Recombinant Fusion Protein Containing Plant Nigellothionin Regulates the Growth of Food-Spoiling Fungus (Aspergillus niger)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Recombinant Peptide Production
2.2.1. Expression Vector Construction
2.2.2. Fusion Protein Expression and Purification
2.3. Solid-Phase Extraction (SPE)
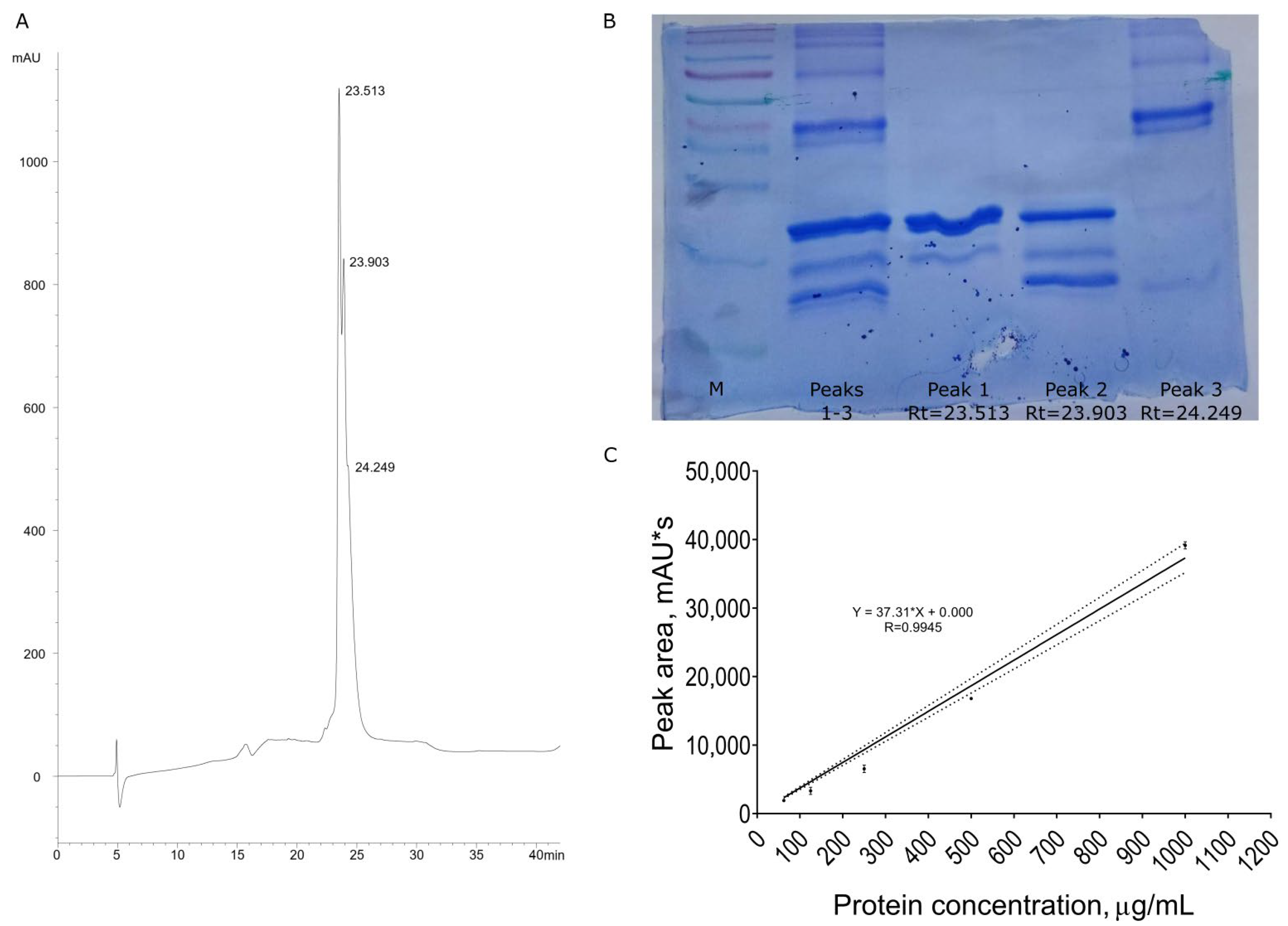
2.4. Trx-NsW2 Purity Confirmation
2.4.1. Analytical Reversed-Phase HPLC
2.4.2. SDS-PAAG Electrophoresis
2.4.3. Edman Sequencing
2.5. Protein Concentration Measurement
2.6. Antifungal Assay
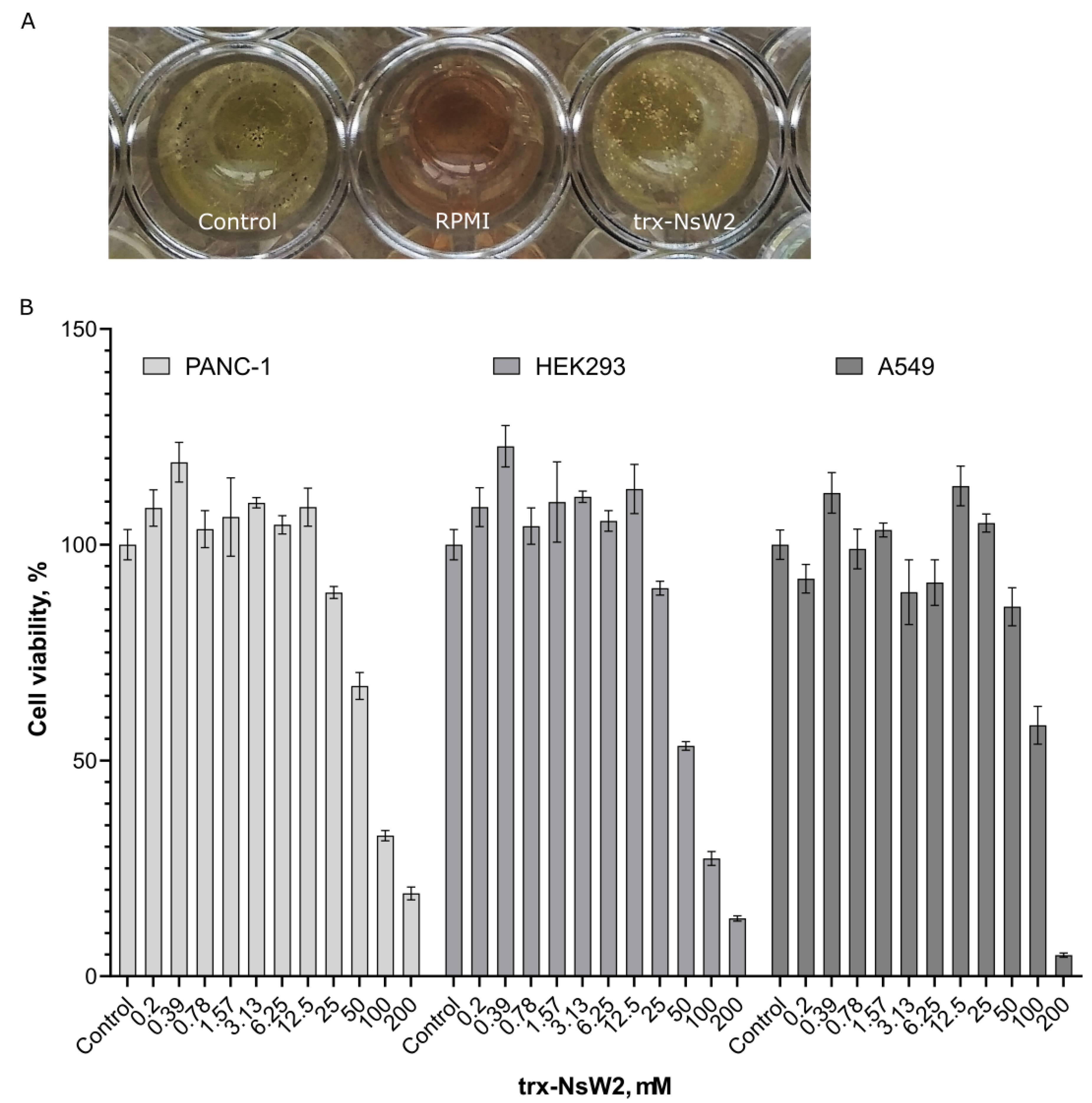
2.7. Cytotoxic Assay
3. Results
3.1. Recombinant Protein Purification
3.2. Determination of Antifungal Activity
3.3. RegulatingFungal Growth and Revealing Cytotoxic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campos, M.L.; De Souza, C.M.; De Oliveira, K.B.S.; Dias, S.C.; Franco, O.L. The role of antimicrobial peptides in plant immunity. J. Exp. Bot. 2018, 69, 4997–5011. [Google Scholar] [CrossRef] [Green Version]
- Tam, J.P.; Wang, S.; Wong, K.H.; Tan, W.L. Antimicrobial peptides from plants. Pharmaceuticals 2015, 8, 711–757. [Google Scholar] [CrossRef] [Green Version]
- Finkina, E.I.; Melnikova, D.N.; Bogdanov, I.V.; Ovchinnikova, T.V. Peptides of the Innate Immune System of Plants. Part II. Biosynthesis, Biological Functions, and Possible Practical Applications. Russ. J. Bioorganic Chem. 2019, 45, 55–65. [Google Scholar] [CrossRef]
- Li, J.; Hu, S.; Jian, W.; Xie, C.; Yang, X. Plant antimicrobial peptides: Structures, functions, and applications. Bot. Stud. 2021, 62, 1–15. [Google Scholar] [CrossRef]
- Astafieva, A.A.; Enyenihi, A.A.; Rogozhin, E.A.; Kozlov, S.A.; Grishin, E.V.; Odintsova, T.I.; Zubarev, R.A.; Egorov, T.A. Novel proline-hydroxyproline glycopeptides from the dandelion (Taraxacum officinale Wigg.) flowers: De novo sequencing and biological activity. Plant Sci. 2015, 238, 323–329. [Google Scholar] [CrossRef]
- Sharma, P.; Kaur, J.; Sharma, G.; Kashyap, P. Plant derived antimicrobial peptides: Mechanism of target, isolation techniques, sources and pharmaceutical applications. J. Food Biochem. 2022, 46, e14348. [Google Scholar] [CrossRef]
