Cellulose Isolation from Tomato Pomace: Part II—Integrating High-Pressure Homogenization in a Cascade Hydrolysis Process for the Recovery of Nanostructured Cellulose and Bioactive Molecules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Chemicals
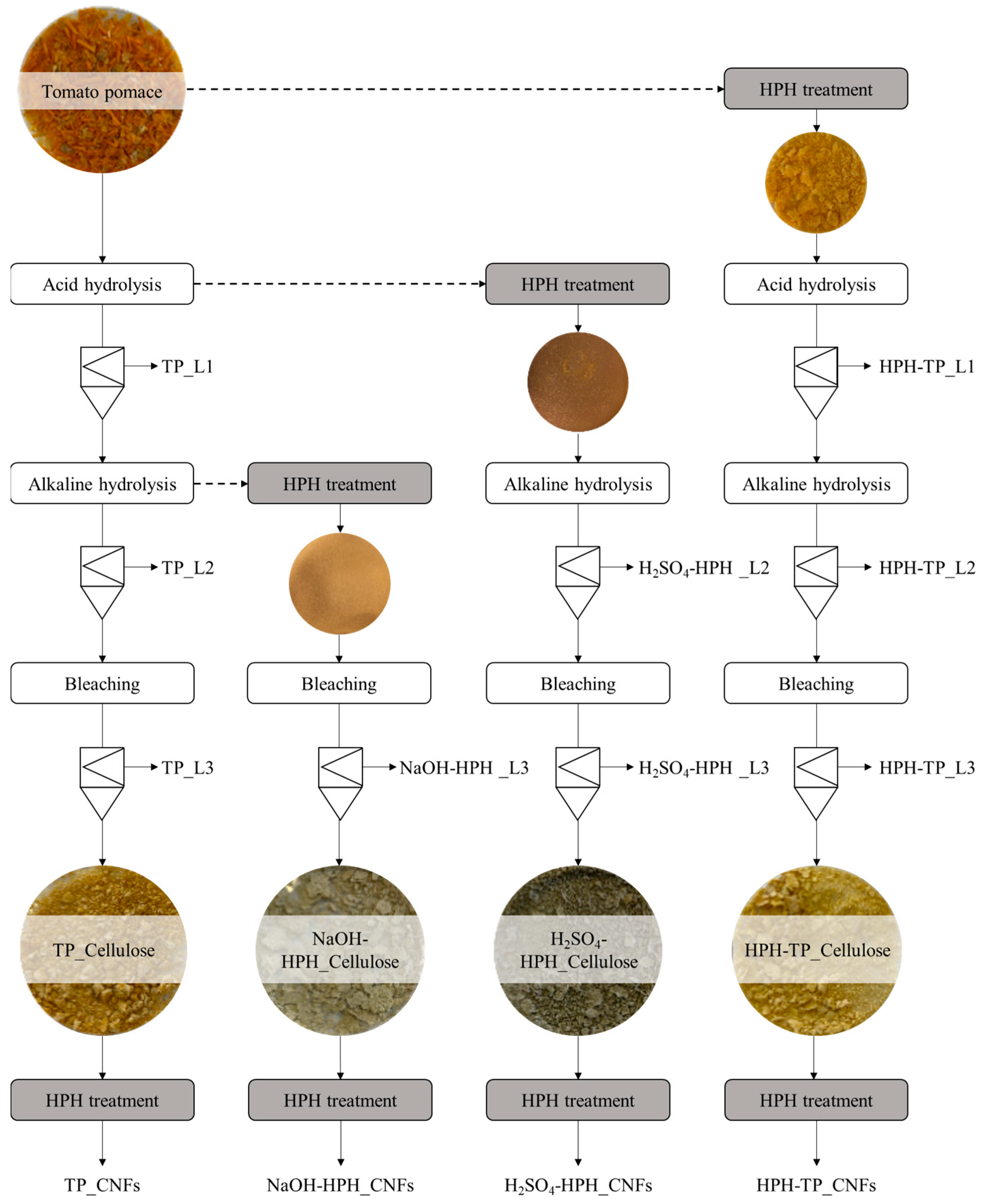
2.3. Isolation of Cellulose from TP
2.4. Isolation of Cellulose Nanofibrillated (CNFs) from TP Cellulose Pulp
2.5. Dyalysis Purification of Side Streams
2.6. Liquor Side Streams Analysis
2.7. Chemico-Physical Characterization
2.7.1. Structural Carbohydrates Determination
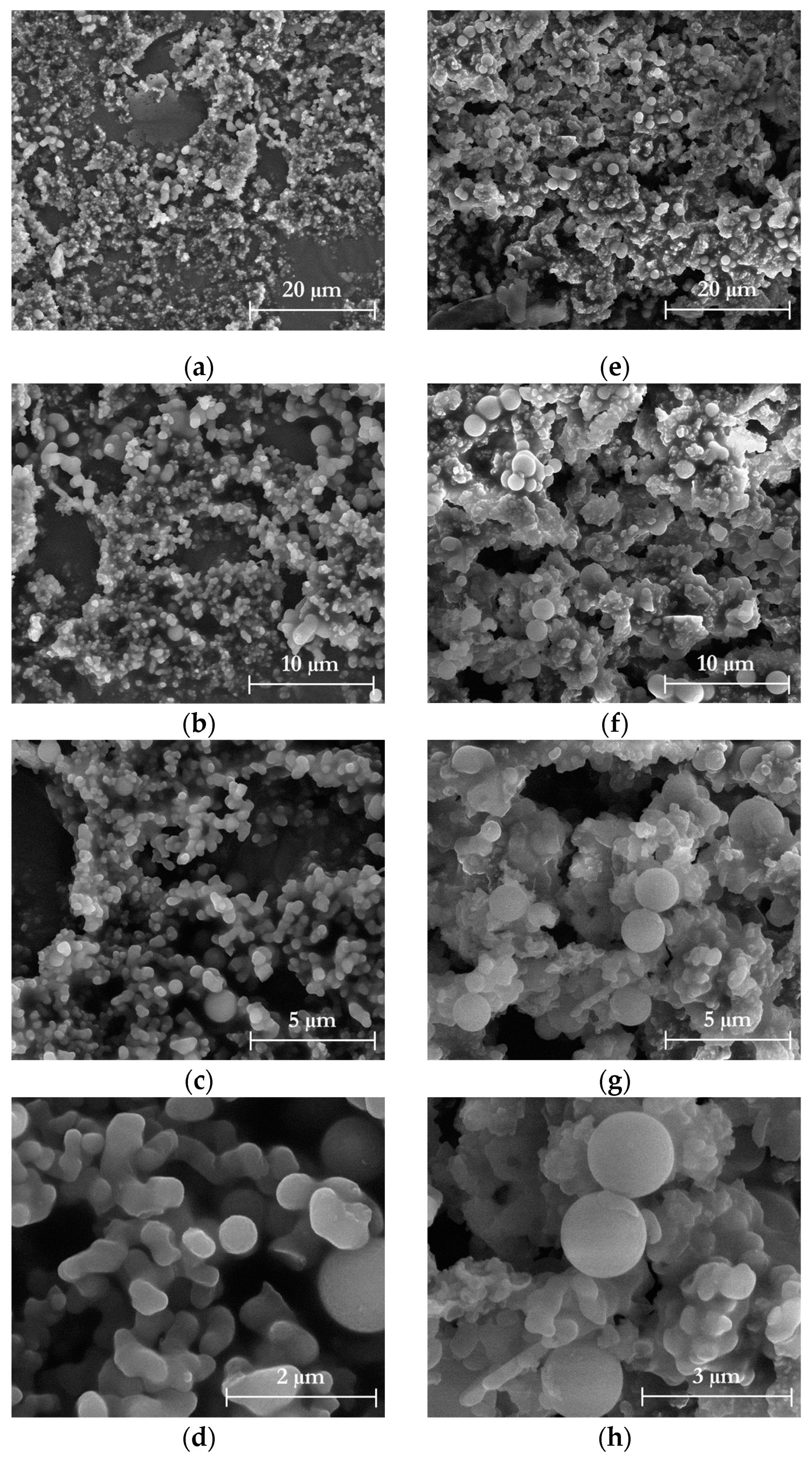
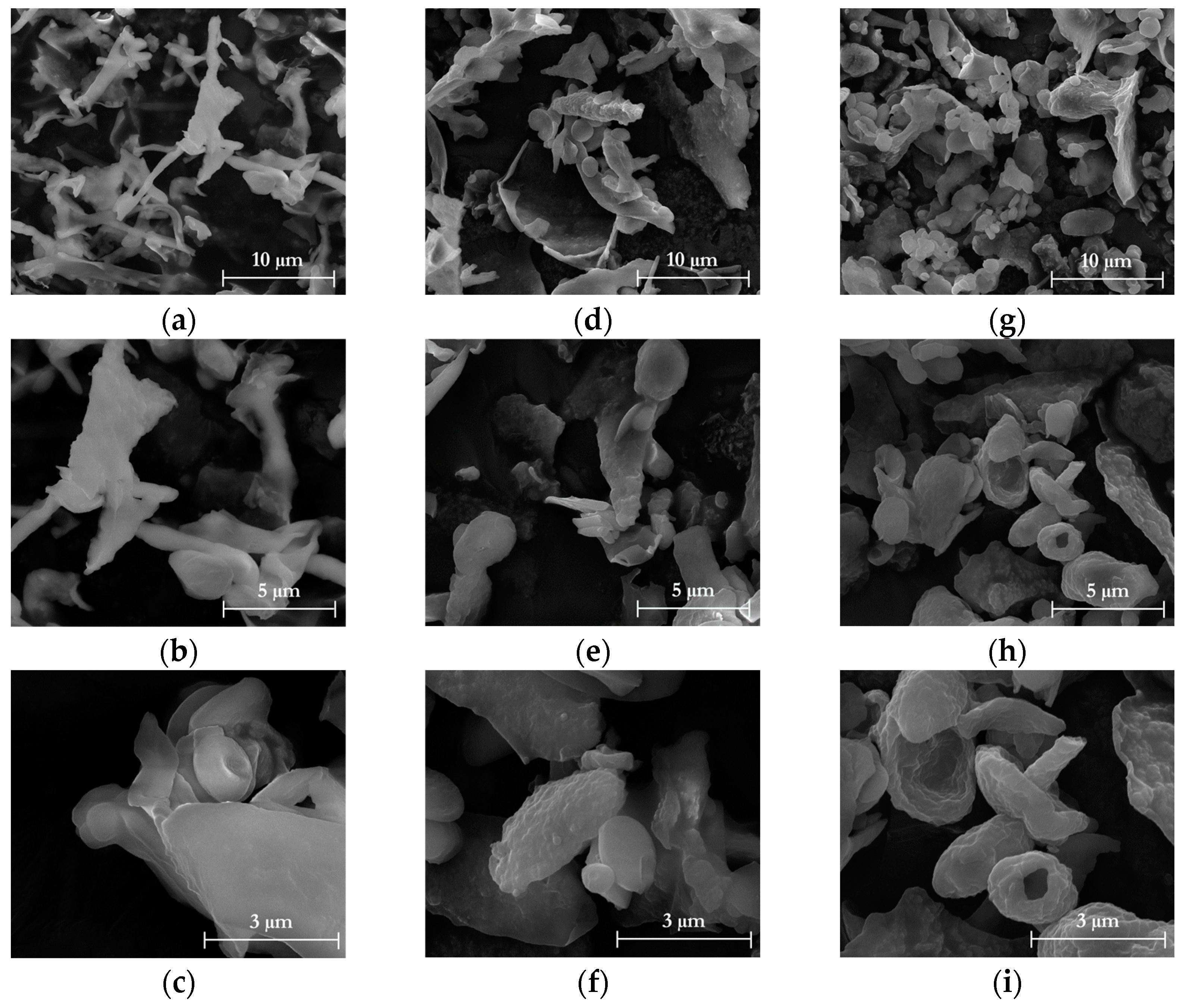
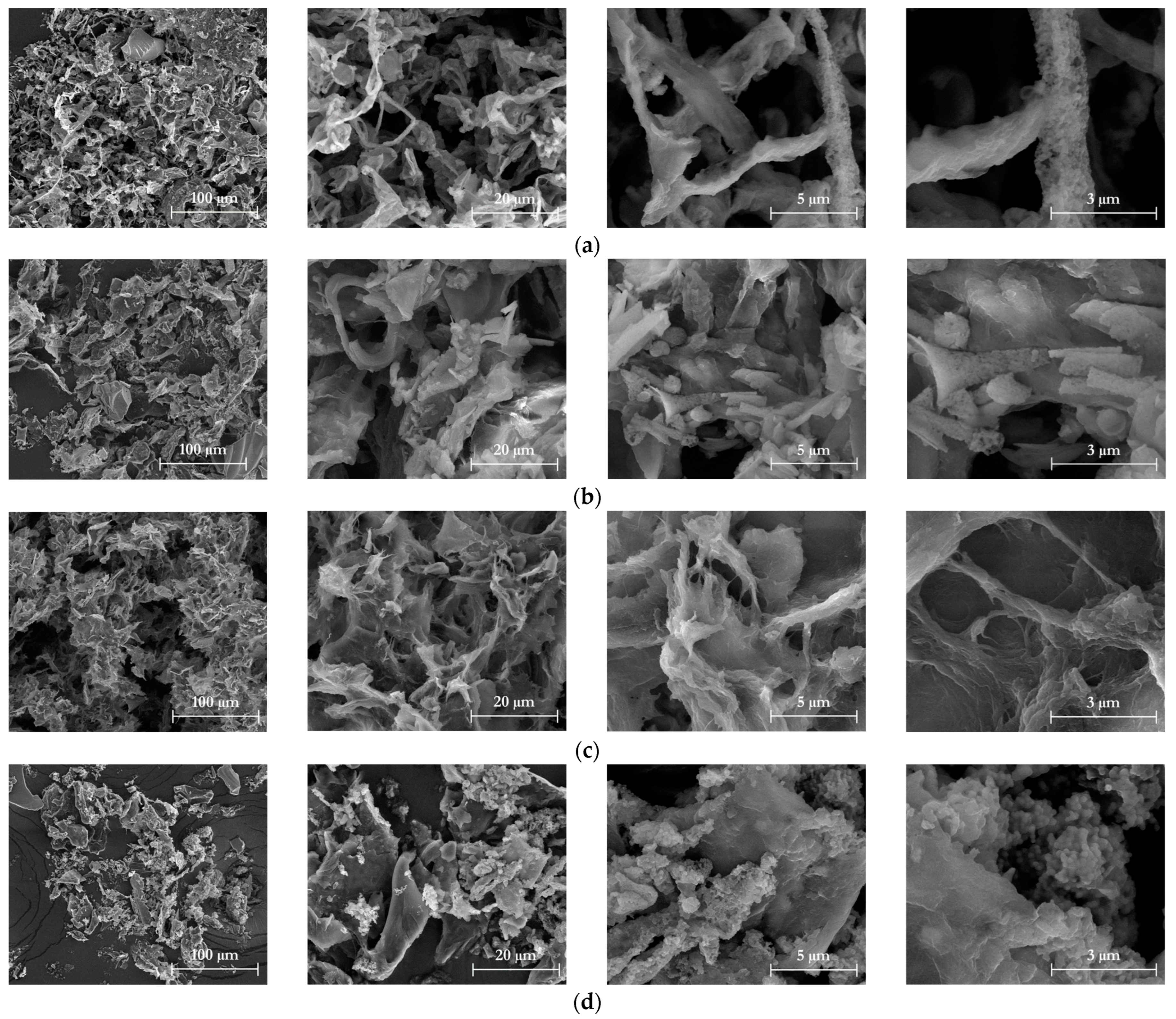
2.7.2. Morphological Properties
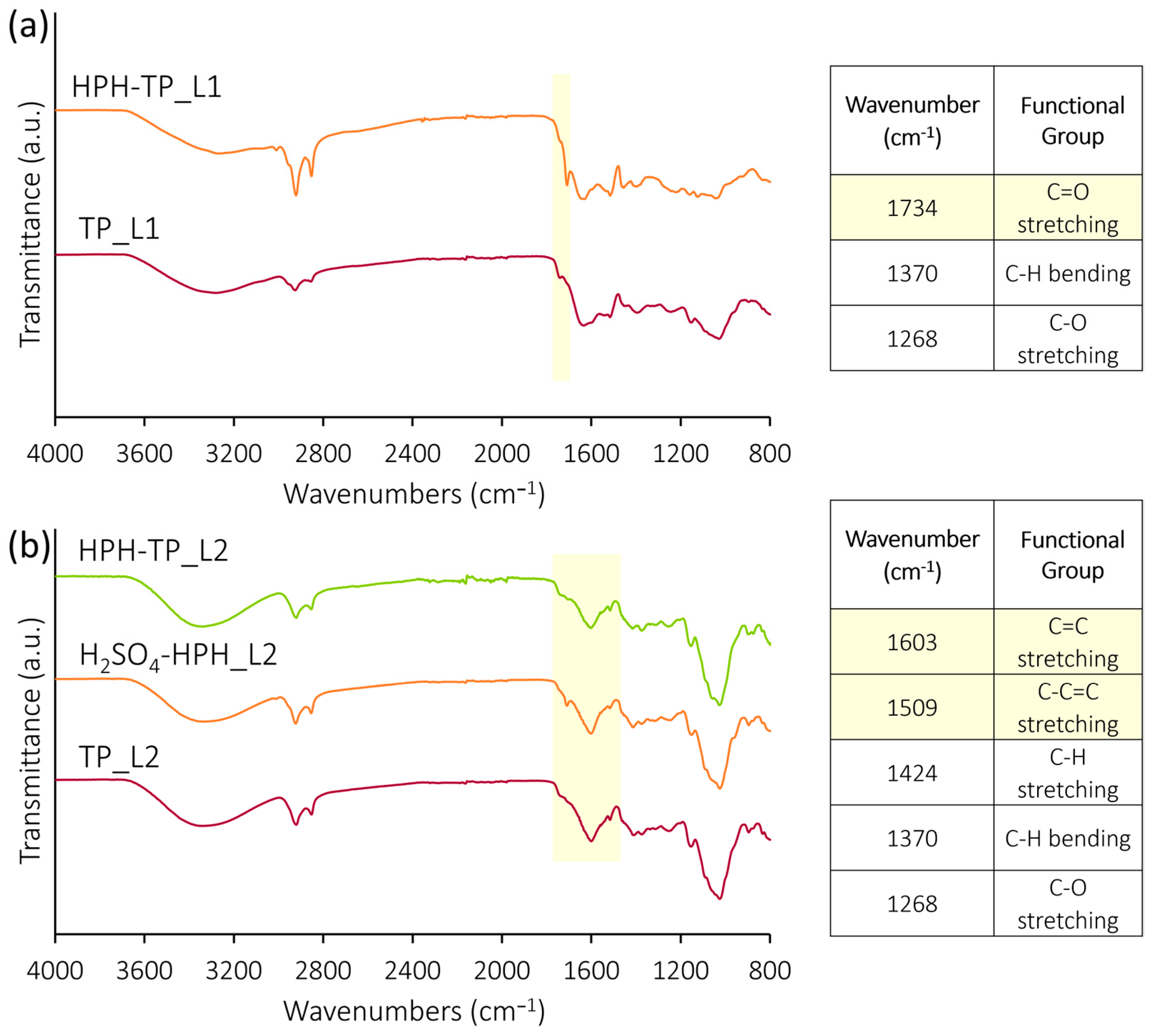
2.7.3. FT-IR Analysis
2.7.4. Particle Size Distribution (PSD)
2.7.5. Contact Angle Analysis
2.7.6. Adsorption Behavior at Water–Oil Interface
2.8. Statistical Analysis
3. Results and Discussion
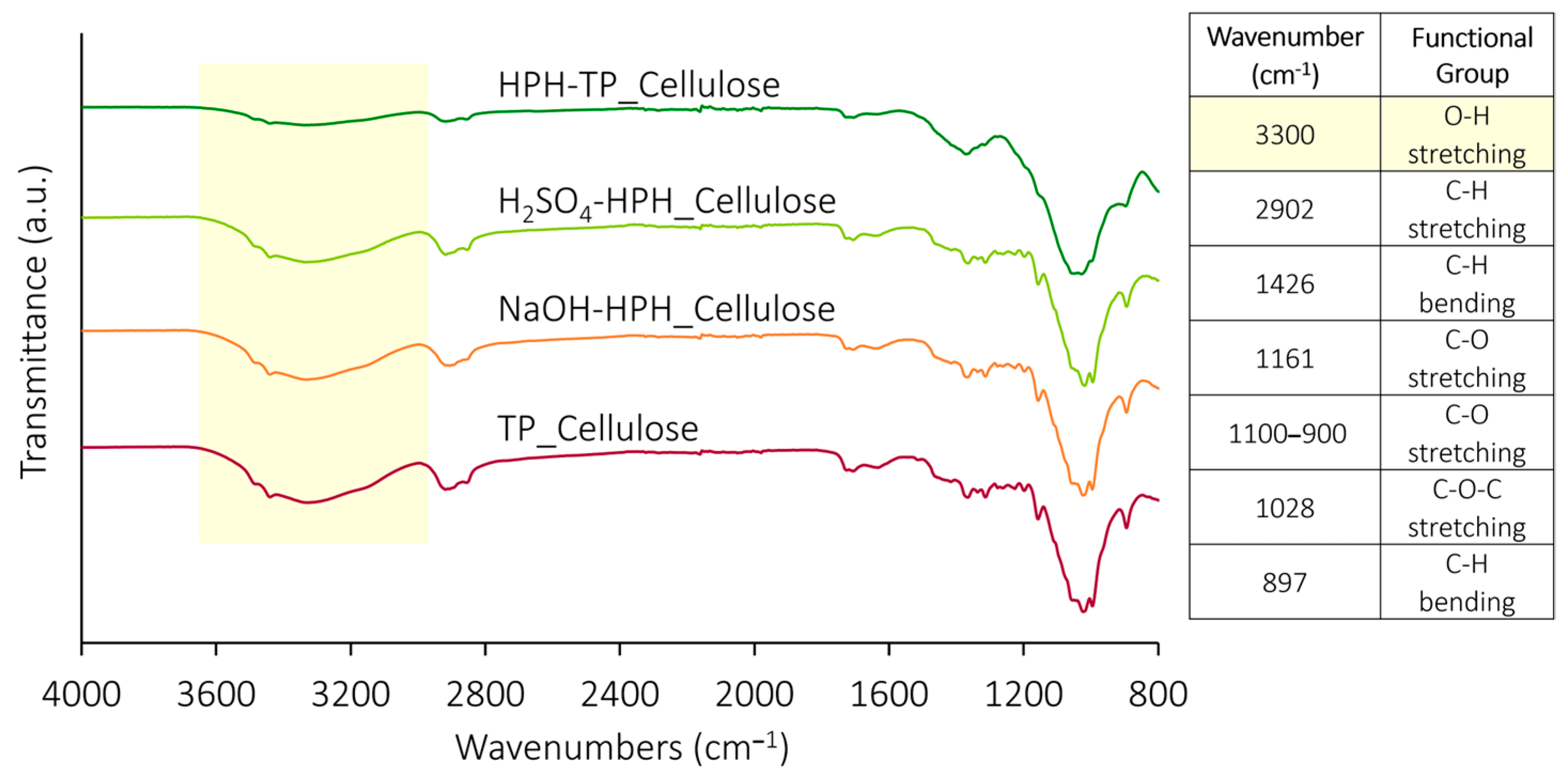
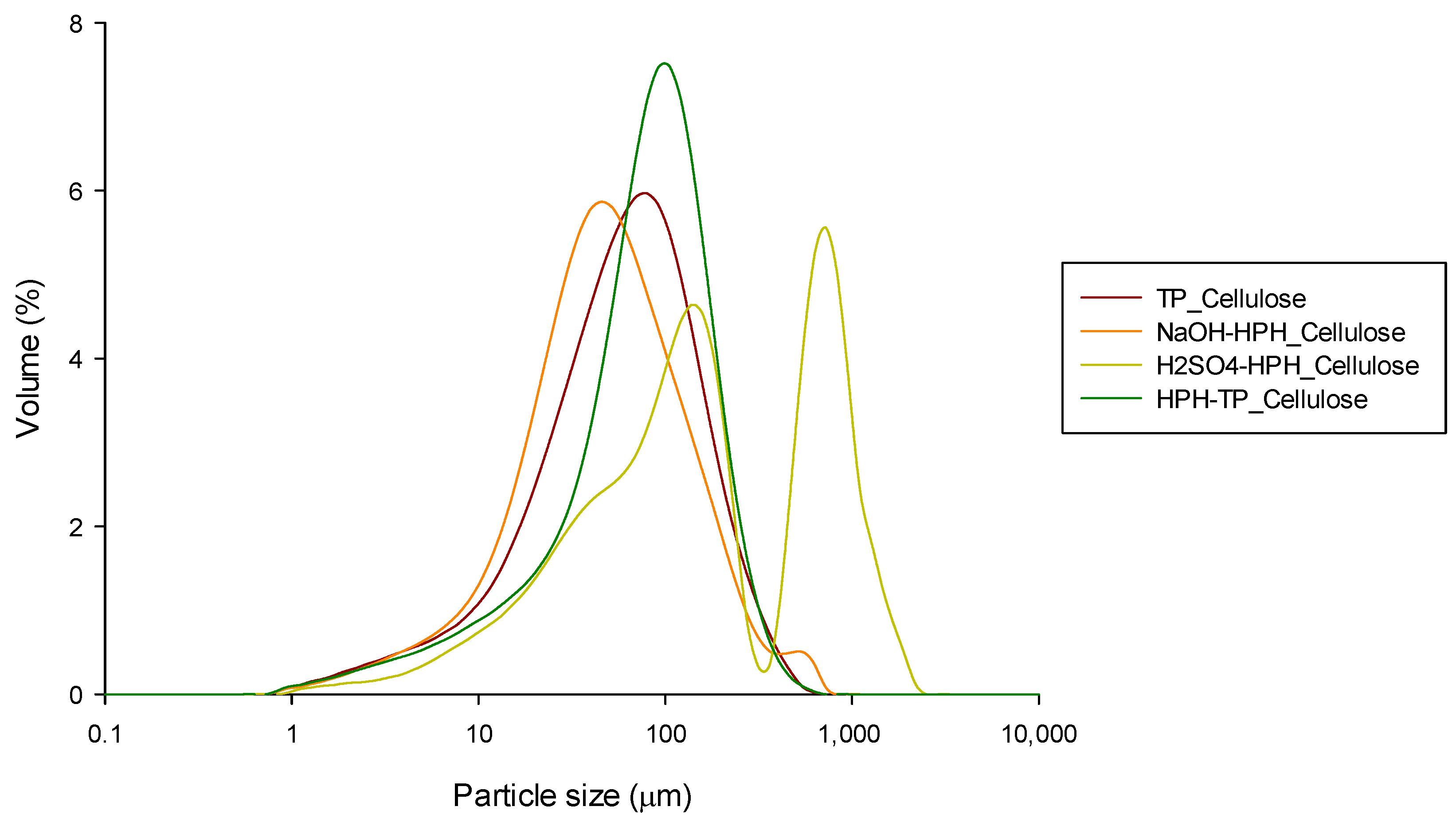
3.1. Chemical and Morphological Characteristics of Isolated Cellulose
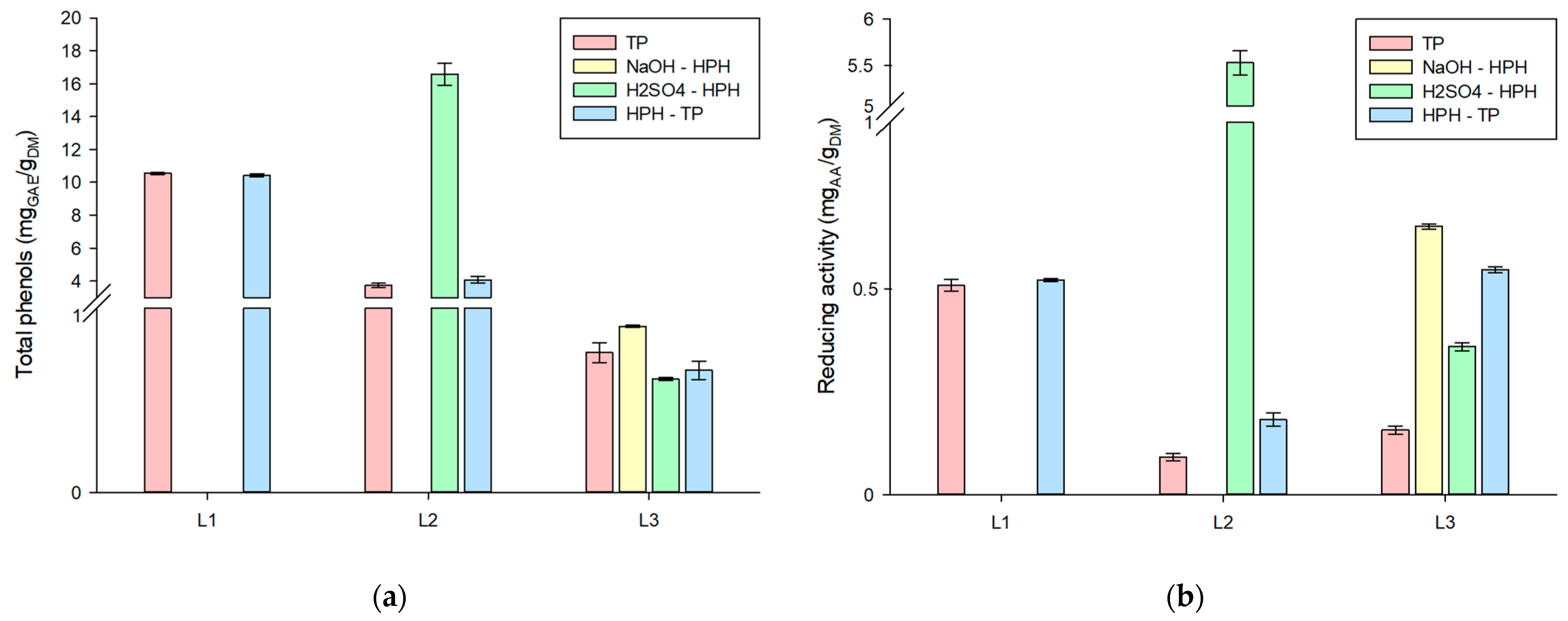
3.2. Side Streams Liquors
Biological Properties
3.3. Structural Carbohydrates and Chemical and Morphological Characteristics
3.4. Chemical and Morphological Characteristics of Isolated Nanocellulose
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamm, B.; Gruber, P.; Kamm, M. Biorefineries-Industrial Processes and Products: Status Quo and Future Directions; Kamm, B., Gruber, P.R., Kamm, M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005. [Google Scholar]
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef] [PubMed]
- Reshmy, R.; Thomas, D.; Philip, E.; Paul, S.A.; Madhavan, A.; Sindhu, R.; Sirohi, R.; Varjani, S.; Pugazhendhi, A.; Pandey, A.; et al. Bioplastic production from renewable lignocellulosic feedstocks: A review. Rev. Environ. Sci. Bio/Technol. 2021, 20, 167–187. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Michelin, M.; Vicente, A.A.; Teixeira, J.A.; Cerqueira, M.Â. Lignocellulosic Materials: Sources and Processing Technologies. In Lignocellulosic Materials and Their Use in Bio-based Packaging. SpringerBriefs in Molecular Science; Springer: Cham, Switzerland, 2018; pp. 13–33. ISBN 978-3-319-92940-8. [Google Scholar]
- Pirozzi, A.; Ferrari, G.; Donsì, F. Cellulose Isolation from Tomato Pomace Pretreated by High-Pressure Homogenization. Foods 2022, 11, 266. [Google Scholar] [CrossRef]
- Carullo, D.; Abera, B.D.; Casazza, A.A.; Donsì, F.; Perego, P.; Ferrari, G.; Pataro, G. Effect of pulsed electric fields and high pressure homogenization on the aqueous extraction of intracellular compounds from the microalgae Chlorella vulgaris. Algal Res. 2018, 31, 60–69. [Google Scholar] [CrossRef]
