | 0.34 ± 0.04 | 0.28 ± 0.02 | |||
| 3 | 0.94 ± 0.04 | 5.07 ± 0.23 | 0.31 ± 0.03 | 0.25 ± 0.04 | |||
| 5 | 0.81 ± 0.05 | 4.16 ± 0.17 | 0.25 ± 0.02 | 0.12 ± 0.02 | |||
| 7 | 0.45 ± 0.03 | 3.50 ± 0.33 | 0.12 ± 0.02 | 0.09 ± 0.01 | |||
| 10 | 0.34 ± 0.04 | 2.40 ± 0.16 | 0.12 ± 0.00 | 0.09 ± 0.01 | |||
| 14 | 0.25 ± 0.04 | 1.75 ± 0.32 | 0.11 ± 0,01 | 0.06 ± 0.01 | |||
| MTC | 0 | 3.58 ± 0.47 | 11.98 ±1.59 | 0.79 ± 0.11 | 0.46 ± 0.01 | 20.0 (leaf) 0.05 (root) | - |
| 1 | 2.89 ± 0.13 | 11.47 ± 1.77 | 0.42 ± 0.04 | 0.23 ± 0.01 | |||
| 2 | 2.56 ± 0.15 | 7.80 ± 13.41 | 0.38 ± 0.01 | 0.21 ± 0.00 | |||
| 3 | 2.40 ± 0.03 | 6.76 ± 2.10 | 0.36 ± 0.02 | 0.20 ± 0.01 | |||
| 5 | 2.08 ± 0.09 | 6.61 ±0.65 | 0.29 ± 0.02 | 0.11 ± 0.00 | |||
| 7 | 1.96 ± 0.06 | 6.27 ±0.05 | 0.25 ± 0.01 | 0.07 ± 0.01 | |||
| 10 | 1.54 ± 0.05 | 4.70 ±11.59 | 0.11 ± 0.00 | 0.03 ± 0.00 | |||
| 14 | 1.31 ± 0.25 | 3.78 ± 6.28 | <0.01 | <0.01 | |||
| TBC | 0 | 8.04 ± 0.11 | 18.03 ± 0.70 | 0.42 ± 0.02 | 0.58 ± 0.05 | 5.0 (leaf) 0.2 (root) | - |
| 1 | 6.56 ± 0.51 | 13.93 ± 1.12 | 0.72 ± 0.11 | 0.55 ± 0.01 | |||
| 2 | 5.55 ± 0.40 | 12.93 ± 0.08 | 0.51 ± 0.00 | 0.48 ± 0.03 | |||
| 3 | 4.52 ± 0.21 | 12.93 ± 0.08 | 0.44 ± 0.04 | 0.38 ± 0.05 | |||
| 5 | 3.74 ± 0.32 | 11.15 ± 1.71 | 0.29 ± 0.01 | 0.29 ± 0.03 | |||
| 7 | 2.95 ± 0.37 | 9.27 ± 0.58 | 0.23 ± 0.01 | 0.20 ± 0.03 | |||
| 10 | 1.42 ± 0.19 | 7.69 ± 0.65 | 0.16 ± 0.03 | 0.14 ± 0.01 | |||
| 14 | 0.83 ± 0.08 | 6.47 ± 0.49 | 0.09 ± 0.01 | 0.10 ± 0.02 | |||
| Pesticide | Radish Leaf | Radish Root | ||||
|---|---|---|---|---|---|---|
| Field 1 | Field 2 | Average | Field 1 | Field 2 | Average | |
| DNC | 6.1 | 6.2 | 6.2 | 8.6 | 4.1 | 6.2 |
| DNT | 4.9 | 5.4 | 5.3 | 6.9 | 3.6 | 4.6 |
| MTC | 10.5 | 9.0 | 9.3 | 4.4 | 2.7 | 3.2 |
| TBC | 4.3 | 10.2 | 8.0 | 5.2 | 5.0 | 5.1 |
| Pesticide | Recommended PHRLs (mg/kg) | MRLs (mg/kg) | ||||
|---|---|---|---|---|---|---|
| 10 Days before | 7 Days before | 5 Days before | 3 Days before | |||
| DNC | Leaf | 5.06 | 3.83 | 3.18 | 2.64 | 2.0 |
