Pulsed Electric Field (PEF) and High-Power Ultrasound (HPU) in the Hurdle Concept for the Preservation of Antioxidant Bioactive Compounds of Strawberry Juice—A Chemometric Evaluation—Part I
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Strawberry Juice Preparation
2.3. Hurdle Concept consisting of Pulsed Electric Field (PEF) and High-Power Ultrasound (HPU) for Strawberry Juice Processing
2.4. Determination of pH and Soluble Solids Content (SSC)
2.5. Extraction of Antioxidant Bioactive Compounds
2.6. Determination of Total Phenolic Content (TPC)
2.7. Determination of Total Hydroxycinnamic Acids (HCA) and Total Flavonols (FL)
2.8. Determination of Condensed Tannins (CT)
2.9. In Vitro Measurement of Antioxidant Activity
2.9.1. 2,2-diphenyl-1-picrylhydrazyl Assay (DPPH)
2.9.2. Ferric Reducing Antioxidant Power Assay (FRAP)
2.10. Statistical Analysis
3. Results and Discussion
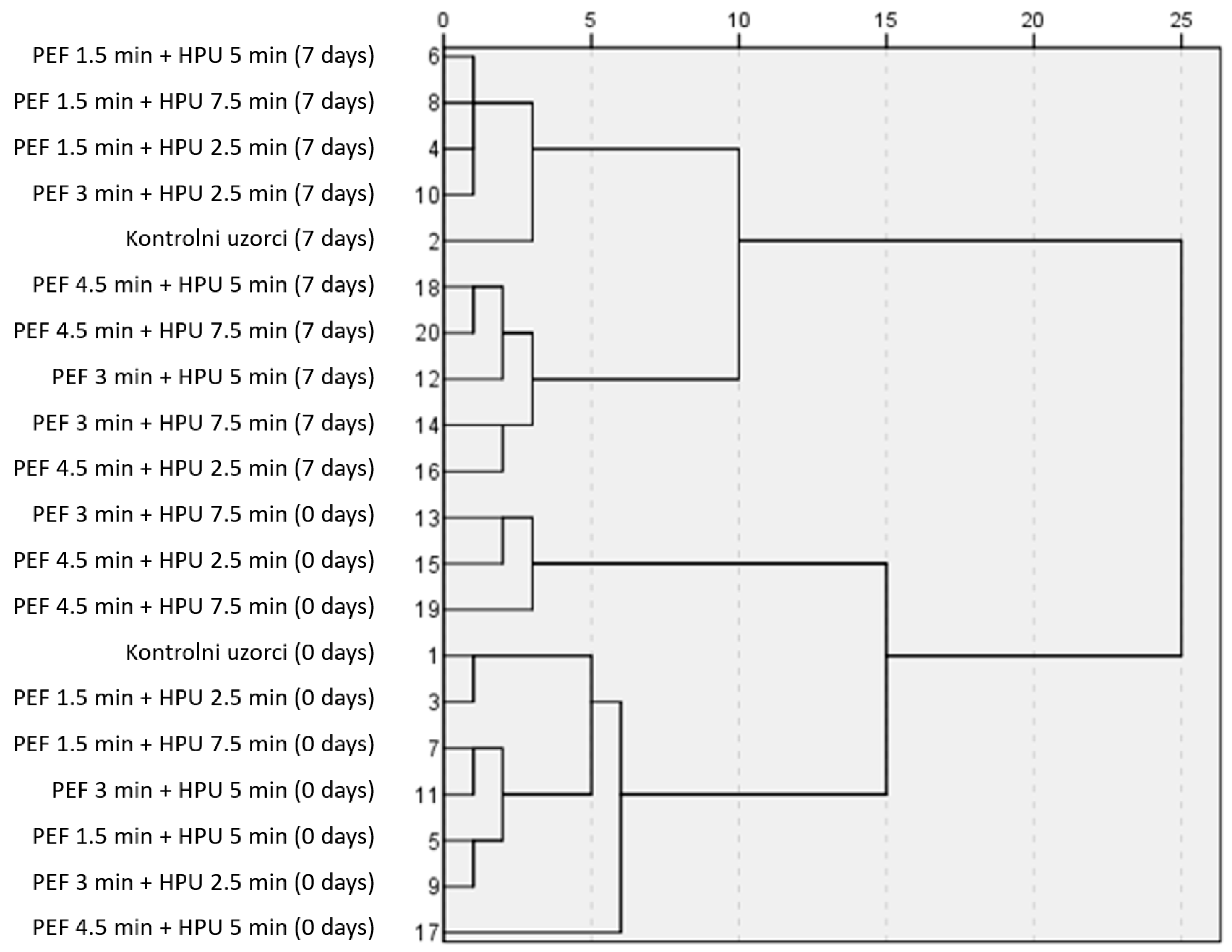
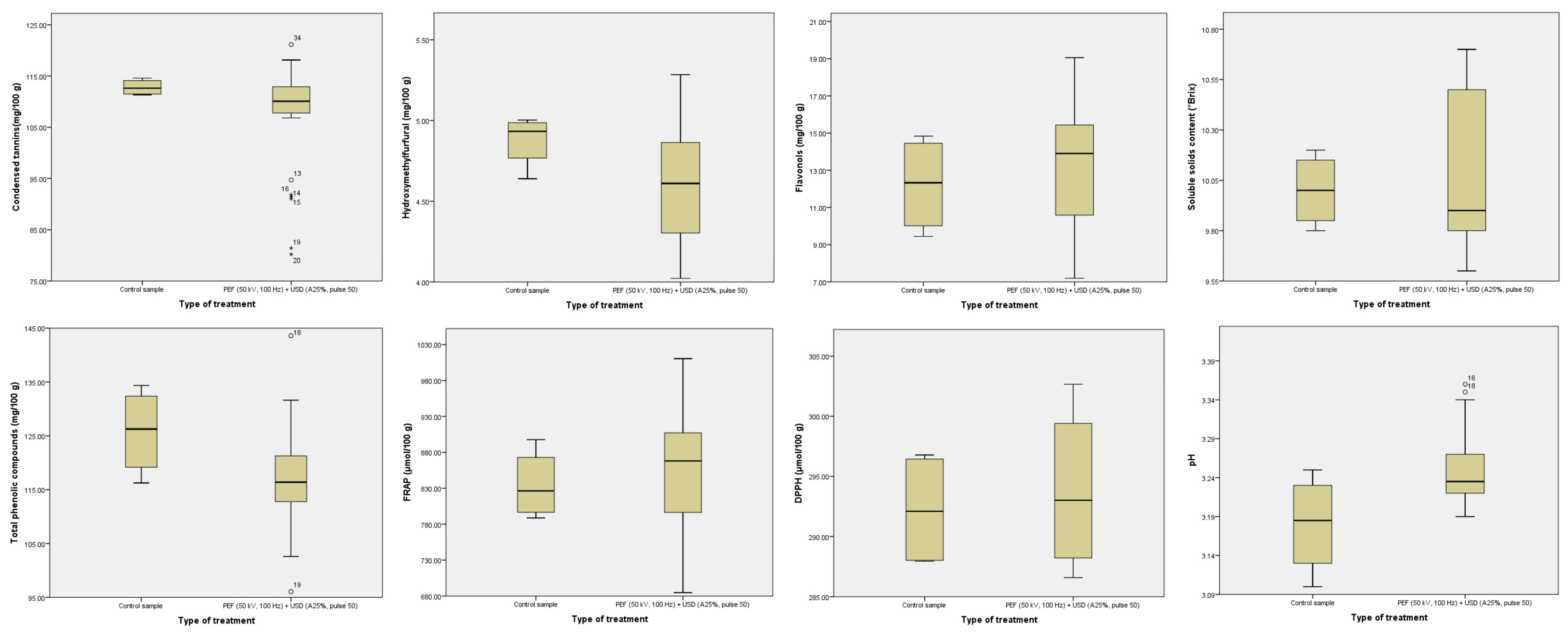
3.1. Use of Chemometrics to Evaluate Hurdle Processed Samples vs. Control Samples
3.2. The Changes in BACs, Antioxidant Activity, SSC, and pH in Strawberry Juices after Hurdle Technology Processing and Storage
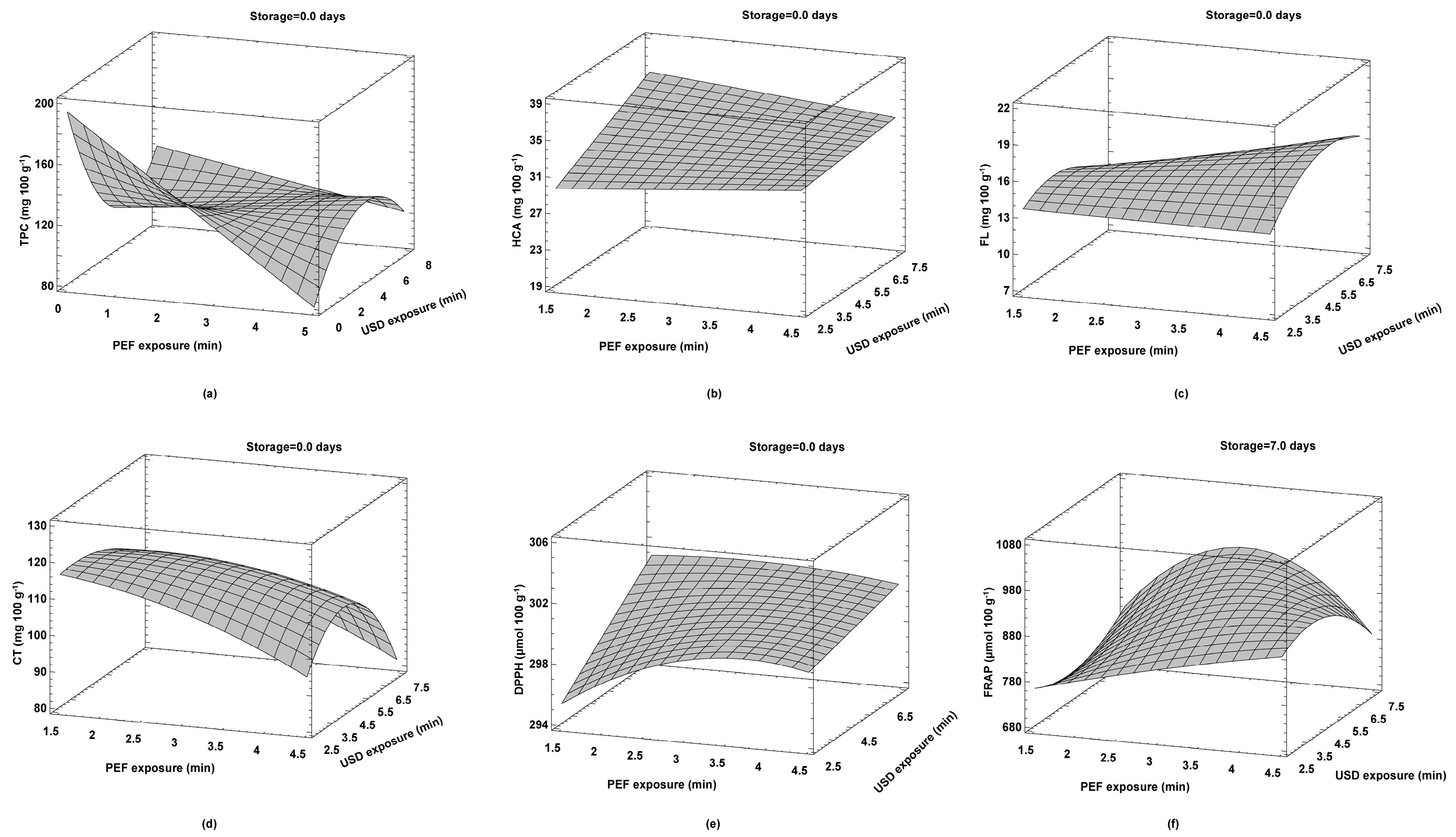
3.3. Optimization of Operating Parameters of Hurdle Technology for Strawberry Juice Treatments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Azam, M.; Ejaz, S.; Naveed Ur Rehman, R.; Khan, M.; Qadri, R. Postharvest Quality Management of Strawberries. In Strawberry—Pre- and Post-Harvest Management Techniques for Higher Fruit Quality; Asao, T., Asaduzzaman, M., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Pap, N.; Fidelis, M.; Azevedo, L.; do Carmo, M.A.V.; Wang, D.; Mocan, A.; Pereira, E.P.R.; Xavier-Santos, D.; Sant’Ana, A.S.; Yang, B.; et al. Berry polyphenols and human health: Evidence of antioxidant, anti-inflammatory, microbiota modulation, and cell-protecting effects. Curr. Opin. Food Sci. 2021, 42, 167–186. [Google Scholar] [CrossRef]
- Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Alvarez-Suarez, J.M.; Afrin, S.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Strawberry as a health promoter: An evidence based review. Food Funct. 2015, 6, 1386–1398. [Google Scholar] [CrossRef] [PubMed]
- Aaby, K.; Mazur, S.; Nes, A.; Skrede, G. Phenolic compounds in strawberry (Fragaria x ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012, 132, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Sabbadini, S.; Giampieri, F. The effects of pre-harvest and post-harvest factors on the nutritional quality of strawberry fruits: A review. J. Berry Res. 2014, 4, 1–10. [Google Scholar] [CrossRef]
