Assessment of Apple Peel Barrier Effect to Pesticide Permeation Using Franz Diffusion Cell and QuEChERS Method Coupled with GC-MS/MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Pesticides’ Penetration Ability
2.3. Apple and Peel Samples Preparation
2.4. Franz Cell Diffusion Tests
2.5. Chromatographic Analysis
2.6. Method Validation and Data Analysis
3. Results and Discussion
3.1. Characteristics of the Apple Peel as a Membrane
3.2. Method Optimization
3.3. Method Validation
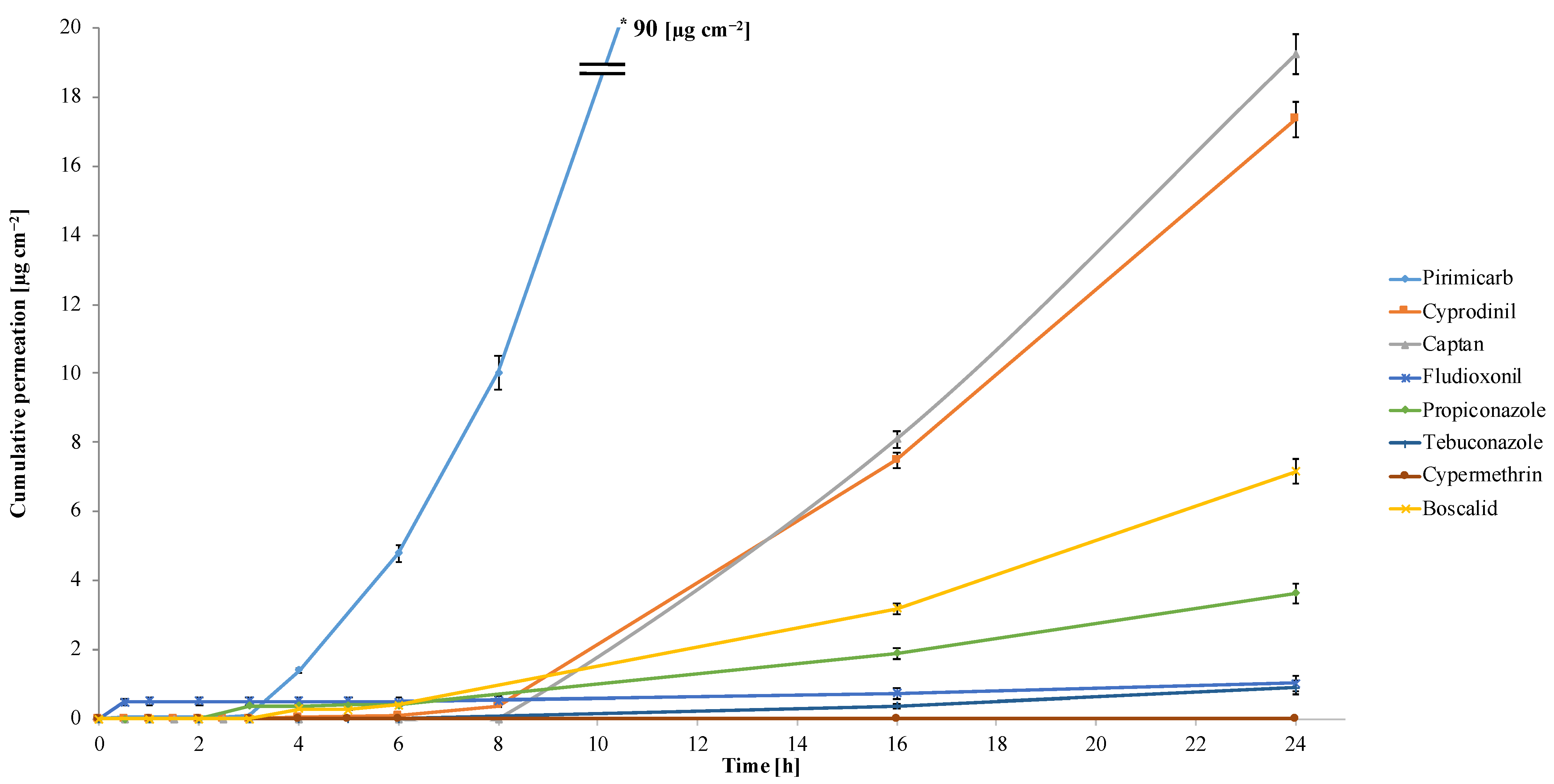
3.4. Pesticide Permeation through the Peel
3.5. Assessment of Pesticide Absorption
4. Conclusions
5. Patents
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Claeys, W.L.; Schmit, J.F.; Bragard, C.; Maghuin-Rogister, G.; Pussemier, L.; Schiffers, B. Exposure of several Belgian consumer groups to pesticide residues through fresh fruit and vegetable consumption. Food Control 2011, 22, 508–516. [Google Scholar] [CrossRef]
- Lozowicka, B. Health risk for children and adults consuming apples with pesticide residue. Sci. Total Environ. 2015, 502, 184–198. [Google Scholar] [CrossRef]
- Poulsen, M.E.; Wenneker, M.; Withagen, J.; Christensen, H.B. Pesticide Residues in Individual versus Composite Samples of Apples after Fine or Coarse Spray Quality Application. Crop Prot. 2012, 35, 5–14. [Google Scholar] [CrossRef]
- Calderon, R.; García-Hernández, J.; Palma, P.; Leyva-Morales, J.B.; Zambrano-Soria, M.; Bastidas-Bastidas, P.J.; Godoy, M. Assessment of pesticide residues in vegetables commonly consumed in Chile and Mexico: Potential impacts for public health. J. Food Compos. Anal. 2022, 108, 104420. [Google Scholar] [CrossRef]
- Carrasco Cabrera, L.; Medina Pastor, P.; Dujardin, B.; Fuertes, R.; Greco, L.; Levorato, S.; Mohimont, L.; Reich, H.; Rossi, G.; Terry, S.; et al. The 2020 European Union report on pesticide residues in food. EFSA J. 2022, 20, e07215. [Google Scholar] [CrossRef]
- Klein, B.P.; Sciences, N. Nutritional consequences of minimal processing of fruits and vegetables. J. Food Qual. 1987, 10, 179–193. [Google Scholar] [CrossRef]
- Chung, S.W.C. How effective are common household preparations on removing pesticide residues from fruit and vegetables? A review. J. Sci. Food Agric. 2018, 98, 2857–2870. [Google Scholar] [CrossRef] [PubMed]