- Parisi, K.; Shafee, T.M.A.; Quimbar, P.; van der Weerden, N.L.; Bleackley, M.R.; Anderson, M.A. The evolution, function and mechanisms of action for plant defensins. Semin. Cell Dev. Biol. 2019, 88, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Stec, B. Plant thionins--the structural perspective. Cell Mol. Life Sci. 2006, 63, 1370–1385. [Google Scholar] [CrossRef] [PubMed]
- Loeza-Angeles, H.; Sagrero-Cisneros, E.; Lara-Zárate, L.; Villagómez-Gómez, E.; López-Meza, J.E.; Ochoa-Zarzosa, A. Thionin Thi2.1 from Arabidopsis thaliana expressed in endothelial cells shows antibacterial, antifungal and cytotoxic activity. Biotechnol. Lett. 2008, 30, 1713–1719. [Google Scholar] [CrossRef]
- Florack, D.E.; Stiekema, W.J. Thionins: Properties, possible biological roles and mechanisms of action. Plant Mol. Biol. 1994, 26, 25–37. [Google Scholar] [CrossRef]
- Vasilchenko, A.S.; Smirnov, A.N.; Zavriev, S.K.; Grishin, E.V.; Vasilchenko, A.V.; Rogozhin, E.A. Novel Thionins from Black Seed (Nigella sativa L.) Demonstrate Antimicrobial Activity. Int. J. Pept. Res. Ther. 2017, 23, 171–180. [Google Scholar] [CrossRef]
- Barashkova, A.S.; Sadykova, V.S.; Salo, V.A.; Zavriev, S.K.; Rogozhin, E.A. Nigellothionins from Black Cumin (Nigella sativa L.) Seeds Demonstrate Strong Antifungal and Cytotoxic Activity. Antibiotics 2021, 10, 166. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W.; Arendrup, M.C.; Hope, W.W.; Flörl, C.; Cuenca-Estrella, M.; Arikan, S.; Barchiesi, F.; et al. EUCAST Technical Note on Aspergillus and amphotericin B, itraconazole, and posaconazole. Clin. Microbiol. Infect. 2012, 18, E248–E250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchelnikova, V.A.; Rogozhin, E.A.; Barashkova, A.S.; Buchelnikov, A.S.; Evstigneev, M.P. C60 Fullerene Clusters Stabilize the Biologically Inactive Form of Topotecan. Chem. Res. Toxicol. 2022, 35, 1482–1492. [Google Scholar] [CrossRef] [PubMed]