- Skupien, K.; Oszmianski, J. Comparison of six cultivars of strawberries (Fragaria x ananassa Duch.) grown in northwest Poland. Eur. Food Res. Technol. 2004, 219, 66–70. [Google Scholar] [CrossRef]
- Bebek Markovinović, A.; Putnik, P.; Duralija, B.; Krivohlavek, A.; Ivešić, M.; Mandić Andačić, I.; Palac Bešlić, I.; Pavlić, B.; Lorenzo, J.M.; Bursać Kovačević, D. Chemometric Valorization of Strawberry (Fragaria x ananassa Duch.) cv. ‘Albion’ for the Production of Functional Juice: The Impact of Physicochemical, Toxicological, Sensory, and Bioactive Value. Foods 2022, 11, 640. [Google Scholar] [CrossRef]
- Cassani, L.; Tomadoni, B.; Moreira, M.R.; Agüero, M.V. Improving Quality Parameters of Functional Strawberry Juices: Optimization of Prebiotic Fiber Enrichment and Geraniol Treatment. Food Bioprocess Technol. 2018, 11, 2110–2124. [Google Scholar] [CrossRef]
- Sui, X.; Bary, S.; Zhou, W. Changes in the color, chemical stability and antioxidant capacity of thermally treated anthocyanin aqueous solution over storage. Food Chem. 2016, 192, 516–524. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Kim, D.O.; Padilla-Zakour, O.I. Jam processing effect on phenolics and antioxidant capacity in anthocyanin-rich fruits: Cherry, plum, and raspberry. J. Food Sci. 2006, 69, S395–S400. [Google Scholar] [CrossRef]
- Zia, S.; Khan, M.R.; Shabbir, M.A.; Aslam Maan, A.; Khan, M.K.I.; Nadeem, M.; Khalil, A.A.; Din, A.; Aadil, R.M. An Inclusive Overview of Advanced Thermal and Nonthermal Extraction Techniques for Bioactive Compounds in Food and Food-related Matrices. Food Rev. Int. 2020, 38, 1166–1196. [Google Scholar] [CrossRef]
- Bursać Kovačević, D.; Maras, M.; Barba, F.J.; Granato, D.; Roohinejad, S.; Mallikarjunan, K.; Montesano, D.; Lorenzo, J.M.; Putnik, P. Innovative technologies for the recovery of phytochemicals from Stevia rebaudiana Bertoni leaves: A review. Food Chem. 2018, 268, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Koubaa, M.; Barba, F.J.; Bursać Kovačević, D.; Putnik, P.; Santos, M.D.; Queirós, R.P.; Moreira, S.A.; Inácio, R.S.; Fidalgo, L.G.; Saraiva, J.A. Pulsed electric field processing of fruit juices. In Fruit Juices; Academic Press: Cambridge, MA, USA, 2018; pp. 437–449. [Google Scholar]
