Significance of Fermentation in Plant-Based Meat Analogs: A Critical Review of Nutrition, and Safety-Related Aspects
Abstract
:1. Introduction
2. Plant-Based Meat Analogs Characterization and the Demand for New Approaches
2.1. Anti-Nutritional Factors
2.2. Protein Allergenicity and Ultra-Processed Food
2.3. Digestibility and Nutrient Deficiency
2.4. Food Spoilage and Pathogens
2.5. Genetically Modified Foods
3. Fermentation and Plant-Based Meat Analogs’ Nutrition and Safety
3.1. Anti-Nutrients
3.2. Allergenicity
3.3. Digestability
3.4. Improve Nutritional Components
3.5. Others
3.6. Nutrition and Health Challenges
4. Fermentation and Plant-Based Meat Analogs’ Sensory Quality
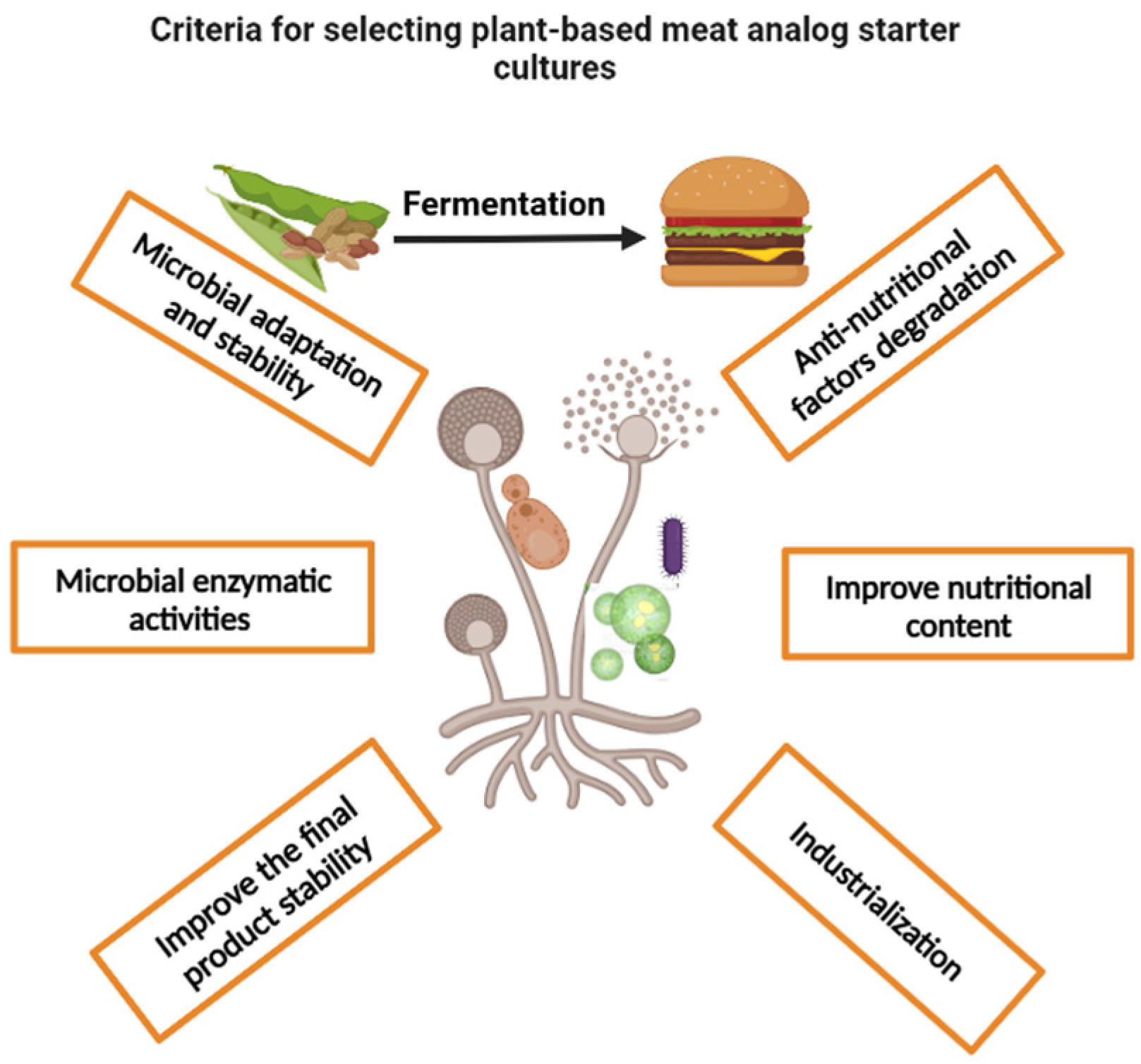
5. Plant-Based Meat Analogs and Starter Culture Technology
- Based on the above discussion, most ingredients used for producing plant-based meat, such as legumes, have significantly high protein, carbohydrate, and fat concentrations, as well as anti-nutritional factors. Starter cultures with strong enzymatic activity, including proteases, lipases, amylases, and phytases, are needed to transform these components. Degradation of these substances improves the digestibility of the final product, as well as reducing the allergenicity and anti-nutritional factor content of plant-based meat, as mentioned above. Furthermore, attention should be given to microorganisms known for producing desired volatile and non-volatile profiles. Conventional and advanced methodologies that have been applied to measure these microbial activities in other areas of study include plate assay, colorimetry, chromatography, microcalorimetry, and sensory tests, all of which can be applied to microbial screening [259,260,261].
- The fermentation of the plant ingredients used in meat analogs has been linked to health benefits including an increase in the level of essential amino acids, omega-3 fatty acids, bioactive compounds, probiotics, and an improvement in the meat analog’s safety and stability. These features have potential physiological roles in the human body and should be considered when screening for suitable starter cultures. Selecting the right microorganisms with such characteristics as the main fermenting microorganism or as the coculture may boost the acceptability of plant-based meat analogs. Laboratory and clinical studies that have been widely applied to test the safety and health benefits of fermented products may be employed in the microbial screening process for plant-based meat analogs [258,262,263].
- Using the available microbial survey data of plant ingredients and meat analogs, the selected strains should be examined for their ability to adapt, compete with the natural microflora, as well as other microbial contaminates and food pathogens, that may present in the raw ingredients during and after processing. An in-depth investigation of microbial safety, and biodegradation capability to toxic compounds include mycotoxins and biogenic amines, should be considered. This criterion can be determined by exposing the selected strains to different stressors (such as high temperature, high salt, pH, and other additives), as well as observing how they react to microflora, foodborne pathogens, and toxic compounds that are frequently found in raw ingredients and processed foods. Successful growth under such stressful conditions is considered a potential indicator of high fermentation performance of the selected isolates. Additionally, factors such as inoculum size, inoculation time, and incubation parameters should be controlled to ensure successful fermentation with desirable results. Similar approaches have been applied to develop starter cultures for other food products [264,265,266].
- Additionally, for commercial applications, selected strains designed for starter culture should be able to be cultivated on available and cheap substrates to lower production costs. In addition, the strains should tolerate downstream processes such as air drying, freeze drying, packaging, and rehydration to ensure stability during storage and handling [267,268].
- With current advancements in molecular techniques, screening and gene editing may be used to increase the capability of the selected isolates to desirably interact with the food matrix. A similar approach was used to improve LAB strains in the meat and dairy fermentation process, which involved no extra risk compared to the use of wild strains [269,270,271]. Genome editing technologies, like CRISPR-Cas9, can be used to eliminate specific DNA sequences from a microbial genome that control mycotoxin or BA biosynthesis, or to add desired genes that biocontrol undesired microorganisms and toxins. Despite the fact that these applications can reduce costs, and improve strain capabilities, using genetically modified organisms (GMOs) in food may trigger public concerns [206,272,273].
- Before starting the development of starter cultures and their commercialization, the Nagoya protocol should be considered. Based on this protocol, prior informed consent and mutually agreed terms must be built by the research provider describing access to the resources and benefit shares [274].
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Font-i-Furnols, M.; Guerrero, L. Consumer Preference, Behavior and Perception about Meat and Meat Products: An Overview. Meat Sci. 2014, 98, 361–371. [Google Scholar] [CrossRef]
- Markets and Markets. 2019. “Plant-Based Protein Market | Industry Size, Share, Analysis, Trends and Forecasts - 2025”. Available online: https://www.marketsandmarkets.com/Market-Reports/plant-based-protein-market-14715651.html (accessed on 20 July 2023).