- Hrynko, I.; Kaczyński, P.; Pietruszyńska, M.; Łozowicka, B. The effect of food thermal processes on the residue concentration of systemic and non-systemic pesticides in apples. Food Control 2023, 143, 109267. [Google Scholar] [CrossRef]
- Rutkowska, E.; Wołejko, E.; Kaczyński, P.; Łuniewski, S.; Łozowicka, B. High and Low Temperature Processing: Effective Tool Reducing Pesticides in/on Apple Used in a Risk Assessment of Dietary Intake Protocol. Chemosphere 2023, 313, 137498. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, B.N.; Mir, M.M.; Sofi, S.A.; Shah, M.A.; Sidiq, T.; Sunooj, K.V.; Hamdani, A.M.; Mousavi Khaneghah, A. Current strategies for the reduction of pesticide residues in food products. J. Food Compos. Anal. 2022, 106, 104274. [Google Scholar] [CrossRef]
- Zhang, A.A.; Sutar, P.P.; Bian, Q.; Fang, X.M.; Ni, J.B.; Xiao, H.W. Pesticide Residue Elimination for Fruits and Vegetables: The Mechanisms, Applications, and Future Trends of Thermal and Non-Thermal Technologies. J. Future Foods 2022, 2, 223–240. [Google Scholar] [CrossRef]
- Omeroglu, P.Y.; Acoglu Celik, B.; Koc Alibasoglu, E. The Effect of Household Food Processing on Pesticide Residues in Oranges (Citrus sinensis). Foods 2022, 11, 3918. [Google Scholar] [CrossRef]
- Yang, T.; Doherty, J.; Zhao, B.; Kinchla, A.J.; Clark, J.M.; He, L. Effectiveness of Commercial and Homemade Washing Agents in Removing Pesticide Residues on and in Apples. J. Agric. Food Chem. 2017, 65, 9744–9752. [Google Scholar] [CrossRef] [PubMed]
- Calvaruso, E.; Cammilleri, G.; Pulvirenti, A.; Lo Dico, G.M.; Lo Cascio, G.; Giaccone, V.; Vitale Badaco, V.; Ciprì, V.; Alessandra, M.M.; Vella, A.; et al. Residues of 165 pesticides in citrus fruits using LC-MS/MS: A study of the pesticides distribution from the peel to the pulp. Nat. Prod. Res. 2020, 34, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.A.; Hayes, A.L.; Butler, R.C. Physicochemical properties of agrochemicals: Their effects on foliar penetration. Pestic. Sci. 1992, 34, 167–182. [Google Scholar] [CrossRef]
- Chen, B.; Johnson, E.J.; Chefetz, B.; Zhu, L.; Xing, B. Sorption of polar and nonpolar aromatic organic contaminants by plant cuticular materials: Role of polarity and accessibility. Environ. Sci. Technol. 2005, 39, 6138–6146. [Google Scholar] [CrossRef]