| Root | 0.12 | 0.09 | 0.08 | 0.06 | 0.05 | |
| DNT | Leaf | 4.26 | 3.11 | 2.53 | 2.05 | 1.5 |
| Root | 0.12 | 0.09 | 0.08 | 0.07 | 0.05 | |
| MTC | Leaf | 33.42 | 28.65 | 25.85 | 23.33 | 20.0 |
| Root | 0.26 | 0.16 | 0.11 | 0.08 | 0.05 | |
| TBC | Leaf | 10.46 | 8.38 | 7.23 | 6.24 | 5.0 |
| Root | 0.61 | 0.44 | 0.35 | 0.28 | 0.2 | |
| (a) | ||||||
| Pesticide | Residue Value (mg/kg) | ADI | EDI (mg/kg b.w./day) | HQ | ||
| DNC | Day 0 | Leaf | 6.30 | 0.0023 | 6.90 × 10−4 | 3.00 × 10−1 |
| Root | 0.29 | 9.99 × 10−5 | 4.34 × 10−2 | |||
| Day 7 | Leaf | 2.28 | 2.50 × 10−4 | 1.09 × 10−1 | ||
| Root | 0.10 | 3.44 × 10−5 | 1.50 × 10−2 | |||
| Day 14 | Leaf | 1.25 | 1.37 × 10−4 | 5.95 × 10−2 | ||
| Root | 0.06 | 2.07 × 10−5 | 8.98 × 10−3 | |||
| DNT | Day 0 | Leaf | 6.21 | 0.02 | 6.80 × 10−4 | 3.40 × 10−2 |
| Root | 0.71 | 2.45 × 10−4 | 1.22 × 10−2 | |||
| Day 7 | Leaf | 1.98 | 2.17 × 10−4 | 1.08 × 10−2 | ||
| Root | 0.11 | 3.79 × 10−5 | 1.89 × 10−3 | |||
| Day 14 | Leaf | 1.00 | 1.10 × 10−4 | 5.48 × 10−3 | ||
| Root | 0.08 | 2.76 × 10−5 | 1.38 × 10−3 | |||
| MTC | Day 0 | Leaf | 7.78 | 0.04 | 8.52 × 10−4 | 2.13 × 10−2 |
| Root | 0.35 | 1.21 × 10−4 | 3.01 × 10−3 | |||
| Day 7 | Leaf | 4.11 | 4.50 × 10−4 | 1.13 × 10−2 | ||
| Root | 0.07 | 2.41 × 10−5 | 6.03 × 10−4 | |||
| Day 14 | Leaf | 2.55 | 2.79 × 10−4 | 6.98 × 10−3 | ||
| Root | <0.001 | <3.44 × 10−7 | <8.61 × 10−6 | |||
| TBC | Day 0 | Leaf | 13.06 | 0.03 | 1.43 × 10−3 | 4.77 × 10−2 |
| Root | 0.71 | 2.45 × 10−4 | 8.15 × 10−3 | |||
| Day 7 | Leaf | 6.11 | 6.69 × 10−4 | 2.23 × 10−2 | ||
| Root | 0.20 | 6.89 × 10−5 | 2.30 × 10−3 | |||
| Day 14 | Leaf | 3.65 | 4.00 × 10−4 | 1.33 × 10−2 | ||
| Root | 0.09 | 3.10 × 10−5 | 1.03 × 10−3 | |||
| (b) | ||||||
| Pesticide | Residue Value (mg/kg) | ADI | EDI (mg/kg b.w./day) | HQ | ||
| DNC | Day 0 | Leaf | 6.30 | 0.0023 | 9.88 × 10−3 | 4.29 |
| Root | 0.29 | 1.97 × 10−4 | 8.57 × 10−2 | |||
| Day 7 | Leaf | 2.28 | 3.57 × 10−3 | 1.55 | ||
| Root | 0.10 | 6.80 × 10−5 | 2.96 × 10−2 | |||
| Day 14 | Leaf | 1.25 | 1.96 × 10−3 | 8.52 × 10−1 | ||