- Bonny, S.P.F.; Gardner, G.E.; Pethick, D.W.; Hocquette, J.-F. What Is Artificial Meat and What Does It Mean for the Future of the Meat Industry? J. Integr. Agric. 2015, 14, 255–263. [Google Scholar] [CrossRef]
- Weinrich, R.; Strack, M.; Neugebauer, F. Consumer Acceptance of Cultured Meat in Germany. Meat Sci. 2020, 162, 107924. [Google Scholar] [CrossRef]
- Hoek, A.C.; Luning, P.A.; Weijzen, P.; Engels, W.; Kok, F.J.; De Graaf, C. Replacement of Meat by Meat Substitutes. A Survey on Person-and Product-Related Factors in Consumer Acceptance. Appetite 2011, 56, 662–673. [Google Scholar] [CrossRef]
- Alexander, P.; Brown, C.; Arneth, A.; Dias, C.; Finnigan, J.; Moran, D.; Rounsevell, M.D.A. Could Consumption of Insects, Cultured Meat or Imitation Meat Reduce Global Agricultural Land Use? Glob. Food Secur. 2017, 15, 22–32. [Google Scholar] [CrossRef]
- Boukid, F. Plant-Based Meat Analogues: From Niche to Mainstream. Eur. Food Res. Technol. 2021, 247, 297–308. [Google Scholar] [CrossRef]
- Xazela, N.M.; Hugo, A.; Marume, U.; Muchenje, V. Perceptions of Rural Consumers on the Aspects of Meat Quality and Health Implications Associated With Meat Consumption. Sustainability 2017, 9, 830. [Google Scholar] [CrossRef]
- Mohamed, Z.; Terano, R.; Yeoh, S.J.; Iliyasu, A. Opinions of Non-Vegetarian Consumers Among the Chinese Community in Malaysia Toward Vegetarian Food and Diets. J. Food Prod. Mark. 2017, 23, 80–98. [Google Scholar] [CrossRef]
- Imran, M.; Liyan, Z. Production of Plant-Based Meat: Functionality, Limitations and Future Prospects. Eur. Food Res. Technol. 2023, 249, 2189–2213. [Google Scholar] [CrossRef]
- Smetana, S.; Mathys, A.; Knoch, A.; Heinz, V. Meat Alternatives: Life Cycle Assessment of Most Known Meat Substitutes. Int. J. Life Cycle Assess. 2015, 20, 1254–1267. [Google Scholar] [CrossRef]
- Fresán, U.; Marrin, D.L.; Mejia, M.A.; Sabaté, J. Water Footprint of Meat Analogs: Selected Indicators According to Life Cycle Assessment. Water 2019, 11, 728. [Google Scholar] [CrossRef]
- Dekkers, B.L.; Boom, R.M.; van der Goot, A.J. Structuring Processes for Meat Analogues. Trends Food Sci. Technol. 2018, 81, 25–36. [Google Scholar] [CrossRef]
- He, J.; Evans, N.M.; Liu, H.; Shao, S. A Review of Research on Plant-based Meat Alternatives: Driving Forces, History, Manufacturing, and Consumer Attitudes. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2639–2656. [Google Scholar] [CrossRef]
- Singh, M.; Trivedi, N.; Enamala, M.K.; Kuppam, C.; Parikh, P.; Nikolova, M.P.; Chavali, M. Plant-Based Meat Analogue (PBMA) as a Sustainable Food: A Concise Review. Eur. Food Res. Technol. 2021, 247, 2499–2526. [Google Scholar] [CrossRef]
- Joshi, V.K.; Kumar, S. Meat Analogues: Plant Based Alternatives to Meat Products-A Review. Int. J. Food Ferment. Technol. 2015, 5, 107–119. [Google Scholar] [CrossRef]
- Kumar, P.; Chatli, M.K.; Mehta, N.; Singh, P.; Malav, O.P.; Verma, A.K. Meat Analogues: Health Promising Sustainable Meat Substitutes. Crit. Rev. Food Sci. Nutr. 2017, 57, 923–932. [Google Scholar] [CrossRef]
- Asgar, M.; Fazilah, A.; Huda, N.; Bhat, R.; Karim, A.A. Nonmeat Protein Alternatives as Meat Extenders and Meat Analogs. Compr. Rev. Food Sci. Food Saf. 2010, 9, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kumar, R.; Sabapathy, S.N.; Bawa, A.S. Functional and Edible Uses of Soy Protein Products. Compr. Rev. Food Sci. Food Saf. 2008, 7, 14–28. [Google Scholar] [CrossRef]
- Bakhsh, A.; Lee, S.-J.; Lee, E.-Y.; Hwang, Y.-H.; Joo, S.-T. Evaluation of Rheological and Sensory Characteristics of Plant-Based Meat Analog with Comparison to Beef and Pork. Food Sci. Anim. Resour. 2021, 41, 983. [Google Scholar] [CrossRef]
- Hu, F.B.; Otis, B.O.; McCarthy, G. Can Plant-Based Meat Alternatives Be Part of a Healthy and Sustainable Diet? JAMA 2019, 322, 1547–1548. [Google Scholar] [CrossRef]
- Ettinger, L.; Falkeisen, A.; Knowles, S.; Gorman, M.; Barker, S.; Moss, R.; McSweeney, M.B. Consumer Perception and Acceptability of Plant-Based Alternatives to Chicken. Foods 2022, 11, 2271. [Google Scholar] [CrossRef] [PubMed]
- Toh, D.W.K.; SRV, A.; Henry, C.J. Unknown Impacts of Plant-Based Meat Alternatives on Long-Term Health. Nat. Food 2022, 3, 90–91. [Google Scholar] [CrossRef]
- Jahn, S.; Furchheim, P.; Strässner, A.-M. Plant-Based Meat Alternatives: Motivational Adoption Barriers and Solutions. Sustainability 2021, 13, 13271. [Google Scholar] [CrossRef]
- Flint, M.; Bowles, S.; Lynn, A.; Paxman, J.R. Novel Plant-Based Meat Alternatives: Future Opportunities and Health Considerations. Proc. Nutr. Soc. 2023, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Santo, R.E.; Kim, B.F.; Goldman, S.E.; Dutkiewicz, J.; Biehl, E.; Bloem, M.W.; Neff, R.A.; Nachman, K.E. Considering Plant-Based Meat Substitutes and Cell-Based Meats: A Public Health and Food Systems Perspective. Front. Sustain. Food Syst. 2020, 4, 134. [Google Scholar] [CrossRef]
- Aganga, A.A.; Tshwenyane, S.O. Feeding Values and Anti-Nutritive Factors of Forage Tree Legumes. Pak. J. Nutr. 2003, 2, 170–177. [Google Scholar] [CrossRef]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef]
- Wood, P.; Tavan, M. A Review of the Alternative Protein Industry. Curr. Opin. Food Sci. 2022, 47, 100869. [Google Scholar] [CrossRef]
- Gänzle, M.G. From Gene to Function: Metabolic Traits of Starter Cultures for Improved Quality of Cereal Foods. Int. J. Food Microbiol. 2009, 134, 29–36. [Google Scholar] [CrossRef]
- Sakandar, H.A.; Hussain, R.; Khan, Q.F.; Zhang, H. Functional Microbiota in Chinese Traditional Baijiu and Mijiu Qu (Starters): A Review. Food Res. Int. 2020, 138, 109830. [Google Scholar] [CrossRef]
- Bourdichon, F.; Casaregola, S.; Farrokh, C.; Frisvad, J.C.; Gerds, M.L.; Hammes, W.P.; Harnett, J.; Huys, G.; Laulund, S.; Ouwehand, A. Food Fermentations: Microorganisms with Technological Beneficial Use. Int. J. Food Microbiol. 2012, 154, 87–97. [Google Scholar] [CrossRef]
- Hansen, E.B. Starter Cultures: Uses in the Food Industry. In Encyclopedia of Food Microbiology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 529–534. [Google Scholar]
- Elhalis, H.; Cox, J.; Frank, D.; Zhao, J. Microbiological and Chemical Characteristics of Wet Coffee Fermentation Inoculated With Hansinaspora Uvarum and Pichia Kudriavzevii and Their Impact on Coffee Sensory Quality. Front. Microbiol. 2021, 12, 713969. [Google Scholar] [CrossRef] [PubMed]
- Cassimiro, D.M.d.J.; Batista, N.N.; Fonseca, H.C.; Naves, J.A.O.; Dias, D.R.; Schwan, R.F. Coinoculation of Lactic Acid Bacteria and Yeasts Increases the Quality of Wet Fermented Arabica Coffee. Int. J. Food Microbiol. 2022, 369, 109627. [Google Scholar] [CrossRef] [PubMed]
- Whittington, H.D.; Dagher, S.F.; Bruno-Bárcena, J.M. Production and Conservation of Starter Cultures: From “Backslopping” to Controlled Fermentations. In How Fermented Foods Feed a Healthy Gut Microbiota; Springer: Berlin/Heidelberg, Germany, 2019; pp. 125–138. [Google Scholar]
- Papaioannou, G.M.; Kosma, I.S.; Dimitreli, G.; Badeka, A.V.; Kontominas, M.G. Effect of Starter Culture, Probiotics, and Flavor Additives on Physico-Chemical, Rheological, and Sensory Properties of Cow and Goat Dessert Yogurts. Eur. Food Res. Technol. 2022, 248, 1191–1202. [Google Scholar] [CrossRef]
- Fiorentini, M.; Kinchla, A.J.; Nolden, A.A. Role of Sensory Evaluation in Consumer Acceptance of Plant-Based Meat Analogs and Meat Extenders: A Scoping Review. Foods 2020, 9, 1334. [Google Scholar] [CrossRef]
- Fresán, U.; Mejia, M.A.; Craig, W.J.; Jaceldo-Siegl, K.; Sabaté, J. Meat Analogs from Different Protein Sources: A Comparison of Their Sustainability and Nutritional Content. Sustainability 2019, 11, 3231. [Google Scholar] [CrossRef]
- Samard, S.; Ryu, G.-H. A Comparison of Physicochemical Characteristics, Texture, and Structure of Meat Analogue and Meats. J. Sci. Food Agric. 2019, 99, 2708–2715. [Google Scholar] [CrossRef]
- Sun, C.; Ge, J.; He, J.; Gan, R.; Fang, Y. Processing, Quality, Safety, and Acceptance of Meat Analogue Products. Engineering 2021, 7, 674–678. [Google Scholar] [CrossRef]
- Younis, K.; Ashfaq, A.; Ahmad, A.; Anjum, Z.; Yousuf, O. A Critical Review Focusing the Effect of Ingredients on the Textural Properties of Plant-Based Meat Products. J. Texture Stud. 2022, 54, 365–382. [Google Scholar] [CrossRef]
- Hamed Hammad Mohammed, H.; Jin, G.; Ma, M.; Khalifa, I.; Shukat, R.; Elkhedir, A.E.; Zeng, Q.; Noman, A.E. Comparative Characterization of Proximate Nutritional Compositions, Microbial Quality and Safety of Camel Meat in Relation to Mutton, Beef, and Chicken. LWT 2020, 118, 108714. [Google Scholar] [CrossRef]