- Li, Y.; Deng, Y.; Chen, B. Sorption of chlorophenols onto fruit cuticles and potato periderm. J. Environ. Sci. 2012, 24, 675–681. [Google Scholar] [CrossRef]
- Fantke, P.; Wieland, P.; Wannaz, C.; Friedrich, R.; Jolliet, O. Dynamics of pesticide uptake into plants: From system functioning to parsimonious modeling. Environ. Modell. Softw. 2013, 40, 316–324. [Google Scholar] [CrossRef]
- Noore, S.; Ramesh, G.; Vendan, S.E.; Nagaraju, V.D. Persistence and diffusion behaviour of chlorpyrifos in five different species of vegetables: A comparative analysis. Ecotoxicol. Environ. Saf. 2021, 217, 112208. [Google Scholar] [CrossRef]
- Xu, X.M.; Yu, S.; Li, R.; Fan, J.; Chen, S.H.; Shen, H.T.; Han, J.L.; Huang, B.F.; Ren, Y.P. Distribution and Migration Study of Pesticides Between Peel and Pulp in Grape by online Gel Permeation Chromatography–Gas Chromatography/Mass Spectrometry. Food Chem. 2012, 135, 161–169. [Google Scholar] [CrossRef]
- Yang, T.; Zhao, B.; Hou, R.; Zhang, Z.; Kinchla, A.J.; Clark, J.M.; He, L. Evaluation of the Penetration of Multiple Classes of Pesticides in Fresh Produce Using Surface-Enhanced Raman Scattering Mapping. J. Food Sci. 2016, 81, T2891–T2901. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.-Y.; Chen, Y.; Wang, S.-Y.; Shi, X.-C.; Herrera-Balandrano, D.D.; Polo, V.; Laborda, P. Peel Diffusion and Antifungal Efficacy of Different Fungicides in Pear Fruit: Structure-Diffusion-Activity Relationships. J. Fungi 2022, 8, 547. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tian, Q.; Li, H.; Dai, L.; Wan, Y.; Wang, M.; Han, B.; Huang, H.; Zhang, Y.; Chen, J. Visualization and Metabolome for the Migration and Distribution Behavior of Pesticides Residue in after-Ripening of Banana. J. Hazard. Mater. 2023, 446, 130665. [Google Scholar] [CrossRef]
- Commission Directive. 2002/63/EC of 11 July 2002 establishing Community methods of sampling for the official control of pesticide residues in and on products of plant and animal origin and repealing Directive 79/700/EEC. Off. J. Eur. Commun. 2002, 187, 30–43. Available online: http://data.europa.eu/eli/dir/2002/63/oj (accessed on 3 July 2023).