- Hubina, A.V.; Pogodaev, A.A.; Sharoyko, V.V.; Vlakh, E.G.; Tennikova, T.B. Self-assembled spin-labeled nanoparticles based on poly(amino acids). React. Funct. Polym. 2016, 100, 173–180. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, F.; Nacev, B.A.; Liu, J.O.; Pei, D. Protein N-terminal processing: Substrate specificity of Escherichia coli and human methionine aminopeptidases. Biochemistry 2010, 49, 5588–5599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kul’ko, V.B.; Kisil, O.V.; Sadykova, V.S.; Mikhailov, V.F.; Vasilieva, I.M.; Shulenina, L.V.; Zasukhina, G.D.; Rogozhin, E.A. Investigation of Thionins from Black Cumin (Nigella sativa L.) Showing Cytotoxic, Regulatory and Antifungal Activity. Antibiot. Chemother. 2016, 61, 8–16. [Google Scholar]
- Lima, A.M.; Azevedo, M.I.G.; Sousa, L.M.; Oliveira, N.S.; Andrade, C.R.; Freitas, C.D.T.; Souza, P.F.N. Plant antimicrobial peptides: An overview about classification, toxicity and clinical applications. Int. J. Biol. Macromol. 2022, 214, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Meyer, V.; Basenko, E.Y.; Benz, J.P.; Braus, G.H.; Caddick, M.X.; Csukai, M.; De Vries, R.P.; Endy, D.; Frisvad, J.C.; Gunde-Cimerman, N.; et al. Growing a circular economy with fungal biotechnology: A white paper. Fungal Biol. Biotechnol. 2020, 71, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Isaac Kirubakaran, S.; Begum, S.M.; Ulaganathan, K.; Sakthivel, N. Characterization of a new antifungal lipid transfer protein from wheat. Plant Physiol. Biochem. 2008, 46, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Istomina, E.A.; Slavokhotova, A.A.; Korostyleva, T.V.; Semina, Y.V.; Shcherbakova, L.A.; Pukhalskij, V.A.; Odintsova, T.I. Genes encoding hevein-like antimicrobial peptides WAMPs in the species of the genus Aegilops L. Russ. J. Genet. 2017, 53, 1320–1327. [Google Scholar] [CrossRef]
- Chang, P.K.; Cary, J.W.; Lebar, M.D. Biosynthesis of conidial and sclerotial pigments in Aspergillus species. Appl. Microbiol. Biotechnol. 2020, 104, 2277–2286. [Google Scholar] [CrossRef]
- Gessler, N.N.; Egorova, A.S.; Belozerskaya, T.A. Melanin pigments of fungi under extreme environmental conditions (Review). Appl. Biochem. Microbiol. 2014, 50, 105–113. [Google Scholar] [CrossRef]
- Krijgsheld, P.; Bleichrodt, R.; van Veluw, G.J.; Wang, F.; Müller, W.H.; Dijksterhuis, J.; Wösten, H.A.B. Development in aspergillus. Stud. Mycol. 2013, 74, 1–29. [Google Scholar] [CrossRef]