- Roe, M.; Pinchen, H.; Church, S.; Finglas, P. McCance and Widdowson’s The Composition of Foods Seventh Summary Edition and Updated Composition of Foods Integrated Dataset. Nutr Bull 2015, 40, 36–39. [Google Scholar] [CrossRef]
- Negrão, C.C.; Mizubuti, I.Y.; Morita, M.C.; Colli, C.; Ida, E.I.; Shimokomaki, M. Biological Evaluation of Mechanically Deboned Chicken Meat Protein Quality. Food Chem. 2005, 90, 579–583. [Google Scholar] [CrossRef]
- Pires, C.V.; Oliveira, M.G.d.A.; Rosa, J.C.; Costa, N.M.B. Nutritional Quality and Chemical Score of Amino Acids from Different Protein Sources. Food Sci. Technol. 2006, 26, 179–187. [Google Scholar] [CrossRef]
- Barrón-Hoyos, J.M.; Archuleta, A.R.; del Refugio Falcón-Villa, M.; Canett-Romero, R.; Cinco-Moroyoqui, F.J.; Romero-Barancini, A.L.; Rueda-Puente, E. Protein Quality Evaluation of Animal Food Proteins by In-Vitro Methodologies. Food Nutr. Sci. 2013, 2013, 376–384. [Google Scholar]
- Ahnan-Winarno, A.D.; Cordeiro, L.; Winarno, F.G.; Gibbons, J.; Xiao, H. Tempeh: A Semicentennial Review on Its Health Benefits, Fermentation, Safety, Processing, Sustainability, and Affordability. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1717–1767. [Google Scholar] [CrossRef]
- Murata, K.; Ikehata, H.; Miyamoto, T. Studies on the Nutritional Value of Tempeh. J. Food Sci. 1967, 32, 580–586. [Google Scholar] [CrossRef]
- Haron, H.B.; Raob, N. Changes in Macronutrient, Total Phenolic and Anti-Nutrient Contentsduring Preparation of Tempeh. J. Nutr. Food Sci. 2014, 4, 265. [Google Scholar]
- Qin, P.; Wang, T.; Luo, Y. A Review on Plant-Based Proteins from Soybean: Health Benefits and Soy Product Development. J. Agric. Food Res. 2022, 7, 100265. [Google Scholar] [CrossRef]
- Eze, N.M.; Okwume, U.G.; Eseadi, C.; Udenta, E.A.; Onyeke, N.G.; Ugwu, E.N.; Akubue, B.N.; Njoku, H.A.; Ezeanwu, A.B. Acceptability and Consumption of Tofu as a Meat Alternative among Secondary School Boarders in Enugu State, Nigeria: Implications for Nutritional Counseling and Education. Medicine 2018, 97, e13155. [Google Scholar] [CrossRef]
- Anwar, D.A.; El-Chaghaby, G. Nutritional Quality, Amino Acid Profiles, Protein Digestibility Corrected Amino Acid Scores and Antioxidant Properties of Fried Tofu and Seitan. Food Environ. Saf. J. 2019, 18, 176–190. [Google Scholar]
- Sim, S.Y.J.; Srv, A.; Chiang, J.H.; Henry, C.J. Plant Proteins for Future Foods: A Roadmap. Foods 2021, 10, 1967. [Google Scholar] [CrossRef]
- Muliani, D.; Nathania, E.; Jeslin, J.; Jayanti, Y.N.; Hadrian, E. Food Innovation: Fungi and Vegetables Potential as A Healthy and Sustainable Meat Substitute. Indones. J. Life Sci. 2021, 3, 1–27. [Google Scholar] [CrossRef]
- Sedgwick, P. Convenience Sampling. Bmj 2013, 347. [Google Scholar] [CrossRef]
- Nigam, P.S.-N. Single Cell Protein: Mycelial Fungi. In Encyclopedia of Food Microbiology; Academic Press: Cambridge, MA, USA, 2000; p. 2034. [Google Scholar]
- Gilani, G.S.; Lee, N. Protein | Sources of Food-grade Protein. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 4873–4879. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E.; Casper, H.H. Molecular Phylogenetic, Morphological, and Mycotoxin Data Support Reidentification of the Quorn Mycoprotein Fungus AsFusarium Venenatum. Fungal Genet. Biol. 1998, 23, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.F.; DePorter, J. Self-Reported Adverse Reactions Associated with Mycoprotein (Quorn-Brand) Containing Foods. Ann. Allergy Asthma Immunol. 2018, 120, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Denny, A.; Aisbitt, B.; Lunn, J. Mycoprotein and Health. Nutr. Bull. 2008, 33, 298–310. [Google Scholar] [CrossRef]
- Akdogan, H. High Moisture Food Extrusion. Int. J. Food Sci. Technol. 1999, 34, 195–207. [Google Scholar] [CrossRef]
- Baune, M.C.; Terjung, N.; Tülbek, M.Ç.; Boukid, F. Textured Vegetable Proteins (TVP): Future Foods Standing on Their Merits as Meat Alternatives. Future Foods 2022, 6, 100181. [Google Scholar] [CrossRef]
- Boye, J.; Wijesinha-Bettoni, R.; Burlingame, B. Protein Quality Evaluation Twenty Years after the Introduction of the Protein Digestibility Corrected Amino Acid Score Method. Br. J. Nutr. 2012, 108, S183–S211. [Google Scholar] [CrossRef]
- Hughes, G.J.; Ryan, D.J.; Mukherjea, R.; Schasteen, C.S. Protein Digestibility-Corrected Amino Acid Scores (PDCAAS) for Soy Protein Isolates and Concentrate: Criteria for Evaluation. J. Agric. Food Chem. 2011, 59, 12707–12712. [Google Scholar] [CrossRef]
- Riaz, M.N. Texturized Vegetable Proteins. Handb. Food Proteins 2011, 395–418. [Google Scholar] [CrossRef]
- Shanthakumar, P.; Klepacka, J.; Bains, A.; Chawla, P.; Dhull, S.B.; Najda, A. The Current Situation of Pea Protein and Its Application in the Food Industry. Molecules 2022, 27, 5354. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, L.A.; Mes, J.J.; Mensink, M.; Wanders, A.J. Protein Quality of Soy and the Effect of Processing: A Quantitative Review. Front. Nutr. 2022, 9, 2148. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J.; Mangels, A.R. Position of the American Dietetic Association: Vegetarian Diets. J. Am. Diet. Assoc. 2009, 109, 1266. [Google Scholar]
- Sha, L.; Xiong, Y.L. Plant Protein-Based Alternatives of Reconstructed Meat: Science, Technology, and Challenges. Trends Food Sci. Technol. 2020, 102, 51–61. [Google Scholar] [CrossRef]
- Williams, P.A.; Phillips, G.O. Chapter 1-Introduction to Food Hydrocolloids. In Woodhead Publishing Series in Food Science, Technology and Nutrition, 3rd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 3–26. ISBN 978-0-12-820104-6. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Böcker, L.; Müssner, C.; Stirnemann, E.; Haberkorn, I.; Adelmann, H.; Handschin, S.; Windhab, E.J.; Mathys, A. Extruded Meat Analogues Based on Yellow, Heterotrophically Cultivated Auxenochlorella Protothecoides Microalgae. Innov. Food Sci. Emerg. Technol. 2020, 59, 102275. [Google Scholar] [CrossRef]
- Wi, G.; Bae, J.; Kim, H.; Cho, Y.; Choi, M.-J. Evaluation of the Physicochemical and Structural Properties and the Sensory Characteristics of Meat Analogues Prepared with Various Non-Animal Based Liquid Additives. Foods 2020, 9, 461. [Google Scholar] [CrossRef]
- McClements, D.J. Development of Next-Generation Nutritionally Fortified Plant-Based Milk Substitutes: Structural Design Principles. Foods 2020, 9, 421. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoscale Nutrient Delivery Systems for Food Applications: Improving Bioactive Dispersibility, Stability, and Bioavailability. J. Food Sci. 2015, 80, N1602–N1611. [Google Scholar] [CrossRef]
- Tyndall, S.M.; Maloney, G.R.; Cole, M.B.; Hazell, N.G.; Augustin, M.A. Critical Food and Nutrition Science Challenges for Plant-Based Meat Alternative Products. Crit. Rev. Food Sci. Nutr. 2022, 1–16. [Google Scholar] [CrossRef]
- McClements, D.J.; Grossmann, L. A Brief Review of the Science behind the Design of Healthy and Sustainable Plant-Based Foods. NPJ Sci. Food 2021, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Elhalis, H.; Chin, X.H.; Chow, Y. Soybean Fermentation: Microbial Ecology and Starter Culture Technology. Crit. Rev. Food Sci. Nutr. 2023, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Hadi, J.; Brightwell, G. Safety of Alternative Proteins: Technological, Environmental and Regulatory Aspects of Cultured Meat, Plant-Based Meat, Insect Protein and Single-Cell Protein. Foods 2021, 10, 1226. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Kwok, K.; Liang, H. Effects of Tea Polyphenols on the Activities of Soybean Trypsin Inhibitors and Trypsin. J. Sci. Food Agric. 2004, 84, 121–126. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, S.; Dar, B.N.; Singh, B. Optimization of Process for Reduction of Antinutritional Factors in Edible Cereal Brans. Food Sci. Technol. Int. 2012, 18, 445–454. [Google Scholar] [CrossRef]
- Rouhana, A.; Adler-Nissen, J.; Cogan, U.R.I.; Frøkiær, H. Heat Inactivation Kinetics of Trypsin Inhibitors during High Temperature-short Time Processing of Soymilk. J. Food Sci. 1996, 61, 265–269. [Google Scholar] [CrossRef]
- Sessa, D.J.; Haney, J.K.; Nelsen, T.C. Inactivation of Soybean Trypsin Inhibitors with Ascorbic Acid plus Copper. J. Agric. Food Chem. 1990, 38, 1469–1474. [Google Scholar] [CrossRef]
- Yuan, S.H.; Chang, S.K.C. Trypsin Inhibitor Activity in Laboratory-Produced and Commercial Soymilk. In Chemistry, Texture, and Flavor of Soy; ACS Publications: Washington, DC, USA; pp. 23–43. ISBN 1947-5918.