- Wang, C.J.; Liu, Z.Q. Foliar Uptake of Pesticides—Present Status and Future Challenge. Pestic. Biochem. Physiol. 2007, 87, 1–8. [Google Scholar] [CrossRef]
- Tankiewicz, M. Determination of Selected Priority Pesticides in High Water Fruits and Vegetables by Modified QuEChERS and GC-ECD with GC-MS/MS Confirmation. Molecules 2019, 24, 417. [Google Scholar] [CrossRef]
- Tankiewicz, M.; Berg, A. Improvement of the QuEChERS Method Coupled with GC–MS/MS for the Determination of Pesticide Residues in Fresh Fruit and Vegetables. Microchem. J. 2022, 181, 107794. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, X.; Jia, Y.; Ho, C.T.; Huang, Q. Applications and Delivery Mechanisms of Hyaluronic Acid used for Topical/Transdermal Delivery—A review. Int. J. Pharm. 2020, 578, 119127. [Google Scholar] [CrossRef]
- OECD. Guidelines for the Testing of Chemicals. In Test No. 428: Skin Absorption: In Vitro Method; OECD Publishing: Paris, France, 2004; Section 4. [Google Scholar] [CrossRef]
- Thakker, K.; Klein, R. Drug release: Topical products. In Specification of Drug Substances and Products: Development and Validation of Analytical Methods, 2nd ed.; Riley, C.M., Rosanske, T.W., Reid, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 505–534. [Google Scholar] [CrossRef]
- U.S. FDA Center for Drug Evaluation and Research, Nonsterile Semisolid Dosage Forms, Scale-Up and Postapproval Changes: Chemistry, Manufacturing, and Controls; In Vitro Release Testing and In Vivo Bioequivalence Documentation. Available online: https://www.fda.gov/media/71141/download (accessed on 3 July 2023).
- Morganti, P.; Ruocco, E.; Wolf, R.; Ruocco, V. Percutaneous absorption and delivery systems. Clin. Dermatol. 2001, 19, 489–501. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- EU Pesticides Database—MRLs. [WWW Document]. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/mrls (accessed on 3 July 2023).
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- European Commission. SANTE/11312/2021, Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/SANTE_11312_2021.pdf (accessed on 3 July 2023).
- Regulation (EU). No 396/2005 on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin, and Amending Council Directive 91/414/EEC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ%3AL%3A2005%3A070%3ATOC (accessed on 3 July 2023).