- Hafid, H.S.; Omar, F.N.; Zhu, J.; Wakisaka, M. Enhanced crystallinity and thermal properties of cellulose from rice husk using acid hydrolysis treatment. Carbohydr. Polym. 2021, 260, 117789. [Google Scholar] [CrossRef] [PubMed]
- Dhali, K.; Ghasemlou, M.; Daver, F.; Cass, P.; Adhikari, B. A review of nanocellulose as a new material towards environmental sustainability. Sci. Total Environ. 2021, 775, 145871. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, H.; Vignolini, S. Recent Progress in Production Methods for Cellulose Nanocrystals: Leading to More Sustainable Processes. Adv. Sustain. Syst. 2022, 6, 2100100. [Google Scholar] [CrossRef]
- Tang, L.; Huang, B.; Ou, W.; Chen, X.; Chen, Y. dan Manufacture of cellulose nanocrystals by cation exchange resin-catalyzed hydrolysis of cellulose. Bioresour. Technol. 2011, 102, 10973–10977. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Yu, G.; Yu, Q.; Li, B.; Mu, X. A novel approach for the preparation of nanocrystalline cellulose by using phosphotungstic acid. Carbohydr. Polym. 2014, 110, 415–422. [Google Scholar] [CrossRef]
- Gao, A.; Chen, H.; Tang, J.; Xie, K.; Hou, A. Efficient extraction of cellulose nanocrystals from waste Calotropis gigantea fiber by SO42−/TiO2 nano-solid superacid catalyst combined with ball milling exfoliation. Ind. Crops Prod. 2020, 152, 112524. [Google Scholar] [CrossRef]
- Kontturi, E.; Meriluoto, A.; Penttilä, P.A.; Baccile, N.; Malho, J.M.; Potthast, A.; Rosenau, T.; Ruokolainen, J.; Serimaa, R.; Laine, J.; et al. Degradation and Crystallization of Cellulose in Hydrogen Chloride Vapor for High-Yield Isolation of Cellulose Nanocrystals. Angew. Chem. Int. Ed. 2016, 55, 14455–14458. [Google Scholar] [CrossRef]
- Leboucher, J.; Bazin, P.; Goux, D.; El Siblani, H.; Travert, A.; Barbulée, A.; Bréard, J.; Duchemin, B. High-yield cellulose hydrolysis by HCl vapor: Co-crystallization, deuterium accessibility and high-temperature thermal stability. Cellulose 2020, 27, 3085–3105. [Google Scholar] [CrossRef]
- Man, Z.; Muhammad, N.; Sarwono, A.; Bustam, M.A.; Kumar, M.V.; Rafiq, S. Preparation of Cellulose Nanocrystals Using an Ionic Liquid. J. Polym. Environ. 2011, 19, 726–731. [Google Scholar] [CrossRef]
- Lazko, J.; Sénéchal, T.; Landercy, N.; Dangreau, L.; Raquez, J.M.; Dubois, P. Well defined thermostable cellulose nanocrystals via two-step ionic liquid swelling-hydrolysis extraction. Cellulose 2014, 21, 4195–4207. [Google Scholar] [CrossRef]