- Luo, Y.; Xie, W.; Xie, C.; Li, Y.; Gu, Z. Impact of Soaking and Phytase Treatments on Phytic Acid, Calcium, Iron and Zinc in Faba Bean Fractions. Int. J. Food Sci. Technol. 2009, 44, 2590–2597. [Google Scholar] [CrossRef]
- Kaleda, A.; Talvistu, K.; Tamm, M.; Viirma, M.; Rosend, J.; Tanilas, K.; Kriisa, M.; Part, N.; Tammik, M.-L. Impact of Fermentation and Phytase Treatment of Pea-Oat Protein Blend on Physicochemical, Sensory, and Nutritional Properties of Extruded Meat Analogs. Foods 2020, 9, 1059. [Google Scholar] [CrossRef]
- Kurek, M.A.; Onopiuk, A.; Pogorzelska-Nowicka, E.; Szpicer, A.; Zalewska, M.; Półtorak, A. Novel Protein Sources for Applications in Meat-Alternative Products—Insight and Challenges. Foods 2022, 11, 957. [Google Scholar] [CrossRef]
- Asif, M.; Qureshi, I.; Bangroo, S.; Mahdi, S.S.; Sheikh, F.A.; Bhat, M.A.; Alie, B.A.; Khan, M.H.; Dar, N.A.; Dar, Z.A.; et al. Reduction of Phytic Acid and Enhancement of Bioavailable Micronutrients in Common Beans (Phaseolus vulgaris L.) in Changing Climatic Scenario BT-Developing Climate Resilient Grain and Forage Legumes; Jha, U.C., Nayyar, H., Agrawal, S.K., Siddique, K.H.M., Eds.; Springer Nature: Singapore, 2022; pp. 59–76. ISBN 978-981-16-9848-4. [Google Scholar]
- El Mecherfi, K.-E.; Todorov, S.D.; Cavalcanti de Albuquerque, M.A.; Denery-Papini, S.; Lupi, R.; Haertlé, T.; Dora Gombossy de Melo Franco, B.; Larré, C. Allergenicity of Fermented Foods: Emphasis on Seeds Protein-Based Products. Foods 2020, 9, 792. [Google Scholar] [CrossRef] [PubMed]
- Gehring, J.; Touvier, M.; Baudry, J.; Julia, C.; Buscail, C.; Srour, B.; Hercberg, S.; Péneau, S.; Kesse-Guyot, E.; Allès, B. Consumption of Ultra-Processed Foods by Pesco-Vegetarians, Vegetarians, and Vegans: Associations with Duration and Age at Diet Initiation. J. Nutr. 2021, 151, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Poti, J.M.; Braga, B.; Qin, B. Ultra-Processed Food Intake and Obesity: What Really Matters for Health—Processing or Nutrient Content? Curr. Obes. Rep. 2017, 6, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Srour, B.; Sellem, L.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Deschasaux, M.; Fassier, P.; Latino-Martel, P.; Beslay, M. Consumption of Ultra-Processed Foods and Cancer Risk: Results from NutriNet-Santé Prospective Cohort. BMJ 2018, 360, k322. [Google Scholar] [CrossRef] [PubMed]
- Johns Hopkins Cancer Biologists Find DNA-Damaging Toxins in Common Plant-Based Foods. Available online: https://phys.org/news/2013-03-cancer-biologists-dna-damaging-toxins-common.html (accessed on 19 August 2023).
- Gallo, M.; Ferrara, L.; Calogero, A.; Montesano, D.; Naviglio, D. Relationships between food and diseases: What to know to ensure food safety. Food Res. Int. 2020, 137, 109414. [Google Scholar] [CrossRef]
- Amchova, P.; Kotolova, H.; Ruda-Kucerova, J. Health Safety Issues of Synthetic Food Colorants. Regul. Toxicol. Pharmacol. 2015, 73, 914–922. [Google Scholar] [CrossRef]
- Fraser, R.Z.; Shitut, M.; Agrawal, P.; Mendes, O.; Klapholz, S. Safety Evaluation of Soy Leghemoglobin Protein Preparation Derived from Pichia Pastoris, Intended for Use as a Flavor Catalyst in Plant-Based Meat. Int. J. Toxicol. 2018, 37, 241–262. [Google Scholar] [CrossRef]
- López, D.N.; Ingrassia, R.; Busti, P.; Wagner, J.; Boeris, V.; Spelzini, D. Effects of Extraction PH of Chia Protein Isolates on Functional Properties. LWT 2018, 97, 523–529. [Google Scholar] [CrossRef]
- Ogawa, Y.; Donlao, N.; Thuengtung, S.; Tian, J.; Cai, Y.; Reginio, F.C.; Ketnawa, S.; Yamamoto, N.; Tamura, M. Impact of Food Structure and Cell Matrix on Digestibility of Plant-Based Food. Curr. Opin. Food Sci. 2018, 19, 36–41. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, Y.; Tan, Y.; Zhang, Z.; McClements, D.J. Digestibility and Gastrointestinal Fate of Meat versus Plant-Based Meat Analogs: An in Vitro Comparison. Food Chem. 2021, 364, 130439. [Google Scholar] [CrossRef]
- Xie, Y.; Cai, L.; Huang, Z.; Shan, K.; Xu, X.; Zhou, G.; Li, C. Plant-Based Meat Analogues Weaken Gastrointestinal Digestive Function and Show Less Digestibility Than Real Meat in Mice. J. Agric. Food Chem. 2022, 70, 12442–12455. [Google Scholar] [CrossRef] [PubMed]
- Erbersdobler, H.F.; Barth, C.A.; Jahreis, G. Legumes in Human Nutrition. Nutrient Content and Protein Quality of Pulses. Ernahr. Umsch. 2017, 64, 134–139. [Google Scholar] [CrossRef]
- Ingenbleek, Y.; Kimura, H. Nutritional Essentiality of Sulfur in Health and Disease. Nutr. Rev. 2013, 71, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Hell, R.; Hillebrand, H. Plant Concepts for Mineral Acquisition and Allocation. Curr. Opin. Biotechnol. 2001, 12, 161–168. [Google Scholar] [CrossRef]
- Krishnan, H.B.; Jez, J.M. Review: The Promise and Limits for Enhancing Sulfur-Containing Amino Acid Content of Soybean Seed. Plant Sci. 2018, 272, 14–21. [Google Scholar] [CrossRef]
- van Vliet, S.; Bain, J.R.; Muehlbauer, M.J.; Provenza, F.D.; Kronberg, S.L.; Pieper, C.F.; Huffman, K.M. A Metabolomics Comparison of Plant-Based Meat and Grass-Fed Meat Indicates Large Nutritional Differences despite Comparable Nutrition Facts Panels. Sci. Rep. 2021, 11, 13828. [Google Scholar] [CrossRef]
- Wu, G. Important Roles of Dietary Taurine, Creatine, Carnosine, Anserine and 4-Hydroxyproline in Human Nutrition and Health. Amino Acids 2020, 52, 329–360. [Google Scholar] [CrossRef]
- Rokicki, J.; Li, L.; Imabayashi, E.; Kaneko, J.; Hisatsune, T.; Matsuda, H. Daily Carnosine and Anserine Supplementation Alters Verbal Episodic Memory and Resting State Network Connectivity in Healthy Elderly Adults. Front. Aging Neurosci. 2015, 7, 219. [Google Scholar] [CrossRef]
- Galili, G.; Amir, R. Fortifying Plants with the Essential Amino Acids Lysine and Methionine to Improve Nutritional Quality. Plant Biotechnol. J. 2013, 11, 211–222. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.-H.; Joo, S.-T. Meat Analog as Future Food: A Review. J. Anim. Sci. Technol. 2020, 62, 111. [Google Scholar] [CrossRef]
- Yu, S.; Zeng, W.; Xu, S.; Zhou, J. Expediting the Growth of Plant-Based Meat Alternatives by Microfluidic Technology: Identification of the Opportunities and Challenges. Curr. Opin. Biotechnol. 2022, 75, 102720. [Google Scholar] [CrossRef] [PubMed]
- Alcorta, A.; Porta, A.; Tárrega, A.; Alvarez, M.D.; Vaquero, M.P. Foods for Plant-Based Diets: Challenges and Innovations. Foods 2021, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Rojo, R.; Vaquero, M.P. Iron Bioavailability from Food Fortification to Precision Nutrition. A Review. Innov. Food Sci. Emerg. Technol. 2019, 51, 126–138. [Google Scholar] [CrossRef]
- Cole, E.; Goeler-Slough, N.; Cox, A.; Nolden, A. Examination of the Nutritional Composition of Alternative Beef Burgers Available in the United States. Int. J. Food Sci. Nutr. 2022, 73, 425–432. [Google Scholar] [CrossRef]
- Harnack, L.; Mork, S.; Valluri, S.; Weber, C.; Schmitz, K.; Stevenson, J.; Pettit, J. Nutrient Composition of a Selection of Plant-Based Ground Beef Alternative Products Available in the United States. J. Acad. Nutr. Diet. 2021, 121, 2401–2408. [Google Scholar] [CrossRef]
- Cutroneo, S.; Angelino, D.; Tedeschi, T.; Pellegrini, N.; Martini, D.; Dall’Asta, M.; Russo, M.D.; Nucci, D.; Moccia, S.; Paolella, G.; et al. Nutritional Quality of Meat Analogues: Results From the Food Labelling of Italian Products (FLIP) Project. Front. Nutr. 2022, 9, 852831. [Google Scholar] [CrossRef]