- Khanal, B.P.; Knoche, M. Mechanical Properties of Apple Skin Are Determined by Epidermis and Hypodermis. J. Am. Soc. Hort. Sci. 2014, 139, 139–147. [Google Scholar] [CrossRef]
- Zhang, Y.L.; You, C.X.; Li, Y.Y.; Hao, Y.J. Advances in Biosynthesis, Regulation, and Function of Apple Cuticular Wax. Front. Plant Sci. 2020, 11, 1165. [Google Scholar] [CrossRef] [PubMed]
- Riccio, R.; Trevisan, M.; Capri, E. Effect of Surface Waxes on the Persistence of Chlorpyrifos-methyl in Apples, Strawberries and Grapefruits. Food Addit. Contam. 2006, 23, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Konarska, A. The structure of the fruit peel in two varieties of Malus domestica Borkh. (Rosaceae) before and after storage. Protoplasma 2013, 250, 701–714. [Google Scholar] [CrossRef]
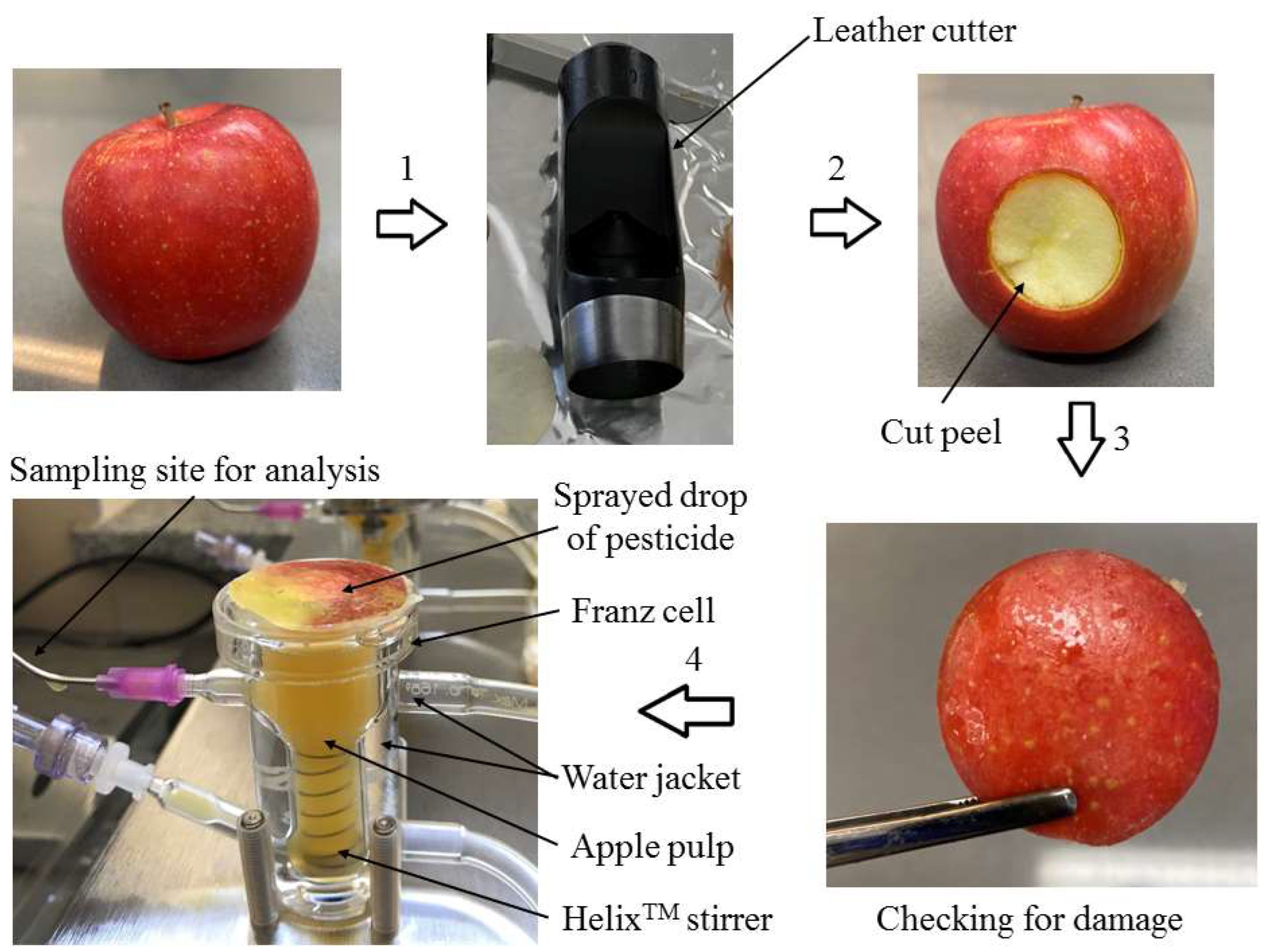
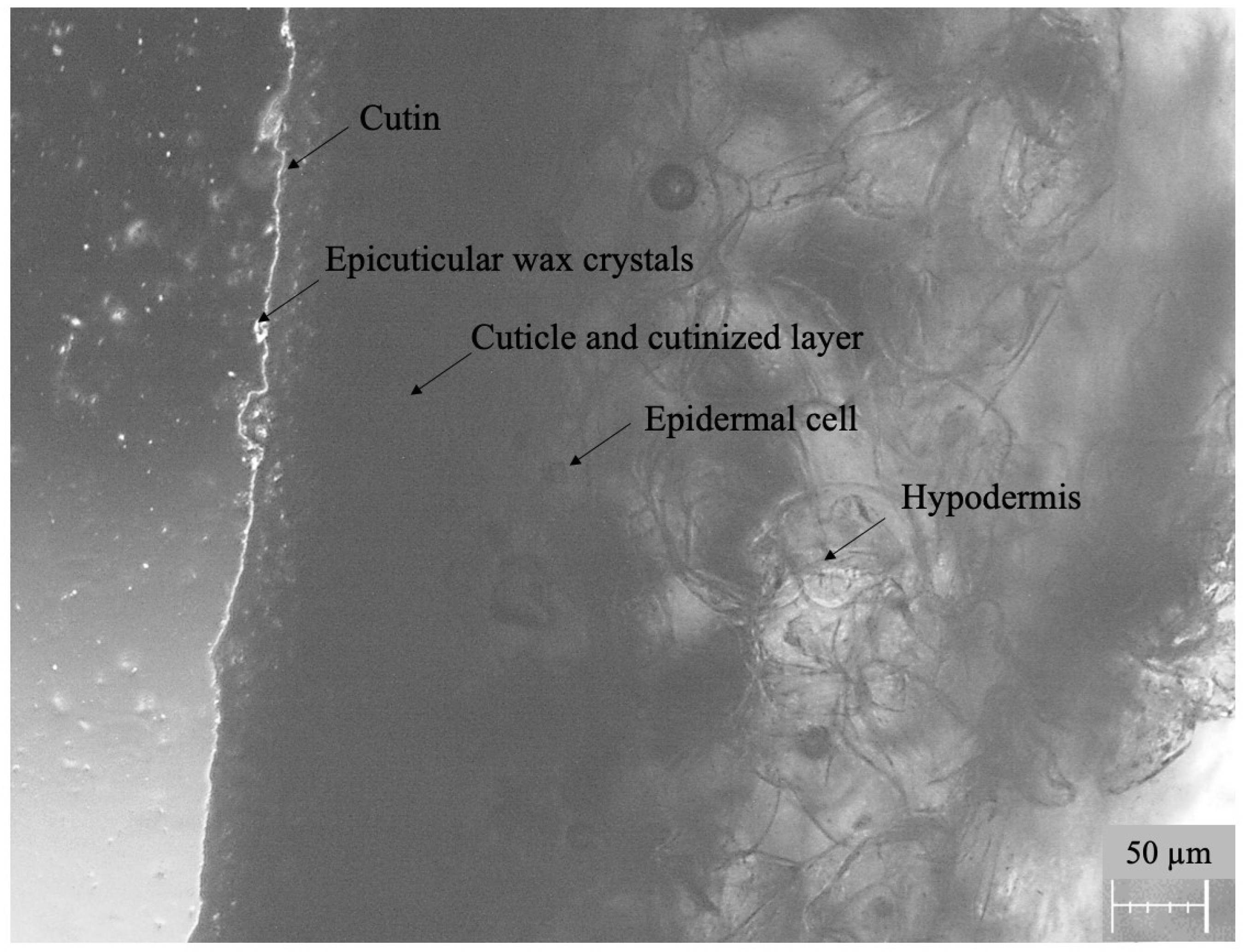
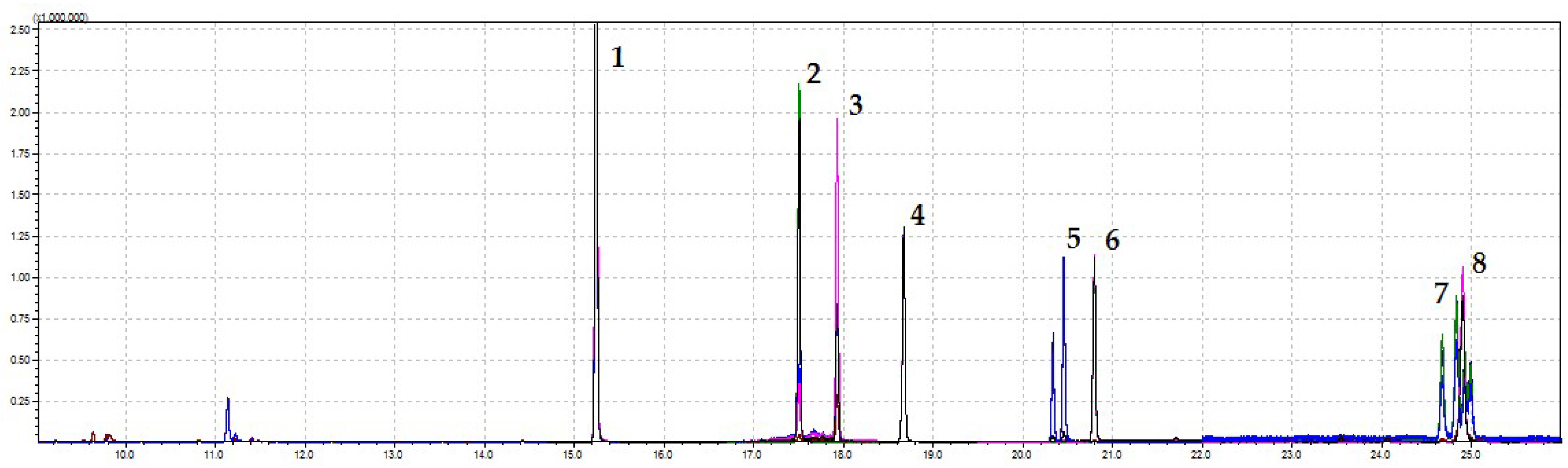
| No. | Analyte | Retention Time [min] | Time Window [min.] | Monitored Ions b [m/z] | Equation | Coefficient of Determination R2 | Limit of Detection LOD [mg kg−1] | Linearity Range [mg kg−1] | Coefficient of Variability CV [%] | Recovery (RSD) [%] of Analyte (n = 5) | Matrix Effect [%] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 mg kg−1 | 1 mg kg−1 | 0.1 mg kg−1 | ||||||||||