- Gabrić, D.; Barba, F.; Roohinejad, S.; Gharibzahedi, S.M.T.; Radojčin, M.; Putnik, P.; Bursać Kovačević, D. Pulsed electric fields as an alternative to thermal processing for preservation of nutritive and physicochemical properties of beverages: A review. J. Food Process Eng. 2018, 41, e12638. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Aaliya, B.; Valiyapeediyekkal Sunooj, K.; Navaf, M.; Parambil Akhila, P.; Sudheesh, C.; Ahmad Mir, S.; Sabu, S.; Sasidharan, A.; Theingi Hlaing, M.; George, J. Recent trends in bacterial decontamination of food products by hurdle technology: A synergistic approach using thermal and non-thermal processing techniques. Food Res. Int. 2021, 147, 110514. [Google Scholar] [CrossRef]
- Arya, S.S.; Nachiappan, N.; Waghmare, R.; Bhat, M.S. Recent progress and future perspectives on non-thermal apple juice processing techniques. Food Prod. Process. Nutr. 2023, 5, 36. [Google Scholar] [CrossRef]
- Krishnan Kesavan, R.; Begum, S.; Das, P.; Nayak, P.K. Hurdle effect of thermosonication and non-thermal processing on the quality characteristics of fruit juices: An overview. J. Food Process Eng. 2023, 46, e14310. [Google Scholar] [CrossRef]
- Jin, T.Z.; Aboelhaggag, R.M. Combined Pulsed Electric Field with Antimicrobial Caps for Extending Shelf Life of Orange Juice. Beverages 2022, 8, 72. [Google Scholar] [CrossRef]
- Shaik, L.; Chakraborty, S. Nonthermal pasteurization of pineapple juice: A review on the potential of achieving microbial safety and enzymatic stability. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4716–4737. [Google Scholar] [CrossRef]
- Mehta, D.; Yadav, S.K. Impact of atmospheric non-thermal plasma and hydrothermal treatment on bioactive compounds and microbial inactivation of strawberry juice: A hurdle technology approach. Food Sci. Technol. Int. 2019, 26, 3–10. [Google Scholar] [CrossRef]
- Yildiz, S.; Pokhrel, P.R.; Unluturk, S.; Barbosa-Cánovas, G.V. Shelf life extension of strawberry juice by equivalent ultrasound, high pressure, and pulsed electric fields processes. Food Res. Int. 2021, 140, 110040. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, G.; Aadil, R.M. Comparison of high temperature-short time and sonication on selected parameters of strawberry juice during room temperature storage. J. Food Sci. Technol. 2019, 57, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, S.; Pokhrel, P.R.; Unluturk, S.; Barbosa-Cánovas, G.V. Changes in Quality Characteristics of Strawberry Juice After Equivalent High Pressure, Ultrasound, and Pulsed Electric Fields Processes. Food Eng. Rev. 2020, 13, 601–612. [Google Scholar] [CrossRef]
- Cassani, L.; Tomadoni, B.; Moreira, M.R.; Ponce, A.; Agüero, M.V. Optimization of inulin:Oligofructose proportion and non-thermal processing to enhance microbiological and sensory properties of fiber-enriched strawberry juice. LWT 2017, 80, 446–455. [Google Scholar] [CrossRef]