- Bryngelsson, S.; Moshtaghian, H.; Bianchi, M.; Hallström, E. Nutritional Assessment of Plant-Based Meat Analogues on the Swedish Market. Int. J. Food Sci. Nutr. 2022, 73, 889–901. [Google Scholar] [CrossRef]
- De Marchi, M.; Costa, A.; Pozza, M.; Goi, A.; Manuelian, C.L. Detailed Characterization of Plant-Based Burgers. Sci. Rep. 2021, 11, 2049. [Google Scholar] [CrossRef]
- McEvoy, C.T.; Temple, N.; Woodside, J. V Vegetarian Diets, Low-Meat Diets and Health: A Review. Public Health Nutr. 2012, 15, 2287–2294. [Google Scholar] [CrossRef]
- García-Maldonado, E.; Gallego-Narbón, A.; Vaquero, M. Son Las Dietas Vegetarianas Nutricionalmente Adecuadas? Una Revisión de La Evidencia Científica. Nutr. Hosp. 2019, 36, 950–961. [Google Scholar]
- Nychas, G.-J.E.; Panagou, E. Microbiological Spoilage of Foods and Beverages. In Woodhead Publishing Series in Food Science, Technology and Nutrition; Kilcast, D., Subramaniam, P., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 3–28. ISBN 978-1-84569-701-3. [Google Scholar]
- Samard, S.; Gu, B.; Ryu, G. Effects of Extrusion Types, Screw Speed and Addition of Wheat Gluten on Physicochemical Characteristics and Cooking Stability of Meat Analogues. J. Sci. Food Agric. 2019, 99, 4922–4931. [Google Scholar] [CrossRef]
- Tóth, A.J.; Dunay, A.; Battay, M.; Illés, C.B.; Bittsánszky, A.; Süth, M. Microbial Spoilage of Plant-Based Meat Analogues. Appl. Sci. 2021, 11, 8309. [Google Scholar] [CrossRef]
- Wild, F.; Czerny, M.; Janssen, A.M.; Kole, A.P.; Zunabovic, M.; Domig, K.J. The Evolution of a Plant-Based Alternative to Meat. Agro Food Industry Hi Tech 2014, 25, 45–49. [Google Scholar]
- Bawa, A.S.; Anilakumar, K.R. Genetically Modified Foods: Safety, Risks and Public Concerns-a Review. J. Food Sci. Technol. 2013, 50, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.; Reichhardt, T.; Abbott, A.; Dickson, D.; Saegusa, A. Long-Term Effect of GM Crops Serves up Food for Thought. Nature 1999, 398, 651–652. [Google Scholar] [CrossRef]
- Conner, A.J.; Jacobs, J.M.E. Genetic Engineering of Crops as Potential Source of Genetic Hazard in the Human Diet. Mutat. Res. /Genet. Toxicol. Environ. Mutagen. 1999, 443, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Schreuders, F.K.G.; Dekkers, B.L.; Bodnár, I.; Erni, P.; Boom, R.M.; van der Goot, A.J. Comparing Structuring Potential of Pea and Soy Protein with Gluten for Meat Analogue Preparation. J. Food Eng. 2019, 261, 32–39. [Google Scholar] [CrossRef]
- Michel, F.; Hartmann, C.; Siegrist, M. Consumers’ Associations, Perceptions and Acceptance of Meat and Plant-Based Meat Alternatives. Food Qual. Prefer. 2021, 87, 104063. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Dekkers, B.; van der Goot, A.J. Plant-Based Meat Analogues. In Sustainable Meat Production and Processing; Elsevier: Amsterdam, The Netherlands, 2019; pp. 103–126. [Google Scholar]
- Mayer Labba, I.-C.; Steinhausen, H.; Almius, L.; Bach Knudsen, K.E.; Sandberg, A.-S. Nutritional Composition and Estimated Iron and Zinc Bioavailability of Meat Substitutes Available on the Swedish Market. Nutrients 2022, 14, 3903. [Google Scholar] [CrossRef]
- Shirai, K.; Revah-Moiseev, S.; García-Garibay, M.; Marshall, V.M. Ability of Some Strains of Lactic Acid Bacteria to Degrade Phytic Acid. Lett. Appl. Microbiol. 1994, 19, 366–369. [Google Scholar] [CrossRef]
- Sutardi; Buckle, K.A. Phytic Acid Changes in Soybeans Fermented by Traditional Inoculum and Six Strains of Rhizopus Oligosporus. J. Appl. Bacteriol. 1985, 58, 539–543. [Google Scholar] [CrossRef]
- Villacrés, E.; Quelal, M.B.; Fernández, E.; Garcìa, G.; Cueva, G.; Rosell, C.M. Impact of Debittering and Fermentation Processes on the Antinutritional and Antioxidant Compounds in Lupinus Mutabilis Sweet. LWT 2020, 131, 109745. [Google Scholar] [CrossRef]
- Owens, J.D.; Allagheny, N.; Kipping, G.; Ames, J.M. Formation of Volatile Compounds during Bacillus Subtilis Fermentation of Soya Beans. J. Sci. Food Agric. 1997, 74, 132–140. [Google Scholar] [CrossRef]
- Shrestha, A.K.; Noomhorm, A. Composition and Functional Properties of Fermented Soybean Flour (Kinema). J. Food Sci. Technol. -Mysore 2001, 38, 467–470. [Google Scholar]
- Shrestha, A.K.; Dahal, N.R.; Ndungutse, V. Bacillus Fermentation of Soybean: A Review. J. Food Sci. Technol. Nepal 2010, 6, 1–9. [Google Scholar] [CrossRef]
- Coda, R.; Melama, L.; Rizzello, C.G.; Curiel, J.A.; Sibakov, J.; Holopainen, U.; Pulkkinen, M.; Sozer, N. Effect of Air Classification and Fermentation by Lactobacillus Plantarum VTT E-133328 on Faba Bean (Vicia faba L.) Flour Nutritional Properties. Int. J. Food Microbiol. 2015, 193, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Roy, F.; Boye, J.I.; Simpson, B.K. Bioactive Proteins and Peptides in Pulse Crops: Pea, Chickpea and Lentil. Food Res. Int. 2010, 43, 432–442. [Google Scholar] [CrossRef]
- Wang, Y.; Sorvali, P.; Laitila, A.; Maina, N.H.; Coda, R.; Katina, K. Dextran Produced in Situ as a Tool to Improve the Quality of Wheat-Faba Bean Composite Bread. Food Hydrocoll 2018, 84, 396–405. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Coda, R.; Säde, E.; Tuomainen, P.; Tenkanen, M.; Katina, K. In Situ Synthesis of Exopolysaccharides by Leuconostoc Spp. and Weissella Spp. and Their Rheological Impacts in Fava Bean Flour. Int. J. Food Microbiol. 2017, 248, 63–71. [Google Scholar] [CrossRef]
- Hu, Y.; Piao, C.; Chen, Y.; Zhou, Y.; Wang, D.; Yu, H.; Xu, B. Soybean Residue (Okara) Fermentation with the Yeast Kluyveromyces Marxianus. Food Biosci. 2019, 31, 100439. [Google Scholar] [CrossRef]
- Vong, W.C.; Liu, S.-Q. Bioconversion of Green Volatiles in Okara (Soybean residue) into Esters by Coupling Enzyme Catalysis and Yeast (Lindnera saturnus) Fermentation. Appl. Microbiol. Biotechnol. 2018, 102, 10017–10026. [Google Scholar] [CrossRef] [PubMed]
- Onwurafor, E.U.; Onweluzo, J.C.; Ezeoke, A.M. Effect of Fermentation Methods on Chemical and Microbial Properties of Mung Bean (Vigna radiata) Flour. Niger. Food J. 2014, 32, 89–96. [Google Scholar] [CrossRef]
- Fardiaz, D.; Markakis, P. Oligosaccharides and Protein Efficiency Ratio of Oncom (Fermented Peanut Press Cake). J. Food Sci. 1981, 46, 1970–1971. [Google Scholar] [CrossRef]
- Xiao, Y.; Xing, G.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Effect of Solid-State Fermentation with Cordyceps Militaris SN-18 on Physicochemical and Functional Properties of Chickpea (Cicer arietinum L.) Flour. LWT-Food Sci. Technol. 2015, 63, 1317–1324. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Przybylski, R.; Sultana, B.; Ashraf, M. Chemical Composition and Antioxidant Activity of Seeds of Different Cultivars of Mungbean. J. Food Sci. 2007, 72, S503–S510. [Google Scholar] [CrossRef]
- Wu, H.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Mung Bean (Vigna radiata) as Probiotic Food through Fermentation with Lactobacillus Plantarum B1-6. LWT-Food Sci. Technol. 2015, 63, 445–451. [Google Scholar] [CrossRef]
- Tangyu, M.; Muller, J.; Bolten, C.J.; Wittmann, C. Fermentation of Plant-Based Milk Alternatives for Improved Flavour and Nutritional Value. Appl. Microbiol. Biotechnol. 2019, 103, 9263–9275. [Google Scholar] [CrossRef]
- Gibson, R.S.; Bailey, K.B.; Gibbs, M.; Ferguson, E.L. A Review of Phytate, Iron, Zinc, and Calcium Concentrations in Plant-Based Complementary Foods Used in Low-Income Countries and Implications for Bioavailability. Food Nutr. Bull. 2010, 31, S134–S146. [Google Scholar] [CrossRef]