| 1. | Boscalid | 24.89 | 22.00–26.00 | 140, 112, 166 | y = 5,578,169x – 250,461 | 0.9996 | 0.0043 | 0.013–10 | 3.9 | 85 (12) | 98 (1.6) | 19 |
| 2. | Captan | 17.92 | 17.70–18.40 | 79, 117, 149 | y = 3,804,694x + 212,979 | 0.9951 | 0.017 | 0.051–10 | 5.6 | 112 (7.8) | 103 (2.6) | 13 |
| 3. | Cypermethrin | 24.61–25.05 | 22.00–26.00 | 163, 181, 127 | y = 1,716,500x + 10,859 | 0.9992 | 0.0057 | 0.017–10 | 3.3 | 89 (8.0) | 99 (6.9) | 11 |
| 4. | Cyprodinil | 17.50 | 17.20–17.70 | 224, 225, 77 | y = 9,308,814x − 9998 | 0.9990 | 0.0072 | 0.022–10 | 3.4 | 88 (9.1) | 97 (4.0) | −12 |
| 5. | Fludioxonil | 18.67 | 18.40–19.50 | 248, 127, 154 | y = 3,559,193x – 423,457 | 0.9993 | 0.0074 | 0.022–10 | 4.8 | 92 (11) | 99 (8.9) | −4.4 |
| 6. | Pirimicarb | 15.24 | 14.70–17.20 | 166, 72, 238 | y = 14,653,557x – 96,622 | 0.9990 | 0.0026 | 0.0078–10 | 1.7 | 93 (4.7) | 97 (5.8) | 16 |
| 7. | Propiconazole | 20.28–20.60 | 19.50–22.00 | 173, 175, 259 | y = 3,471,709x – 309,348 | 0.9995 | 0.0060 | 0.018–10 | 3.0 | 98 (10) | 98 (1.2) | −18 |