- Bebek Markovinović, A.; Putnik, P.; Bičanić, P.; Brdar, D.; Duralija, B.; Pavlić, B.; Milošević, S.; Rocchetti, G.; Lucini, L.; Bursać Kovačević, D. A chemometric investigation on the functional potential in high power ultrasound (HPU) processed strawberry juice made from fruits harvested at two stages of ripeness. Molecules 2022, 28, 138. [Google Scholar] [CrossRef]
- Bebek Markovinović, A.; Putnik, P.; Stulić, V.; Batur, L.; Duralija, B.; Pavlić, B.; Vukušić Pavičić, T.; Herceg, Z.; Bursać Kovačević, D. The application and optimization of HIPEF technology in the processing of juice from strawberries harvested at two stages of ripeness. Foods 2022, 11, 1997. [Google Scholar] [CrossRef]
- Yuan, B.; Danao, M.-G.C.; Stratton, J.E.; Weier, S.A.; Weller, C.L.; Lu, M. High pressure processing (HPP) of aronia berry purée: Effects on physicochemical properties, microbial counts, bioactive compounds, and antioxidant capacities. Innov. Food Sci. Emerg. Technol. 2018, 47, 249–255. [Google Scholar] [CrossRef]
- Howard, L.R.; Clark, J.R.; Brownmiller, C. Antioxidant capacity and phenolic content in blueberries as affected by genotype and growing season. J. Sci. Food Agric. 2003, 83, 1238–1247. [Google Scholar] [CrossRef]
- Sun, B.; Ricardo-da-Silva, J.M.; Spranger, I. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Shortle, E.; O’Grady, M.N.; Gilroy, D.; Furey, A.; Quinn, N.; Kerry, J.P. Influence of extraction technique on the anti-oxidative potential of hawthorn (Crataegus monogyna) extracts in bovine muscle homogenates. Meat Sci. 2014, 98, 828–834. [Google Scholar] [CrossRef]
- Benzie, I.F.F. An automated, specific, spectrophotometric method for measuring ascorbic acid in plasma (EFTSA). Clin. Biochem. 1996, 29, 111–116. [Google Scholar] [CrossRef]
- Maza, M.A.; Pereira, C.; Martínez, J.M.; Camargo, A.; Álvarez, I.; Raso, J. PEF treatments of high specific energy permit the reduction of maceration time during vinification of Caladoc and Grenache grapes. Innov. Food Sci. Emerg. Technol. 2020, 63, 102375. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Ye, J.; Vanga, S.K.; Raghavan, V. Influence of high-intensity ultrasound on bioactive compounds of strawberry juice: Profiles of ascorbic acid, phenolics, antioxidant activity and microstructure. Food Control 2019, 96, 128–136. [Google Scholar] [CrossRef]