- Arbab Sakandar, H.; Chen, Y.; Peng, C.; Chen, X.; Imran, M.; Zhang, H. Impact of Fermentation on Antinutritional Factors and Protein Degradation of Legume Seeds: A Review. Food Rev. Int. 2021, 39, 1227–1249. [Google Scholar] [CrossRef]
- Tovar, L.E.R.; Gänzle, M.G. Degradation of Wheat Germ Agglutinin during Sourdough Fermentation. Foods 2021, 10, 340. [Google Scholar] [CrossRef]
- Tian, S.; Sun, Y.; Chen, Z.; Yang, Y.; Wang, Y. Functional Properties of Polyphenols in Grains and Effects of Physicochemical Processing on Polyphenols. J. Food Qual 2019, 2019, 2793973. [Google Scholar] [CrossRef]
- Molfetta, M.; Morais, E.G.; Barreira, L.; Bruno, G.L.; Porcelli, F.; Dugat-Bony, E.; Bonnarme, P.; Minervini, F. Protein Sources Alternative to Meat: State of the Art and Involvement of Fermentation. Foods 2022, 11, 2065. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Caggianiello, G.; Kleerebezem, M.; Spano, G. Exopolysaccharides Produced by Lactic Acid Bacteria: From Health-Promoting Benefits to Stress Tolerance Mechanisms. Appl. Microbiol. Biotechnol. 2016, 100, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Biscola, V.; de Olmos, A.R.; Choiset, Y.; Rabesona, H.; Garro, M.S.; Mozzi, F.; Chobert, J.-M.; Drouet, M.; Haertlé, T.; Franco, B.D.G.d.M. Soymilk Fermentation by Enterococcus Faecalis VB43 Leads to Reduction in the Immunoreactivity of Allergenic Proteins β-Conglycinin (7S) and Glycinin (11S). Benef. Microbes 2017, 8, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Meinlschmidt, P.; Schweiggert-Weisz, U.; Eisner, P. Soy Protein Hydrolysates Fermentation: Effect of Debittering and Degradation of Major Soy Allergens. LWT-Food Sci. Technol. 2016, 71, 202–212. [Google Scholar] [CrossRef]
- Rizzello, C.G.; De Angelis, M.; Coda, R.; Gobbetti, M. Use of Selected Sourdough Lactic Acid Bacteria to Hydrolyze Wheat and Rye Proteins Responsible for Cereal Allergy. Eur. Food Res. Technol. 2006, 223, 405–411. [Google Scholar] [CrossRef]
- Yang, A.; Zuo, L.; Cheng, Y.; Wu, Z.; Li, X.; Tong, P.; Chen, H. Degradation of Major Allergens and Allergenicity Reduction of Soybean Meal through Solid-State Fermentation with Microorganisms. Food Funct. 2018, 9, 1899–1909. [Google Scholar] [CrossRef]
- Scholz, M.B.d.S.; Prudencio, S.H.; Kitzberger, C.S.G.; Silva, R.S. dos S.F. da Physico-Chemical Characteristics and Sensory Attributes of Coffee Beans Submitted to Two Post-Harvest Processes. J. Food Meas. Charact. 2019, 13, 831–839. [Google Scholar] [CrossRef]
- Sun, X.; Shan, X.; Yan, Z.; Zhang, Y.; Guan, L. Prediction and Characterization of the Linear IgE Epitopes for the Major Soybean Allergen β-Conglycinin Using Immunoinformatics Tools. Food Chem. Toxicol. 2013, 56, 254–260. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, Y.; Liu, Y.; Chen, X.; Jiang, M.; Rui, X. Lactic Acid Bacteria Fermented Soy β-Conglycinin: Assessment of Structural Conformational Feature and Immunoglobulin E Reactivity. LWT 2023, 173, 114246. [Google Scholar] [CrossRef]
- Frias, J.; Song, Y.S.; Martínez-Villaluenga, C.; De Mejia, E.G.; Vidal-Valverde, C. Immunoreactivity and Amino Acid Content of Fermented Soybean Products. J. Agric. Food Chem. 2008, 56, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Jannathulla, R.; Dayal, J.S.; Vasanthakumar, D.; Ambasankar, K.; Muralidhar, M. Effect of Fungal Fermentation on Apparent Digestibility Coefficient for Dry Matter, Crude Protein and Amino Acids of Various Plant Protein Sources in Penaeus Vannamei. Aquac. Nutr. 2018, 24, 1318–1329. [Google Scholar] [CrossRef]
- Ahmed, A.; Zulkifli, I.; Farjam, A.S.; Abdullah, N.; Liang, J.B.; Awad, E.A. Effect of Solid State Fermentation on Nutrient Content and Ileal Amino Acids Digestibility of Canola Meal in Broiler Chickens. Ital. J. Anim. Sci. 2014, 13, 3293. [Google Scholar] [CrossRef]
- Yousif, N.E.; El Tinay, A.H. Effect of Fermentation on Sorghum Protein Fractions and in Vitro Protein Digestibility. Plant Foods Hum. Nutr. 2001, 56, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.A. Changes in Sorghum Enzyme Inhibitors, Phytic Acid, Tannins and in Vitro Protein Digestibility Occurring during Khamir (Local Bread) Fermentation. Food Chem. 2004, 88, 129–134. [Google Scholar] [CrossRef]
- Li, J.; Zhou, R.; Ren, Z.; Fan, Y.; Hu, S.; Zhuo, C.; Deng, Z. Improvement of Protein Quality and Degradation of Allergen in Soybean Meal Fermented by Neurospora Crassa. LWT 2019, 101, 220–228. [Google Scholar] [CrossRef]
- Yadav, S.; Khetarpaul, N. Indigenous Legume Fermentation: Effect on Some Antinutrients and in-Vitro Digestibility of Starch and Protein. Food Chem. 1994, 50, 403–406. [Google Scholar] [CrossRef]
- Cronk, T.C.; Steinkraus, K.H.; Hackler, L.R.; Mattick, L.R. Indonesian Tapé Ketan Fermentation. Appl. Environ. Microbiol. 1977, 33, 1067–1073. [Google Scholar] [CrossRef]
- Hou, J.-W.; Yu, R.-C.; Chou, C.-C. Changes in Some Components of Soymilk during Fermentation with Bifidobacteria. Food Res. Int. 2000, 33, 393–397. [Google Scholar] [CrossRef]
- Song, Y.-S.; Frías, J.; Martinez-Villaluenga, C.; Vidal-Valdeverde, C.; de Mejia, E.G. Immunoreactivity Reduction of Soybean Meal by Fermentation, Effect on Amino Acid Composition and Antigenicity of Commercial Soy Products. Food Chem. 2008, 108, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.-F.; Shi, Y.-H.; Sun, J.; Le, G.-W. Evaluation of Peanut Flour Fermented with Lactic Acid Bacteria as a Probiotic Food. Food Sci. Technol. Int. 2007, 13, 469–475. [Google Scholar] [CrossRef]
- Jannathulla, R.; Dayal, J.S.; Vasanthakumar, D.; Ambasankar, K.; Muralidhar, M. Effect of fermentation methods on amino acids, fiber fractions and anti nutritional factors in different plant protein sources and essential amino acid index for Penaeus (Litopenaeus) vannamei. Indian J. Fish. 2017, 64, 60341. [Google Scholar] [CrossRef]
- Handa, C.L.; de Lima, F.S.; Guelfi, M.F.G.; da Silva Fernandes, M.; Georgetti, S.R.; Ida, E.I. Parameters of the Fermentation of Soybean Flour by Monascus Purpureus or Aspergillus Oryzae on the Production of Bioactive Compounds and Antioxidant Activity. Food Chem. 2019, 271, 274–283. [Google Scholar] [CrossRef]
- Jeon, H.-L.; Yang, S.-J.; Son, S.-H.; Kim, W.-S.; Lee, N.-K.; Paik, H.-D. Evaluation of Probiotic Bacillus Subtilis P229 Isolated from Cheonggukjang and Its Application in Soybean Fermentation. LWT 2018, 97, 94–99. [Google Scholar] [CrossRef]
- Cui, J.; Xia, P.; Zhang, L.; Hu, Y.; Xie, Q.; Xiang, H. A Novel Fermented Soybean, Inoculated with Selected Bacillus, Lactobacillus and Hansenula Strains, Showed Strong Antioxidant and Anti-Fatigue Potential Activity. Food Chem. 2020, 333, 127527. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, A.; Singh, R.; Pandey, K.D. Endophytic Bacteria: A New Source of Bioactive Compounds. 3 Biotech 2017, 7, 315. [Google Scholar] [CrossRef]
- Lyu, X.; Lee, J.; Chen, W.N. Potential Natural Food Preservatives and Their Sustainable Production in Yeast: Terpenoids and Polyphenols. J. Agric. Food Chem. 2019, 67, 4397–4417. [Google Scholar] [CrossRef]
- Wang, J.; Guleria, S.; Koffas, M.A.G.; Yan, Y. Microbial Production of Value-Added Nutraceuticals. Curr. Opin. Biotechnol. 2016, 37, 97–104. [Google Scholar] [CrossRef]
- Ha, L.T.N. Phenolic Compounds and Human Health Benefits. Vietnam J. Agri. Sci. 2016, 14, 1107–1118. [Google Scholar]
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P.K. Microbial Metabolites in Nutrition, Healthcare and Agriculture. 3 Biotech 2017, 7, 15. [Google Scholar] [CrossRef]