| 8. | Tebuconazole | 20.79 | 19.50–22.00 | 125, 250, 70 | y = 3,441,188x – 515,312 | 0.9987 | 0.0044 | 0.013–10 | 3.4 | 93 (6.8) | 99 (4.3) | 10 |
| No. | Analyte | Maximum Residue Limits (MRLs) in Apples [mg kg−1] | Acceptable Daily Intake (ADI) [mg kg−1 Body Weight per Day] | Acute Reference Dose (ARfD) [mg kg−1 Body Weight] | Mean Concentration Values ± SD (with p Value *) in Apples after 24 h of Permeation [mg kg−1] (n = 3) | Cumulative Permeation after 24 h of Permeation [µg cm−2] | Absorption Flux [µg cm−2 per Hour] | Total Absorption [%] |
|---|---|---|---|---|---|---|---|---|
| 1. | Boscalid | 2.0 | 0.040 | not applicable | 1.8 ± 0.011 (p = 0.000015) | 7.2 | 0.30 | 2.5 |
| 2. | Captan | 10 | 0.10 | 0.30 | 4.8 ± 0.039 (p = 0.000011) | 19 | 0.79 | 6.8 |
| 3. | Cypermethrin | 1.0 | 0.0050 | 0.0050 | <LOQ | <LOQ | <LOQ | <LOQ |
| 4. | Cyprodinil | 2.0 | 0.030 | not applicable | 4.4 ± 0.028 (p = 0.000008) | 17 | 0.71 | 6.2 |
| 5. | Fludioxonil | 5.0 | 0.37 | not applicable | 0.26 ± 0.0074 (p = 0.003373) | 1.1 | 0.050 | 0.36 |
| 6. | Pirimicarb | 0.5 | 0.035 | 0.10 | 23 ± 0.13 (p = 0.000010) | 90 | 3.8 | 32 |