- Marshall, M.A.; Timberlake, W.E. Aspergillus nidulans wetA activates spore-specific gene expression. Mol. Cell. Biol. 1991, 11, 55. [Google Scholar] [CrossRef] [Green Version]
- Mikhailov, V.F.; Shishkina, A.A.; Vasilyeva, I.M.; Shulenina, L.V.; Raeva, N.F.; Rogozhin, E.A.; Startsev, M.I.; Zasukhina, G.D.; Gromov, S.P.; Alfimov, M.V. Comparative analysis of natural and synthetic antimutagens as regulators of gene expression in human cells under exposure to ionizing radiation. Russ. J. Genet. 2015, 51, 130–137. [Google Scholar] [CrossRef]
- Clifton, L.A.; Sanders, M.; Kinane, C.; Arnold, T.; Edler, K.J.; Neylon, C.; Green, R.J.; Frazier, R.A. The role of protein hydrophobicity in thionin–phospholipid interactions: A comparison of α1 and α2-purothionin adsorbed anionic phospholipid monolayers. Phys. Chem. Chem. Phys. 2012, 14, 13569–13579. [Google Scholar] [CrossRef] [PubMed]
- Majewski, J.; Stec, B. X-ray scattering studies of model lipid membrane interacting with purothionin provide support for a previously proposed mechanism of membrane lysis. Eur. Biophys. J. 2010, 39, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barashkova, A.S.; Ryazantsev, D.Y.; Zhuravleva, A.S.; Sharoyko, V.V.; Rogozhin, E.A. Recombinant Fusion Protein Containing Plant Nigellothionin Regulates the Growth of Food-Spoiling Fungus (Aspergillus niger). Foods 2023, 12, 3002. https://doi.org/10.3390/foods12163002
Barashkova AS, Ryazantsev DY, Zhuravleva AS, Sharoyko VV, Rogozhin EA. Recombinant Fusion Protein Containing Plant Nigellothionin Regulates the Growth of Food-Spoiling Fungus (Aspergillus niger). Foods. 2023; 12(16):3002. https://doi.org/10.3390/foods12163002
Chicago/Turabian StyleBarashkova, Anna S., Dmitry Yu. Ryazantsev, Anna S. Zhuravleva, Vladimir V. Sharoyko, and Eugene A. Rogozhin. 2023. "Recombinant Fusion Protein Containing Plant Nigellothionin Regulates the Growth of Food-Spoiling Fungus (Aspergillus niger)" Foods 12, no. 16: 3002. https://doi.org/10.3390/foods12163002
APA StyleBarashkova, A. S., Ryazantsev, D. Y., Zhuravleva, A. S., Sharoyko, V. V., & Rogozhin, E. A. (2023). Recombinant Fusion Protein Containing Plant Nigellothionin Regulates the Growth of Food-Spoiling Fungus (Aspergillus niger). Foods, 12(16), 3002. https://doi.org/10.3390/foods12163002