- Putnik, P.; Pavlić, B.; Šojić, B.; Zavadlav, S.; Žuntar, I.; Kao, L.; Kitonić, D.; Kovačević, D.B. Innovative hurdle technologies for the preservation of functional fruit juices. Foods 2020, 9, 699. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ahmed, Z.; Ahmad, N.; Aadil, R.M.; Rahaman, A.; Roobab, U.; Rehman, A.; Siddique, R.; Zeng, X.A.; Siddeeg, A. Novel processing techniques and spinach juice: Quality and safety improvements. J. Food Sci. 2020, 85, 1018–1026. [Google Scholar] [CrossRef]
- Makroo, H.A.; Saxena, J.; Rastogi, N.K.; Srivastava, B. Ohmic heating assisted polyphenol oxidase inactivation of watermelon juice: Effects of the treatment on pH, lycopene, total phenolic content, and color of the juice. J. Food Process. Preserv. 2017, 41, e13271. [Google Scholar] [CrossRef]
- Assiry, A. Degradation kinetics of ascorbic acid during ohmic heating with stainless steel electrodes. J. Appl. Electrochem. 2003, 33, 187–196. [Google Scholar] [CrossRef]
- Sulaiman, A.; Farid, M.; Silva, F.V.M. Strawberry puree processed by thermal, high pressure, or power ultrasound: Process energy requirements and quality modeling during storage. Food Sci. Technol. Int. 2016, 23, 293–309. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-W.; Zeng, X.-A.; Ngadi, M. Enhanced extraction of phenolic compounds from onion by pulsed electric field (PEF). J. Food Process. Preserv. 2018, 42, e13755. [Google Scholar] [CrossRef]
- Bursać Kovačević, D.; Bilobrk, J.; Buntić, B.; Bosiljkov, T.; Karlović, S.; Rocchetti, G.; Lucini, L.; Barba, F.J.; Lorenzo, J.M.; Putnik, P. High-power ultrasound altered the polyphenolic content and antioxidant capacity in cloudy apple juice during storage. J. Food Process. Preserv. 2019, 43, e14023. [Google Scholar] [CrossRef]
- Adekunte, A.O.; Tiwari, B.K.; Cullen, P.J.; Scannell, A.G.M.; O’Donnell, C.P. Effect of sonication on colour, ascorbic acid and yeast inactivation in tomato juice. Food Chem. 2010, 122, 500–507. [Google Scholar] [CrossRef]
- Sobral, M.M.C.; Nunes, C.; Maia, A.; Ferreira, P.; Coimbra, M.A. Conditions for producing long shelf life fruit salads processed using mild pasteurization. LWT-Food Sci. Technol. 2017, 85, 316–323. [Google Scholar] [CrossRef]
- Nguyen, C.; Nguyen, H. Ultrasonic Effects on the Quality of Mulberry Juice. Beverages 2018, 4, 56. [Google Scholar] [CrossRef]
- El Darra, N.; Grimi, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Pulsed electric field, ultrasound, and thermal pretreatments for better phenolic extraction during red fermentation. Eur. Food Res. Technol. 2012, 236, 47–56. [Google Scholar] [CrossRef]