- Gonçalves, A.P.; Oliveira, E.; Mattedi, S.; José, N.M. Separation of cellulose nanowhiskers from microcrystalline cellulose with an aqueous protic ionic liquid based on ammonium and hydrogensulphate. Sep. Purif. Technol. 2018, 196, 200–207. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, B.; Xia, Q.; Meng, J.; Chen, W.; Liu, S.; Wang, Q.; Liu, Y.; Li, J.; Yu, H. Efficient Cleavage of Strong Hydrogen Bonds in Cotton by Deep Eutectic Solvents and Facile Fabrication of Cellulose Nanocrystals in High Yields. ACS Sustain. Chem. Eng. 2017, 5, 7623–7631. [Google Scholar] [CrossRef]
- Fan, Q.; Jiang, C.; Wang, W.; Bai, L.; Chen, H.; Yang, H.; Wei, D.; Yang, L. Eco-friendly extraction of cellulose nanocrystals from grape pomace and construction of self-healing nanocomposite hydrogels. Cellulose 2020, 27, 2541–2553. [Google Scholar] [CrossRef]
- Ma, Y.; Xia, Q.; Liu, Y.; Chen, W.; Liu, S.; Wang, Q.; Liu, Y.; Li, J.; Yu, H. Production of Nanocellulose Using Hydrated Deep Eutectic Solvent Combined with Ultrasonic Treatment. ACS Omega 2019, 4, 8539–8547. [Google Scholar] [CrossRef]
- Siqueira, G.A.; Dias, I.K.R.; Arantes, V. Exploring the action of endoglucanases on bleached eucalyptus kraft pulp as potential catalyst for isolation of cellulose nanocrystals. Int. J. Biol. Macromol. 2019, 133, 1249–1259. [Google Scholar] [CrossRef]
- Cui, S.; Zhang, S.; Ge, S.; Xiong, L.; Sun, Q. Green preparation and characterization of size-controlled nanocrystalline cellulose via ultrasonic-assisted enzymatic hydrolysis. Ind. Crop. Prod. 2016, 83, 346–352. [Google Scholar] [CrossRef]
- Camargo, L.A.; Pereira, S.C.; Correa, A.C.; Farinas, C.S.; Marconcini, J.M.; Mattoso, L.H.C. Feasibility of Manufacturing Cellulose Nanocrystals from the Solid Residues of Second-Generation Ethanol Production from Sugarcane Bagasse. Bioenergy Res. 2016, 9, 894–906. [Google Scholar] [CrossRef]
- Piras, C.C.; Fernández-Prieto, S.; De Borggraeve, W.M. Ball milling: A green technology for the preparation and functionalisation of nanocellulose derivatives. Nanoscale Adv. 2019, 1, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Konan, D.; Koffi, E.; Ndao, A.; Peterson, E.C.; Rodrigue, D.; Feng, W.; Konan, D.; Koffi, E.; Ndao, A.; Peterson, E.C.; et al. An Overview of Extrusion as a Pretreatment Method of Lignocellulosic Biomass. Energies 2022, 15, 3002. [Google Scholar] [CrossRef]
- Salimi, S.; Sotudeh-Gharebagh, R.; Zarghami, R.; Chan, S.Y.; Yuen, K.H. Production of Nanocellulose and Its Applications in Drug Delivery: A Critical Review. ACS Sustain. Chem. Eng. 2019, 7, 15800–15827. [Google Scholar] [CrossRef]