| Root | 0.06 | 4.08 × 10−5 | 1.77 × 10−2 | |||
| DNT | Day 0 | Leaf | 6.21 | 0.02 | 9.73 × 10−3 | 4.87 × 10−1 |
| Root | 0.71 | 4.83 × 10−4 | 2.41 × 10−2 | |||
| Day 7 | Leaf | 1.98 | 3.10 × 10−3 | 1.55 × 10−1 | ||
| Root | 0.11 | 7.28 × 10−5 | 3.74 × 10−3 | |||
| Day 14 | Leaf | 1.00 | 1.57 × 10−3 | 7.84 × 10−2 | ||
| Root | 0.08 | 5.44 × 10−5 | 2.72 × 10−3 | |||
| MTC | Day 0 | Leaf | 7.78 | 0.04 | 1.22 × 10−2 | 3.05 × 10−1 |
| Root | 0.35 | 2.38 × 10−4 | 5.95 × 10−3 | |||
| Day 7 | Leaf | 4.11 | 6.44 × 10−3 | 1.61 × 10−1 | ||
| Root | 0.07 | 4.76 × 10−5 | 1.19 × 10−3 | |||
| Day 14 | Leaf | 2.55 | 4.00 × 10−3 | 9.99 × 10−2 | ||
| Root | <0.001 | <6.80 × 10−7 | <1.70 × 10−5 | |||
| TBC | Day 0 | Leaf | 13.06 | 0.03 | 2.05 × 10−2 | 6.82 × 10−1 |
| Root | 0.71 | 4.83 × 10−4 | 1.61 × 10−2 | |||
| Day 7 | Leaf | 6.11 | 9.58 × 10−3 | 3.19 × 10−1 | ||
| Root | 0.20 | 1.36 × 10−4 | 4.53 × 10−3 | |||
| Day 14 | Leaf | 3.65 | 5.72 × 10−3 | 1.91 × 10−1 | ||
| Root | 0.09 | 6.12 × 10−5 | 2.04 × 10−3 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, Y.; Song, M.-H.; Yu, J.-W.; Lee, J.-H. Dissipation Kinetics and Risk Assessment of Diniconazole, Dinotefuran, Metconazole, and Tebuconazole in Raphanus sativus L. Foods 2023, 12, 2846. https://doi.org/10.3390/foods12152846
Kwak Y, Song M-H, Yu J-W, Lee J-H. Dissipation Kinetics and Risk Assessment of Diniconazole, Dinotefuran, Metconazole, and Tebuconazole in Raphanus sativus L. Foods. 2023; 12(15):2846. https://doi.org/10.3390/foods12152846
Chicago/Turabian StyleKwak, Yunseon, Min-Ho Song, Ji-Woo Yu, and Ji-Ho Lee. 2023. "Dissipation Kinetics and Risk Assessment of Diniconazole, Dinotefuran, Metconazole, and Tebuconazole in Raphanus sativus L." Foods 12, no. 15: 2846. https://doi.org/10.3390/foods12152846
APA StyleKwak, Y., Song, M.-H., Yu, J.-W., & Lee, J.-H. (2023). Dissipation Kinetics and Risk Assessment of Diniconazole, Dinotefuran, Metconazole, and Tebuconazole in Raphanus sativus L. Foods, 12(15), 2846. https://doi.org/10.3390/foods12152846