- Roobab, U.; Aadil, R.M.; Madni, G.M.; Bekhit, A.E.-D. The impact of nonthermal technologies on the microbiological quality of juices: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 437–457. [Google Scholar] [CrossRef]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochemistry 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Koraqi, H.; Petkoska, A.T.; Khalid, W.; Sehrish, A.; Ambreen, S.; Lorenzo, J.M. Optimization of the extraction conditions of antioxidant phenolic compounds from strawberry fruits (Fragaria x ananassa Duch.) using response surface methodology. Food Anal. Methods 2023, 16, 1030–1042. [Google Scholar] [CrossRef]
| Sample of Juice | Storage (Days) | Processing | PEF Exposure (min) | HPU Exposure (min) |
|---|---|---|---|---|
| 1 | 0 | Control | / | / |
| 2 | 0 | PEF + HPU | 1.5 | 2.5 |
| 3 | 0 | PEF + HPU | 1.5 | 5 |
| 4 | 0 | PEF + HPU | 1.5 | 7.5 |
| 5 | 0 | PEF + HPU | 3 | 2.5 |
| 6 | 0 | PEF + HPU | 3 | 5 |
| 7 | 0 | PEF + HPU | 3 | 7.5 |
| 8 | 0 | PEF + HPU | 4.5 | 2.5 |
| 9 | 0 | PEF + HPU | 4.5 | 5 |
| 10 | 0 | PEF + HPU | 4.5 | 7.5 |
| 11 | 7 | Control | / | / |
| 12 | 7 | PEF + HPU | 1.5 | 2.5 |
| 13 | 7 | PEF + HPU | 1.5 | 5 |
| 14 | 7 | PEF + HPU | 1.5 | 7.5 |
| 15 | 7 | PEF + HPU | 3 | 2.5 |
| 16 | 7 | PEF + HPU | 3 | 5 |
| 17 | 7 | PEF + HPU | 3 | 7.5 |
| 18 | 7 | PEF + HPU | 4.5 | 2.5 |
| 19 | 7 | PEF + HPU | 4.5 | 5 |
| 20 | 7 | PEF + HPU | 4.5 | 7.5 |
| TPC | HCA | FL | CT | DPPH | FRAP | SSC | pH | |
|---|---|---|---|---|---|---|---|---|
| Chi-Square | 3.417 | 1.829 | 0.52 | 2.35 | 0.813 | 0.587 | 0.134 | 4.64 |
| df | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Sig. | 0.07 | 0.18 | 0.47 | 0.13 | 0.37 | 0.44 | 0.71 | 0.03 * |
| n | TPC | HCA | FL | CT | DPPH | FRAP | SSC | pH | |
|---|---|---|---|---|---|---|---|---|---|
| Storage | p = 0.39 ‡ | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p = 0.02 † | p = 0.26 ‡ | p ≤ 0.01 † | |
| 0 days | 18 | 118.54 ± 0.99 a | 32.58 ± 0.40 a | 14.26 ± 0.27 a | 104.15 ± 0.33 b | 299.30 ± 0.13 a | 866.54 ± 7.66 a | 10.11 ± 0.01 a | 3.28 ± 0.01 a |
| 7 days | 18 | 117.31 ± 0.99 a | 23.49 ± 0.40 b | 12.50 ± 0.27 b | 111.18 ± 0.33 a | 288.57 ± 0.13 b | 838.29 ± 7.66 b | 10.13 ± 0.01 a | 3.22 ± 0.01 b |
| PEF | p = 0.48 ‡ | p = 0.02 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p = 0.02 † | |
| 1.5 min | 12 | 118.19 ± 1.22 a | 26.79 ± 0.49 b | 14.15 ± 0.33 a | 110.96 ± 0.40 a | 292.77 ± 0.15 b | 830.50 ± 9.38 b | 9.85 ± 0.02 c | 3.23 ± 0.01 b |
| 3 min | 12 | 118.85 ± 1.22 a | 28.86 ± 0.49 a | 13.57 ± 0.33 a | 109.94 ± 0.40 a | 294.69 ± 0.15 a | 879.81 ± 9.38 a | 10.01 ± 0.02 b | 3.26 ± 0.01 a |