- Romero-Zúñiga, G.Y.; González-Morones, P.; Sánchez-Valdés, S.; Yáñez-Macías, R.; Sifuentes-Nieves, I.; García-Hernández, Z.; Hernández-Hernández, E. Microwave Radiation as Alternative to Modify Natural Fibers: Recent Trends and Opportunities—A Review. J. Nat. Fibers 2021, 19, 7594–7610. [Google Scholar] [CrossRef]
- Liyanage, S.; Acharya, S.; Parajuli, P.; Shamshina, J.L.; Abidi, N. Production and Surface Modification of Cellulose Bioproducts. Polymers 2021, 13, 3433. [Google Scholar] [CrossRef]
- Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Emerging technologies for the production of nanocellulose from lignocellulosic biomass. Carbohydr. Polym. 2022, 285, 119258. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Heo, M.H.; Lee, H.; Lee, H.H.; Jeong, H.; Kim, Y.W.; Shin, J. Facile and eco-friendly extraction of cellulose nanocrystals via electron beam irradiation followed by high-pressure homogenization. Green Chem. 2018, 20, 2596–2610. [Google Scholar] [CrossRef]
- Coccaro, N.; Ferrari, G.; Donsì, F. Understanding the break-up phenomena in an orifice-valve high pressure homogenizer using spherical bacterial cells (Lactococcus lactis) as a model disruption indicator. J. Food Eng. 2018, 236, 60–71. [Google Scholar] [CrossRef]
- Donsì, F.; Ferrari, G.; Maresca, P. High-Pressure Homogenization for Food Sanitization; Academic Press: Cambridge, MA, USA, 2009; ISBN 9780123741240. [Google Scholar]
- Martínez-Monteagudo, S.I.; Yan, B.; Balasubramaniam, V.M. Engineering Process Characterization of High-Pressure Homogenization—From Laboratory to Industrial Scale. Food Eng. Rev. 2017, 9, 143–169. [Google Scholar] [CrossRef]
- Carullo, D.; Donsì, F.; Ferrari, G. Influence of high-pressure homogenization on structural properties and enzymatic hydrolysis of milk proteins. LWT 2020, 130, 109657. [Google Scholar] [CrossRef]
- Nabi, M.; Zhang, G.; Li, F.; Zhang, P.; Wu, Y.; Tao, X.; Bao, S.; Wang, S.; Chen, N.; Ye, J.; et al. Enhancement of high pressure homogenization pretreatment on biogas production from sewage sludge: A review. Desalin. Water Treat. 2020, 175, 341–351. [Google Scholar] [CrossRef]
- Vinchhi, P.; Patel, J.K.; Patel, M.M. High-pressure homogenization techniques for nanoparticles. In Emerging Technologies for Nanoparticle Manufacturing; Springer: Berlin/Heidelberg, Germany, 2021; pp. 263–285. ISBN 9783030507039. [Google Scholar]
- Donsì, F. Applications of Nanoemulsions in Foods. In Nanoemulsions; Academic Press: Cambridge, MA, USA, 2018; pp. 349–377. ISBN 9780128118382. [Google Scholar]
- Gali, L.; Bedjou, F.; Ferrari, G.; Donsì, F. Formulation and Characterization of Zein/Gum Arabic Nanoparticles for the Encapsulation of a Rutin-Rich Extract from Ruta chalepensis L. Food Chem. 2022, 367, 129982. [Google Scholar] [CrossRef]