- Chuah, H.Q.; Tang, P.L.; Ang, N.J.; Tan, H.Y. Submerged Fermentation Improves Bioactivity of Mulberry Fruits and Leaves. Chin. Herb. Med. 2021, 13, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Bei, Q.; Chen, G.; Lu, F.; Wu, S.; Wu, Z. Enzymatic Action Mechanism of Phenolic Mobilization in Oats (Avena sativa L.) during Solid-State Fermentation with Monascus Anka. Food Chem. 2018, 245, 297–304. [Google Scholar] [CrossRef]
- Gao, M.-Z.; Cui, Q.; Wang, L.-T.; Meng, Y.; Yu, L.; Li, Y.-Y.; Fu, Y.-J. A Green and Integrated Strategy for Enhanced Phenolic Compounds Extraction from Mulberry (Morus alba L.) Leaves by Deep Eutectic Solvent. Microchem. J. 2020, 154, 104598. [Google Scholar] [CrossRef]
- Rekha, C.R.; Vijayalakshmi, G. Bioconversion of Isoflavone Glycosides to Aglycones, Mineral Bioavailability and Vitamin B Complex in Fermented Soymilk by Probiotic Bacteria and Yeast. J. Appl. Microbiol. 2010, 109, 1198–1208. [Google Scholar] [CrossRef]
- Greiner, R.; Konietzny, U. Phytase for Food Application. Food Technol. Biotechnol. 2006, 44, 125–140. [Google Scholar]
- Claxson, A.; Morris, C.; Blake, D.; Sirén, M.; Halliwell, B.; Gustafsson, T.; Löfkvist, B.; Bergelin, I. The Anti-Inflammatory Effects Ofd-Myo-Inositol-1.2.6-Trisphosphate (PP56) on Animal Models of Inflammation. Agents Actions 1990, 29, 68–70. [Google Scholar] [CrossRef]
- Carrington, A.L.; Calcutt, N.A.; Ettlinger, C.B.; Gustafsson, T.; Tomlinson, D.R. Effects of Treatment with Myo-Inositol or Its 1,2,6-Trisphosphate (PP56) on Nerve Conduction in Streptozotocin-Diabetes. Eur. J. Pharmacol. 1993, 237, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Matos, Â.P. The Impact of Microalgae in Food Science and Technology. J. Am. Oil Chem. Soc. 2017, 94, 1333–1350. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A Potential Alternative to Health Supplementation for Humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Xia, S.; Xue, Y.; Xue, C.; Jiang, X.; Li, J. Structural and Rheological Properties of Meat Analogues from Haematococcus Pluvialis Residue-Pea Protein by High Moisture Extrusion. LWT 2022, 154, 112756. [Google Scholar] [CrossRef]
- Stiles, W.A.V.; Styles, D.; Chapman, S.P.; Esteves, S.; Bywater, A.; Melville, L.; Silkina, A.; Lupatsch, I.; Grünewald, C.F.; Lovitt, R. Using Microalgae in the Circular Economy to Valorise Anaerobic Digestate: Challenges and Opportunities. Bioresour. Technol. 2018, 267, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Shirai, F.; Kunii, K.; Sato, C.; Teramoto, Y.; Mizuki, E.; Murao, S.; Nakayama, S. Cultivation of Microalgae in the Solution from the Desalting Process of Soy Sauce Waste Treatment and Utilization of the Algal Biomass for Ethanol Fermentation. World J. Microbiol. Biotechnol. 1998, 14, 839–842. [Google Scholar] [CrossRef]
- Koutra, E.; Grammatikopoulos, G.; Kornaros, M. Selection of Microalgae Intended for Valorization of Digestate from Agro-Waste Mixtures. Waste Manag. 2018, 73, 123–129. [Google Scholar] [CrossRef]
- Chong, J.W.R.; Khoo, K.S.; Yew, G.Y.; Leong, W.H.; Lim, J.W.; Lam, M.K.; Ho, Y.-C.; Ng, H.S.; Munawaroh, H.S.H.; Show, P.L. Advances in Production of Bioplastics by Microalgae Using Food Waste Hydrolysate and Wastewater: A Review. Bioresour. Technol. 2021, 342, 125947. [Google Scholar] [CrossRef]
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F. Microalgae as Multi-Functional Options in Modern Agriculture: Current Trends, Prospects and Challenges. Biotechnol. Adv. 2018, 36, 1255–1273. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Cerdá-Bernad, D.; Pastor, J.-J.; Frutos, M.-J. Non-Dairy Fermented Beverages as Potential Carriers to Ensure Probiotics, Prebiotics, and Bioactive Compounds Arrival to the Gut and Their Health Benefits. Nutrients 2020, 12, 1666. [Google Scholar] [CrossRef]
- Boukid, F.; Hassoun, A.; Zouari, A.; Tülbek, M.Ç.; Mefleh, M.; Aït-Kaddour, A.; Castellari, M. Fermentation for Designing Innovative Plant-Based Meat and Dairy Alternatives. Foods 2023, 12, 1005. [Google Scholar] [CrossRef]
- Yan, W.; Jia, X.; Zhang, Q.; Chen, H.; Zhu, Q.; Yin, L. Interpenetrating Polymer Network Hydrogels of Soy Protein Isolate and Sugar Beet Pectin as a Potential Carrier for Probiotics. Food Hydrocoll. 2021, 113, 106453. [Google Scholar] [CrossRef]
- Marasas, W.F.O.; Gelderblom, W.C.A.; Shephard, G.S.; Vismer, H.F. Mycotoxins: A Global Problem. In Mycotoxins: Detection Methods, Management, Public Health and Agricultural Trade; CABI: Wallingford, UK, 2008; pp. 29–39. [Google Scholar]
- Lee, J.; Her, J.Y.; Lee, K.G. Reduction of Aflatoxins (B1, B2, G1, and G2) in Soybean-Based Model Systems. Food Chem. 2015, 189, 45–51. [Google Scholar] [CrossRef]
- Tian, F.; Woo, S.Y.; Lee, S.Y.; Park, S.B.; Im, J.H.; Chun, H.S. Mycotoxins in Soybean-Based Foods Fermented with Filamentous Fungi: Occurrence and Preventive Strategies. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5131–5152. [Google Scholar] [CrossRef] [PubMed]
- Petchkongkaew, A.; Taillandier, P.; Gasaluck, P.; Lebrihi, A. Isolation of Bacillus Spp. from Thai Fermented Soybean (Thua-nao): Screening for Aflatoxin B1 and Ochratoxin A Detoxification. J. Appl. Microbiol. 2008, 104, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Bahuguna, A.; Lee, J.S.; Sood, A.; Han, S.S.; Chun, H.S.; Kim, M. Degradation Mechanism of Aflatoxin B1 and Aflatoxin G1 by Salt Tolerant Bacillus Albus YUN5 Isolated from ‘Doenjang’, a Traditional Korean Food. Food Res. Int. 2023, 165, 112479. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspectives of Their Co-Occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef] [PubMed]
- Linares, D.M.; Martín, M.; Ladero, V.; Alvarez, M.A.; Fernández, M. Biogenic Amines in Dairy Products. Crit. Rev. Food Sci. Nutr. 2011, 51, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Pircher, A.; Bauer, F.; Paulsen, P. Formation of Cadaverine, Histamine, Putrescine and Tyramine by Bacteria Isolated from Meat, Fermented Sausages and Cheeses. Eur. Food Res. Technol. 2007, 226, 225–231. [Google Scholar] [CrossRef]
- Marino, M.; Maifreni, M.; Moret, S.; Rondinini, G. The Capacity of Enterobacteriaceae Species to Produce Biogenic Amines in Cheese. Lett. Appl. Microbiol. 2000, 31, 169–173. [Google Scholar] [CrossRef]
- Fernández, M.; Hudson, J.A.; Korpela, R.; de los Reyes-Gavilán, C.G. Impact on Human Health of Microorganisms Present in Fermented Dairy Products: An Overview. BioMed Res. Int. 2015, 2015, 412714. [Google Scholar] [CrossRef]
- Bermúdez, R.; Lorenzo, J.; Fonseca, S.; Franco, I.; Carballo, J. Strains of Staphylococcus and Bacillus Isolated from Traditional Sausages as Producers of Biogenic Amines. Front. Microbiol. 2012, 3, 151. [Google Scholar] [CrossRef]
- Ladero, V.; Rattray, F.P.; Mayo, B.; Martín, M.C.; Fernández, M.; Alvarez, M.A. Sequencing and Transcriptional Analysis of the Biosynthesis Gene Cluster of Putrescine-Producing Lactococcus Lactis. Appl. Environ. Microbiol. 2011, 77, 6409–6418. [Google Scholar] [CrossRef]