| 4.5 min | 12 | 116.75 ± 1.22 a | 28.46 ± 0.49 a | 12.44 ± 0.33 b | 102.09 ± 0.40 b | 294.34 ± 0.15 a | 846.92 ± 9.38 b | 10.49 ± 0.02 a | 3.26 ± 0.01 a |
| HPU | p = 0.02 † | p = 0.63 ‡ | p = 0.02 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p = 0.26 ‡ | |
| 2.5 min | 12 | 118.51 ± 1.22 a,b | 28.42 ± 0.49 a | 13.31 ± 0.33 a,b | 107.96 ± 0.40 b | 293.54 ± 0.15 b | 854.81 ± 9.38 a | 10.11 ± 0.02 b | 3.25 ± 0.01 a |
| 5 min | 12 | 120.24 ± 1.22 a | 27.84 ± 0.49 a | 14.14 ± 0.33 a | 111.46 ± 0.40 a | 293.62 ± 0.15 b | 877.46 ± 9.38 a | 10.07 ± 0.02 b | 3.25 ± 0.01 a |
| 7.5 min | 12 | 115.04 ± 1.22 b | 27.84 ± 0.49 a | 12.70 ± 0.33 b | 103.57 ± 0.40 c | 294.64 ± 0.15 a | 824.96 ± 9.38 b | 10.18 ± 0.02 a | 3.26 ± 0.01 a |
| PEF + HPU (hurdle) | p = 0.07 ‡ | p = 0.59 ‡ | p = 0.03 † | p ≤ 0.01 † | p ≤ 0.01 † | p =0.78 ‡ | p = 0.03 † | p = 0.76 ‡ | |
| 1.5 min + 2.5 min | 12 | 122.79 ± 1.90 a | 26.14 ± 0.84 a | 12.80 ± 0.76 b | 113.62 ± 0.52 a | 290.94 ± 0.22 c | 840.84 ± 17.48 a | 9.93 ± 0.04 a | 3.23 ± 0.01 a |
| 1.5 min + 5 min | 12 | 115.91 ± 1.90 a | 26.81 ± 0.84 a | 16.36 ± 0.76 a | 110.47 ± 0.52 b | 293.09 ± 0.22 b | 825.53 ± 17.48 a | 9.88 ± 0.04 a | 3.23 ± 0.01 a |
| 1.5 min + 7.5 min | 12 | 115.86 ± 1.90 a | 27.41 ± 0.84 a | 13.29 ± 0.76 b | 108.78 ± 0.52 b | 294.30 ± 0.22 a | 825.14 ± 17.48 a | 9.75 ± 0.04 b | 3.23 ± 0.01 a |
| PEF + HPU (hurdle) | p = 0.39 ‡ | p = 0.25 ‡ | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p = 0.09 ‡ | p ≤ 0.01 † | p = 0.76 ‡ | |
| 3 min + 2.5 min | 12 | 118.18 ± 2.15 a | 29.23 ± 0.82 a | 15.19 ± 0.41 a | 110.25 ± 0.90 a | 294.94 ± 0.25 a | 836.0 5± 19.66 a | 9.75 ± 0.03 b | 3.25 ± 0.01 a |
| 3 min + 5 min | 12 | 116.99 ± 2.15 a | 27.63 ± 0.82 a | 11.95 ± 0.41 c | 113.32 ± 0.90 a | 293.64 ± 0.25 b | 895.61 ± 19.66 a | 9.80 ± 0.03 b | 3.25 ± 0.01 a |
| 3 min + 7.5 min | 12 | 121.37 ± 2.15 a | 29.72 ± 0.82 a | 13.55 ± 0.41 b | 106.25 ± 0.90 b | 295.48 ± 0.25 a | 907.78 ± 19.66 a | 10.48 ± 0.03 a | 3.28 ± 0.01 a |
| PEF + HPU (hurdle) | p = 0.02 † | p = 0.08 ‡ | p = 0.02 † | p ≤ 0.01 † | p = 0.36 ‡ | p ≤ 0.01 † | p ≤ 0.01 † | p = 0.76 ‡ | |
| 4.5 min + 2.5 min | 12 | 114.56 ± 2.26 b | 29.89 ± 0.90 a | 11.93 ± 0.51 b | 99.99 ± 0.62 b | 294.75 ± 0.32 a | 887.54 ± 9.99 a | 10.65 ± 0.03 a | 3.26 ± 0.01 a |
| 4.5 min + 5 min | 12 | 127.80 ± 2.26 a | 29.07 ± 0.90 a | 14.11 ± 0.51 a | 110.6 ± 0.62 a | 294.13 ± 0.32 a | 911.25 ± 9.99 a | 10.53 ± 0.03 b | 3.27 ± 0.01 a |
| 4.5 min + 7.5 min | 12 | 107.90 ± 2.26 b | 26.40 ± 0.90 a | 11.27 ± 0.51 b | 95.69 ± 0.62 c | 294.15 ± 0.32 a | 741.97 ± 9.99 b | 10.30 ± 0.03 c | 3.26 ± 0.01 a |