- Lenhart, V.; Quodbach, J.; Kleinebudde, P. Fibrillated Cellulose via High Pressure Homogenization: Analysis and Application for Orodispersible Films. AAPS PharmSciTech 2020, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Onyianta, A.J.; O’Rourke, D.; Perrin, G.; Popescu, C.M.; Saw, L.H.; Cai, Z.; Dorris, M. A process for deriving high quality cellulose nanofibrils from water hyacinth invasive species. Cellulose 2020, 27, 3727–3740. [Google Scholar] [CrossRef]
- Davoudpour, Y.; Hossain, M.S.; Abdul Khalil, H.P.S.; Mohamad Haafiz, M.K.; Mohd Ishak, Z.A.; Hassan, A.; Sarker, Z.I. Optimization of high pressure homogenization parameters for the isolation of cellulosic nanofibers using response surface methodology. Ind. Crop. Prod. 2015, 74, 381–387. [Google Scholar] [CrossRef]
- Urbonaviciene, D.; Viskelis, P. The cis-lycopene isomers composition in supercritical CO2 extracted tomato by-products. LWT-Food Sci. Technol. 2017, 85, 517–523. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G. Sustainable valorisation of tomato pomace: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 172–187. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Bhuyan, D.J.; Punia, S.; Grasso, S.; Sá, A.G.A.; Carciofi, B.A.M.; Arrutia, F.; Changan, S.; Radha; et al. Tomato (Solanum lycopersicum L.) seed: A review on bioactives and biomedical activities. Biomed. Pharmacother. 2021, 142, 112018. [Google Scholar] [CrossRef]
- Saba, N.; Jawaid, M. Recent advances in nanocellulose-based polymer nanocomposites. In Cellulose-Reinforced Nanofibre Composites: Production, Properties and Applications; Woodhead Publishing: Sawston, UK, 2017; ISBN 9780081009659. [Google Scholar]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, T.; Iwata, T.; Kumamoto, Y.; Isogai, A. Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromolecules 2009, 10, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalil, H.P.S.; Bhat, A.H.; Ireana Yusra, A.F. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated cellulose, a new cellulose product: Properties, uses, and commercial potential. J. Appl. Polym. Sci. Appl. Polym. Symp. 1983, 37, 815–827. [Google Scholar]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Pirozzi, A.; Capuano, R.; Avolio, R.; Gentile, G.; Ferrari, G.; Donsì, F. O/W pickering emulsions stabilized with cellulose nanofibrils produced through different mechanical treatments. Foods 2021, 10, 1886. [Google Scholar] [CrossRef]