| 7. | Propiconazole | 0.010 | 0.040 | 0.10 | 0.92 ± 0.0089 (p = 0.000021) | 3.6 | 0.15 | 1.3 |
| 8. | Tebuconazole | 0.30 | 0.030 | 0.030 | 0.23 ± 0.0011 (p = 0.000031) | 0.91 | 0.040 | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tankiewicz, M. Assessment of Apple Peel Barrier Effect to Pesticide Permeation Using Franz Diffusion Cell and QuEChERS Method Coupled with GC-MS/MS. Foods 2023, 12, 3220. https://doi.org/10.3390/foods12173220
Tankiewicz M. Assessment of Apple Peel Barrier Effect to Pesticide Permeation Using Franz Diffusion Cell and QuEChERS Method Coupled with GC-MS/MS. Foods. 2023; 12(17):3220. https://doi.org/10.3390/foods12173220
Chicago/Turabian StyleTankiewicz, Maciej. 2023. "Assessment of Apple Peel Barrier Effect to Pesticide Permeation Using Franz Diffusion Cell and QuEChERS Method Coupled with GC-MS/MS" Foods 12, no. 17: 3220. https://doi.org/10.3390/foods12173220
APA StyleTankiewicz, M. (2023). Assessment of Apple Peel Barrier Effect to Pesticide Permeation Using Franz Diffusion Cell and QuEChERS Method Coupled with GC-MS/MS. Foods, 12(17), 3220. https://doi.org/10.3390/foods12173220