| Dataset average | 36 | 117.93 ± 0.70 | 28.03 ± 0.28 | 13.38 ± 0.19 | 107.66 ± 0.23 | 293.93 ± 0.09 | 852.41 ± 5.42 | 10.12 ± 0.01 | 3.25 ± 0.01 |
| Storage | PEF Exposure | HPU Exposure | TPC 1 | HCA 2 | FL 3 | CT 4 | DPPH 5 | FRAP 6 | SSC 7 | pH | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Storage | 1 | 0 | 0 | −0.07 | −0.85 * | −0.3 | 0.37 * | −0.96 * | −0.19 | 0.03 | −0.73 * |
| PEF exposure | 1 | 0 | −0.07 | 0.13 | −0.24 | −0.38 * | 0.11 | 0.09 | 0.75 * | 0.30 * | |
| HPU exposure | 1 | −0.17 | −0.05 | −0.08 | −0.19 | 0.08 | −0.16 | 0.08 | 0.09 | ||
| TPC | 1 | 0.08 | 0.14 | 0.69 * | 0 | 0.74 * | 0.09 | 0.19 | |||
| HCA | 1 | 0.38 * | −0.40 * | 0.90 * | 0.18 | 0.08 | 0.68 * | ||||
| FL | 1 | −0.05 | 0.35 * | −0.05 | −0.19 | 0.16 | |||||
| CT | 1 | −0.46 * | 0.54 * | −0.33 | −0.43 * | ||||||
| DPPH | 1 | 0.17 | 0.04 | 0.74 * | |||||||
| FRAP | 1 | 0.25 | 0.26 | ||||||||
| SSC | 1 | 0.28 | |||||||||
| pH | 1 |
| TPC | HCA | FL | CT | DPPH | FRAP | |
|---|---|---|---|---|---|---|
| Storage (Days) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 7.0 |
| PEF treatment (min) | 1.5 | 1.5 | 4.5 | 1.5 | 2.7 | 3.0 |
| HPU treatment (min) | 2.5 | 7.5 | 5.8 | 3.2 | 7.5 | 7.5 |
| Optimal quantity (mg 100 g−1) | 125.81 | 35.56 | 17.36 | 116.72 | 301.22 | 958.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bebek Markovinović, A.; Stulić, V.; Putnik, P.; Birkić, A.; Jambrović, M.; Šaško, D.; Ljubičić, J.; Pavlić, B.; Herceg, Z.; Bursać Kovačević, D. Pulsed Electric Field (PEF) and High-Power Ultrasound (HPU) in the Hurdle Concept for the Preservation of Antioxidant Bioactive Compounds of Strawberry Juice—A Chemometric Evaluation—Part I. Foods 2023, 12, 3172. https://doi.org/10.3390/foods12173172
Bebek Markovinović A, Stulić V, Putnik P, Birkić A, Jambrović M, Šaško D, Ljubičić J, Pavlić B, Herceg Z, Bursać Kovačević D. Pulsed Electric Field (PEF) and High-Power Ultrasound (HPU) in the Hurdle Concept for the Preservation of Antioxidant Bioactive Compounds of Strawberry Juice—A Chemometric Evaluation—Part I. Foods. 2023; 12(17):3172. https://doi.org/10.3390/foods12173172
Chicago/Turabian StyleBebek Markovinović, Anica, Višnja Stulić, Predrag Putnik, Anamaria Birkić, Maja Jambrović, Dolores Šaško, Josipa Ljubičić, Branimir Pavlić, Zoran Herceg, and Danijela Bursać Kovačević. 2023. "Pulsed Electric Field (PEF) and High-Power Ultrasound (HPU) in the Hurdle Concept for the Preservation of Antioxidant Bioactive Compounds of Strawberry Juice—A Chemometric Evaluation—Part I" Foods 12, no. 17: 3172. https://doi.org/10.3390/foods12173172