- Pirozzi, A.; Del Grosso, V.; Ferrari, G.; Donsì, F. Edible coatings containing oregano essential oil nanoemulsion for improving postharvest quality and shelf life of tomatoes. Foods 2020, 9, 1605. [Google Scholar] [CrossRef]
- Mauriello, E.; Ferrari, G.; Donsì, F. Effect of formulation on properties, stability, carvacrol release and antimicrobial activity of carvacrol emulsions. Colloids Surf. B Biointerfaces 2021, 197, 111424. [Google Scholar] [CrossRef]
- Fu, T.-K.; Wang, Y.-H.; Li, J.-H.; Wei, X.-Y.; Wang, F. Effect of High Pressure Homogenization (HPH) on The Rheological Properties of Pineapple Leaf Cellulose/[BMIM]Cl Solution. In Proceedings of the International Conference on Mechanics Design, Manufacturing and Automation (MDM), Porto, Portugal, 4 January 2017. [Google Scholar]
- Yan, Z.; Yi, C.; Liu, T.; Yang, J.; Ma, H.; Sha, L.; Guo, D.; Zhao, H.; Zhang, X.; Wang, W. Effect of Lignin containing Highly Fibrillated Cellulose on the Adsorption Behavior of an Organic Dye. BioResources 2021, 16, 6560–6576. [Google Scholar] [CrossRef]
- Larrea-Wachtendorff, D.; Tabilo-Munizaga, G.; Moreno-Osorio, L.; Villalobos-Carvajal, R.; Pérez-Won, M. Protein Changes Caused by High Hydrostatic Pressure (HHP): A Study Using Differential Scanning Calorimetry (DSC) and Fourier Transform Infrared (FTIR) Spectroscopy. Food Eng. Rev. 2015, 7, 222–230. [Google Scholar]
- Larrea-Wachtendorff, D.; Tabilo-Munizaga, G.; Ferrari, G. Potato starch hydrogels produced by high hydrostatic pressure (HHP): A first approach. Polymers 2019, 11, 1673. [Google Scholar] [CrossRef]
- Wu, C.; McClements, D.J.; He, M.; Zheng, L.; Tian, T.; Teng, F.; Li, Y. Preparation and characterization of okara nanocellulose fabricated using sonication or high-pressure homogenization treatments. Carbohydr. Polym. 2021, 255, 117364. [Google Scholar] [CrossRef]
- Ling, Z.; Tang, W.; Su, Y.; Shao, L.; Wang, P.; Ren, Y.; Huang, C. Promoting enzymatic hydrolysis of aggregated bamboo crystalline cellulose by fast microwave-assisted dicarboxylic acid deep eutectic solvents pretreatments. Bioresour. Technol. 2021, 333, 125122. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Renard, C.M.G.C.; Bureau, S.; Le Bourvellec, C. Revisiting the contribution of ATR-FTIR spectroscopy to characterize plant cell wall polysaccharides. Carbohydr. Polym. 2021, 262, 117935. [Google Scholar] [CrossRef]
- Kubovský, I.; Kačíková, D.; Kačík, F. Structural Changes of Oak Wood Main Components Caused by Thermal Modification. Polymers 2020, 12, 485. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Li, M.; Pu, Y.; Ragauskas, A.J.; Yoo, C.G. Observation of Potential Contaminants in Processed Biomass Using Fourier Transform Infrared Spectroscopy. Appl. Sci. 2020, 10, 4345. [Google Scholar] [CrossRef]
- Guo, J.; Filpponen, I.; Johansson, L.S.; Heiβler, S.; Li, L.; Levkin, P.; Rojas, O.J. Micro-patterns on nanocellulose films and paper by photo-induced thiol–yne click coupling: A facile method toward wetting with spatial resolution. Cellulose 2018, 25, 367–375. [Google Scholar]
- Xu, Y.; Yang, S.; Zhao, P.; Wu, M.; Song, X.; Ragauskas, A.J. Effect of endoglucanase and high-pressure homogenization post-treatments on mechanically grinded cellulose nanofibrils and their film performance. Carbohydr. Polym. 2021, 253, 117253. [Google Scholar] [CrossRef]
- Hassan, N.S.; Badri, K.H. Lignin recovery from alkaline hydrolysis and glycerolysis of oil palm fiber. AIP Conf. Proc. 2014, 1614, 433–438. [Google Scholar]
- Cheng, S.; Huang, A.; Wang, S.; Zhang, Q. Effect of different heat treatment temperatures on the chemical composition and structure of chinese fir wood. BioResources 2016, 11, 4006–4016. [Google Scholar]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar]
- Xu, F.; Sun, J.X.; Sun, R.; Fowler, P.; Baird, M.S. Comparative study of organosolv lignins from wheat straw. Ind. Crop. Prod. 2006, 23, 180–193. [Google Scholar]
- Wei, Y.; Chen, W.; Liu, C.; Wang, H. Facial synthesis of adsorbent from hemicelluloses for Cr(VI) adsorption. Molecules 2021, 26, 1443. [Google Scholar] [PubMed]
- Yang, Y.; Zhang, M.; Zhao, J.; Wang, D. Effects of particle size on biomass pretreatment and hydrolysis performances in bioethanol conversion. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Tamayo Tenorio, A.; Gieteling, J.; Nikiforidis, C.V.; Boom, R.M.; van der Goot, A.J. Interfacial properties of green leaf cellulosic particles. Food Hydrocoll. 2017, 71, 8–16. [Google Scholar]
- Lei, Y.; Zhang, X.; Li, J.; Chen, Y.; Liang, H.; Li, Y.; Li, B.; Luo, X.; Pei, Y.; Liu, S. Nanocellulose from bamboo shoots as perfect Pickering stabilizer: Effect of the emulsification process on the interfacial and emulsifying properties. Food Biosci. 2022, 46, 101596. [Google Scholar]
- Du, K.; Glogowski, E.; Emrick, T.; Russell, T.P.; Dinsmore, A.D. Adsorption energy of nano- and microparticles at liquid-liquid interfaces. Langmuir 2010, 26, 12518–12522. [Google Scholar]
- Bao, C.; Chen, X.; Liu, C.; Liao, Y.; Huang, Y.; Hao, L.; Yan, H.; Lin, Q. Extraction of cellulose nanocrystals from microcrystalline cellulose for the stabilization of cetyltrimethylammonium bromide-enhanced Pickering emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125442. [Google Scholar]
- Bi, C.H.; Yan, Z.M.; Wang, P.L.; Alkhatib, A.; Zhu, J.Y.; Zou, H.C.; Sun, D.Y.; Zhu, X.D.; Gao, F.; Shi, W.T.; et al. Effect of high pressure homogenization treatment on the rheological properties of citrus peel fiber/corn oil emulsion. J. Sci. Food Agric. 2020, 100, 3658–3665. [Google Scholar]
- Hua, X.; Xu, S.; Wang, M.; Chen, Y.; Yang, H.; Yang, R. Effects of high-speed homogenization and high-pressure homogenization on structure of tomato residue fibers. Food Chem. 2017, 232, 443–449. [Google Scholar] [PubMed]
- Zhu, X.; Lundberg, B.; Cheng, Y.; Shan, L.; Xing, J.; Peng, P.; Chen, P.; Huang, X.; Li, D.; Ruan, R. Effect of high-pressure homogenization on the flow properties of citrus peel fibers. J. Food Process Eng. 2018, 41, e12659. [Google Scholar]

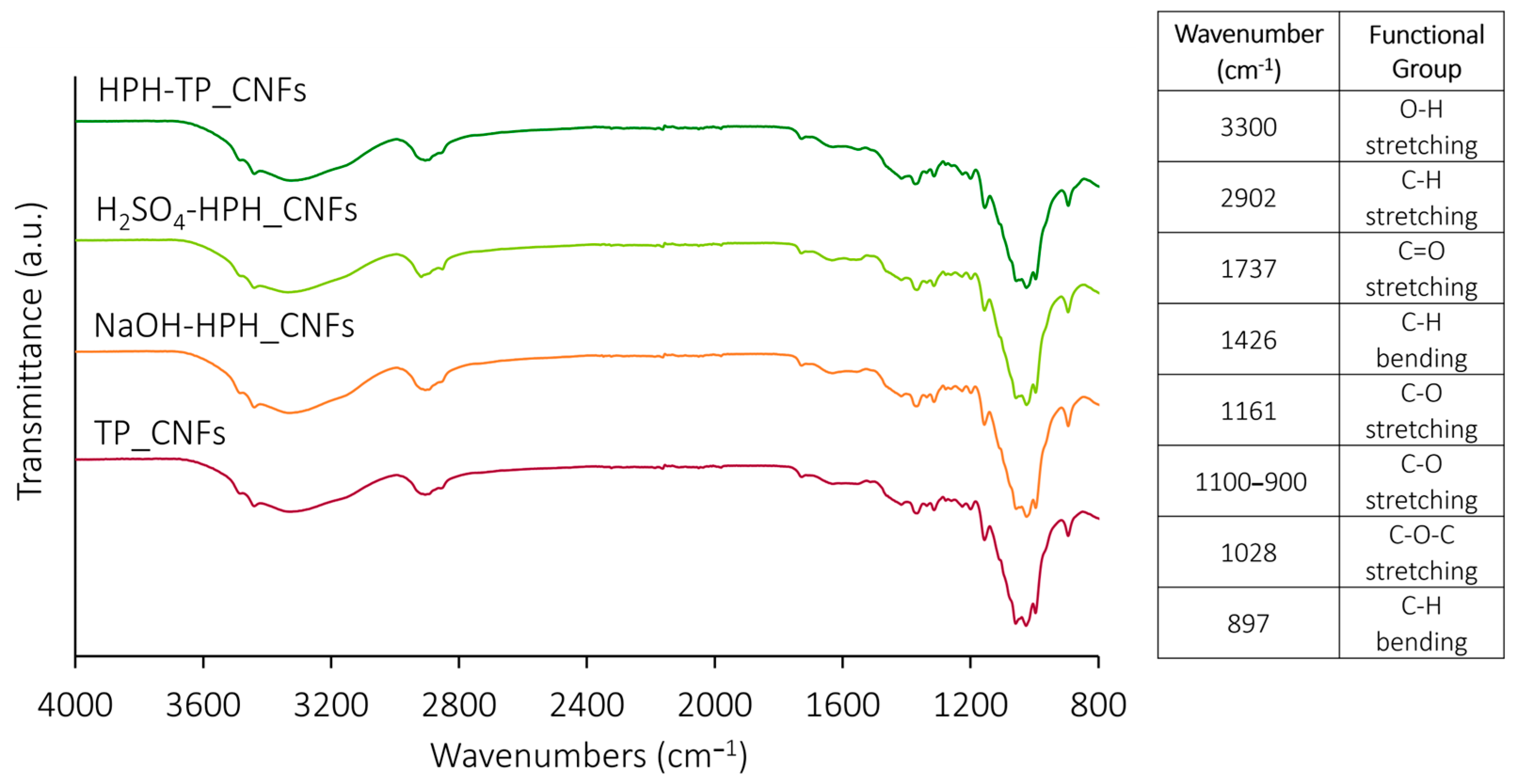

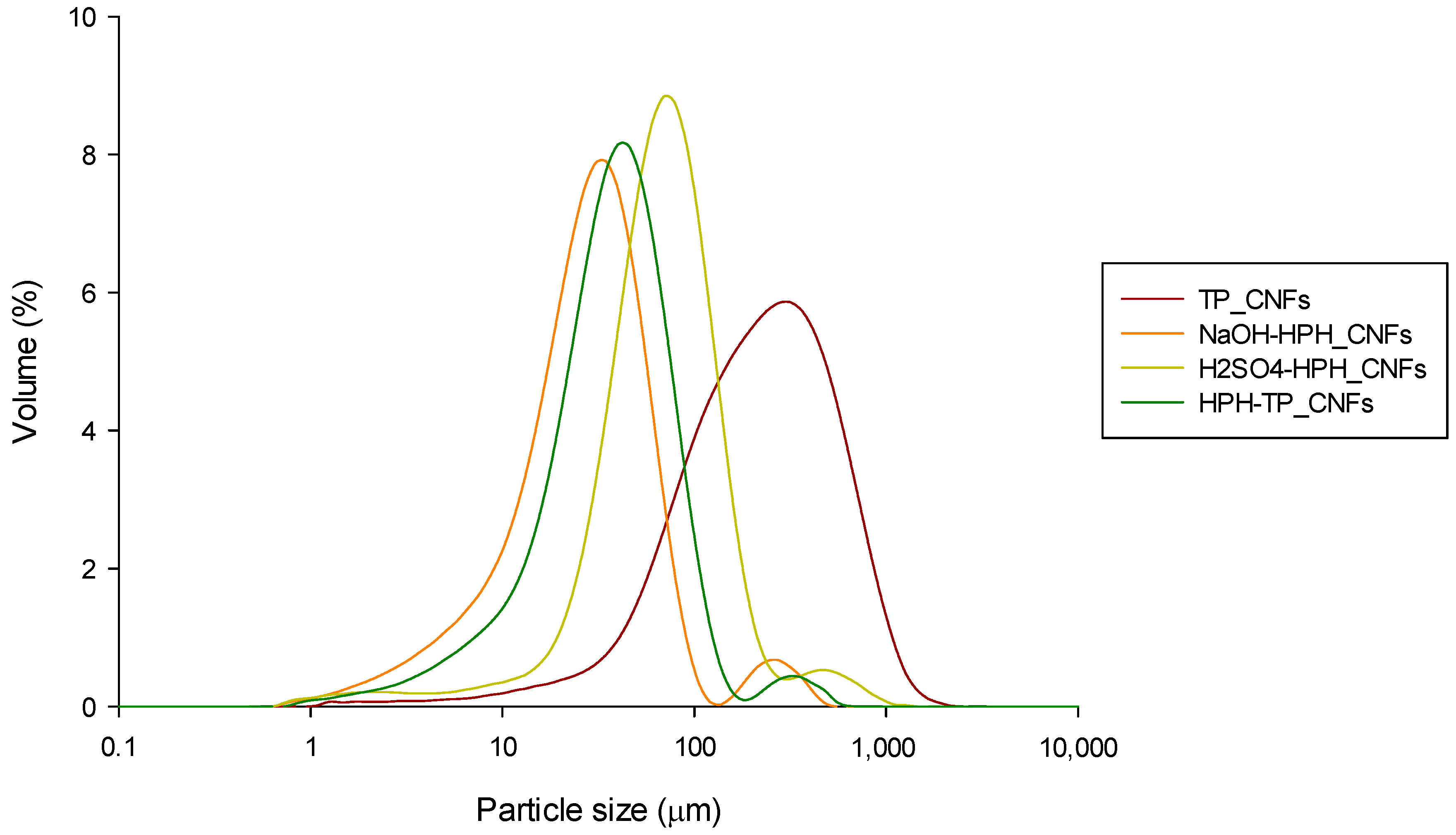
| TP_Cellulose | NaOH-HPH_Cellulose | H2SO4-HPH_Cellulose | HPH-TP_Cellulose | |
|---|---|---|---|---|
| Cellulose (%Residue fiber) | (29.33 ± 1.52) * | 31.51 ± 0.17 | 32.12 ± 0.24 | (37.72 ± 0.79) * |
| Hemicellulose (%Residue fiber) | (18.74 ± 0.52) * | 2.17 ± 0.07 | 2.18 ± 0.10 | (4.62 ± 0.65) * |
| Lignin (%Residue fiber) | 0.74 ± 0.03 | 0.94 ± 0.03 | 0.97 ± 0.08 | 0.76 ± 0.02 |
| Cumulative yield (%DM) | 61.72 ± 0.50 | 65.47 ± 0.36 | 65.07 ± 0.51 | 74.38 ± 0.41 |
| γ0 (mN/m) | γ∞ (mN/m) | τr (s) | R2 | |
|---|---|---|---|---|
| TP_CNFs | 14.14 ± 0.44 b | 11.39 ± 0.10 a | 333.33 ± 1.02 a | 0.9851 |
| NaOH-HPH_CNFs | 16.04 ± 0.62 a | 12.34 ± 0.39 b | 333.33 ± 0.98 a | 0.9887 |
| H2SO4-HPH_CNFs | 15.28 ± 0.39 a | 11.77 ± 0.11 a | 333.33 ± 0.79 a | 0.9836 |
| HPH-TP_CNFs | 13.30 ± 0.41 b | 10.74 ± 0.27 a | 333.33 ± 0.87 a | 0.9813 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirozzi, A.; Olivieri, F.; Castaldo, R.; Gentile, G.; Donsì, F. Cellulose Isolation from Tomato Pomace: Part II—Integrating High-Pressure Homogenization in a Cascade Hydrolysis Process for the Recovery of Nanostructured Cellulose and Bioactive Molecules. Foods 2023, 12, 3221. https://doi.org/10.3390/foods12173221
Pirozzi A, Olivieri F, Castaldo R, Gentile G, Donsì F. Cellulose Isolation from Tomato Pomace: Part II—Integrating High-Pressure Homogenization in a Cascade Hydrolysis Process for the Recovery of Nanostructured Cellulose and Bioactive Molecules. Foods. 2023; 12(17):3221. https://doi.org/10.3390/foods12173221
Chicago/Turabian StylePirozzi, Annachiara, Federico Olivieri, Rachele Castaldo, Gennaro Gentile, and Francesco Donsì. 2023. "Cellulose Isolation from Tomato Pomace: Part II—Integrating High-Pressure Homogenization in a Cascade Hydrolysis Process for the Recovery of Nanostructured Cellulose and Bioactive Molecules" Foods 12, no. 17: 3221. https://doi.org/10.3390/foods12173221