- Fernández, M.; Linares, D.M.; Alvarez, M.A. Sequencing of the Tyrosine Decarboxylase Cluster of Lactococcus Lactis IPLA 655 and the Development of a PCR Method for Detecting Tyrosine Decarboxylating Lactic Acid Bacteria. J. Food Prot. 2004, 67, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Bunčić, S.; Paunović, L.j.; Teodorović, V.; Radišić, D.; Vojinović, G.; Smiljanić, D.; Baltić, M. Effects of Gluconodeltalactone and Lactobacillus Plantarum on the Production of Histamine and Tyramine in Fermented Sausages. Int. J. Food Microbiol. 1993, 17, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Maijala, R.L.; Eerola, S.H.; Aho, M.A.; Hirn, J.A. The Effect of GDL-Induced PH Decrease on the Formation of Biogenic Amines in Meat. J. Food Prot. 1993, 56, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Luo, W.; Fu, M.; Yu, Y.; Wu, J.; Xu, Y.; Li, L. Effects of Selected Bacillus Strains on the Biogenic Amines, Bioactive Ingredients and Antioxidant Capacity of Shuidouchi. Int. J. Food Microbiol. 2023, 388, 110084. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, S. The Importance of Amine-Degrading Enzymes on the Biogenic Amine Degradation in Fermented Foods: A Review. Process Biochem. 2020, 99, 331–339. [Google Scholar] [CrossRef]
- García-Ruiz, A.; González-Rompinelli, E.M.; Bartolomé, B.; Moreno-Arribas, M.V. Potential of Wine-Associated Lactic Acid Bacteria to Degrade Biogenic Amines. Int. J. Food Microbiol. 2011, 148, 115–120. [Google Scholar] [CrossRef]
- Beneduce, L.; Romano, A.; Capozzi, V.; Lucas, P.; Barnavon, L.; Bach, B.; Vuchot, P.; Grieco, F.; Spano, G. Biogenic Amine in Wines. Ann. Microbiol. 2010, 60, 573–578. [Google Scholar] [CrossRef]
- Capozzi, V.; Russo, P.; Ladero, V.; Fernandez, M.; Fiocco, D.; Alvarez, M.A.; Grieco, F.; Spano, G. Biogenic Amines Degradation by Lactobacillus Plantarum: Toward a Potential Application in Wine. Front. Microbiol. 2012, 3, 122. [Google Scholar] [CrossRef]
- Bäumlisberger, M.; Moellecken, U.; König, H.; Claus, H. The Potential of the Yeast Debaryomyces Hansenii H525 to Degrade Biogenic Amines in Food. Microorganisms 2015, 3, 839–850. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Probiotic Fermentation of Plant Based Products: Possibilities and Opportunities. Crit. Rev. Food Sci. Nutr. 2012, 52, 183–199. [Google Scholar] [CrossRef]
- Su, L.-W.; Cheng, Y.-H.; Hsiao, F.S.-H.; Han, J.-C.; Yu, Y.-H. Optimization of Mixed Solid-State Fermentation of Soybean Meal by Lactobacillus Species and Clostridium Butyricum. Pol. J. Microbiol. 2018, 67, 297. [Google Scholar] [CrossRef]
- Fuhrmeister, H.; Meuser, F. Impact of Processing on Functional Properties of Protein Products from Wrinkled Peas. J. Food Eng. 2003, 56, 119–129. [Google Scholar] [CrossRef]
- Taherian, A.R.; Mondor, M.; Labranche, J.; Drolet, H.; Ippersiel, D.; Lamarche, F. Comparative Study of Functional Properties of Commercial and Membrane Processed Yellow Pea Protein Isolates. Food Res. Int. 2011, 44, 2505–2514. [Google Scholar] [CrossRef]
- Li, X.; Li, J. The Flavor of Plant-Based Meat Analogues. Cereal Foods World 2020, 65, 40. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.-C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA Food Classification and the Trouble with Ultra-Processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef]
- Rehrah, D.; Ahmedna, M.; Goktepe, I.; Yu, J. Extrusion Parameters and Consumer Acceptability of a Peanut-Based Meat Analogue. Int. J. Food Sci. Technol. 2009, 44, 2075–2084. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Dekkers, B.; van der Goot, A.J. Functionality of Ingredients and Additives in Plant-Based Meat Analogues. In Foods 10, No. 3: 600; Elsevier: Amsterdam, The Netherlands, 2021; pp. 103–126. [Google Scholar]
- Sakai, K.; Sato, Y.; Okada, M.; Yamaguchi, S. Synergistic Effects of Laccase and Pectin on the Color Changes and Functional Properties of Meat Analogs Containing Beet Red Pigment. Sci. Rep. 2022, 12, 1168. [Google Scholar] [CrossRef]
- Bohrer, B.M. An Investigation of the Formulation and Nutritional Composition of Modern Meat Analogue Products. Food Sci. Hum. Wellness 2019, 8, 320–329. [Google Scholar] [CrossRef]
- Tuccillo, F.; Wang, Y.; Edelmann, M.; Lampi, A.-M.; Coda, R.; Katina, K. Fermentation Conditions Affect the Synthesis of Volatile Compounds, Dextran, and Organic Acids by Weissella Confusa A16 in Faba Bean Protein Concentrate. Foods 2022, 11, 3579. [Google Scholar] [CrossRef]
- Piao, M.Y.; Lee, H.J.; Yong, H.I.; Beak, S.-H.; Kim, H.J.; Jo, C.; Wiryawan, K.G.; Baik, M. Comparison of Reducing Sugar Content, Sensory Traits, and Fatty Acids and Volatile Compound Profiles of the Longissimus Thoracis among Korean Cattle, Holsteins, and Angus Steers. Asian-Australas J. Anim. Sci. 2019, 32, 126–136. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Chen, D.; Yu, B.; Yin, J.; Huang, Z. Effects of Dietary Apple Polyphenol Supplementation on Carcass Traits, Meat Quality, Muscle Amino Acid and Fatty Acid Composition in Finishing Pigs. Food Funct. 2019, 10, 7426–7434. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-J.; Oh, S.-H.; Pridmore, R.D.; Juillerat, M.A.; Lee, C.-H. Purification and Characterization of Proteases from Bacillus Amyloliquefaciens Isolated from Traditional Soybean Fermentation Starter. J. Agric. Food Chem. 2003, 51, 7664–7670. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Tang, B.; Wang, Q.; Xu, Z.; Sun, L.; Ma, J.; Li, S.; Xu, H.; Lei, P. The Bio-Processing of Soybean Dregs by Solid State Fermentation Using a Poly γ-Glutamic Acid Producing Strain and Its Effect as Feed Additive. Bioresour. Technol. 2019, 291, 121841. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.K.; Tamang, J.P. Changes in the Microbial Profile and Proximate Composition during Natural and Controlled Fermentations of Soybeans to Produce Kinema. Food Microbiol. 1995, 12, 317–325. [Google Scholar] [CrossRef]
- Abbas, H.; Hiol, A.; Deyris, V.; Comeau, L. Isolation and Characterization of an Extracellular Lipase from Mucor Sp Strain Isolated from Palm Fruit. Enzym. Microb. Technol. 2002, 31, 968–975. [Google Scholar] [CrossRef]
- Hiol, A.; Jonzo, M.D.; Rugani, N.; Druet, D.; Sarda, L.; Comeau, L.C. Purification and Characterization of an Extracellular Lipase from a Thermophilic Rhizopus Oryzae Strain Isolated from Palm Fruit. Enzym. Microb. Technol. 2000, 26, 421–430. [Google Scholar] [CrossRef]
- Stødkilde, L.; Ambye-Jensen, M.; Jensen, S.K. Biorefined Organic Grass-Clover Protein Concentrate for Growing Pigs: Effect on Growth Performance and Meat Fatty Acid Profile. Anim. Feed. Sci. Technol. 2021, 276, 114943. [Google Scholar] [CrossRef]
- Gu, B.Y.; Kim, M.H.; Ryu, G.H. Fermentation of Texturized Vegetable Proteins Extruded at Different Moisture Contents: Effect on Physicochemical, Structural, and Microbial Properties. Food. Sci. Biotechnol. 2020, 29, 897–907. [Google Scholar] [CrossRef]
- Day, L. Proteins from Land Plants–Potential Resources for Human Nutrition and Food Security. Trends Food Sci. Technol. 2013, 32, 25–42. [Google Scholar] [CrossRef]
- McCarthy, N.A.; Kennedy, D.; Hogan, S.A.; Kelly, P.M.; Thapa, K.; Murphy, K.M.; Fenelon, M.A. Emulsification Properties of Pea Protein Isolate Using Homogenization, Microfluidization and Ultrasonication. Food Res. Int. 2016, 89, 415–421. [Google Scholar] [CrossRef]
- Ruxton, C.H.S.; McMillan, B. The Impact of Mycoprotein on Blood Cholesterol Levels: A Pilot Study. Br. Food J. 2010, 112, 1092–1101. [Google Scholar] [CrossRef]
- Souza Filho, P.F.; Nair, R.B.; Andersson, D.; Lennartsson, P.R.; Taherzadeh, M.J. Vegan-Mycoprotein Concentrate from Pea-Processing Industry Byproduct Using Edible Filamentous Fungi. Fungal Biol. Biotechnol. 2018, 5, 5. [Google Scholar] [CrossRef]
- Upcraft, T.; Tu, W.-C.; Johnson, R.; Finnigan, T.; Van Hung, N.; Hallett, J.; Guo, M. Protein from Renewable Resources: Mycoprotein Production from Agricultural Residues. Green Chem. 2021, 23, 5150–5165. [Google Scholar] [CrossRef]
- Williamson, D.A.; Geiselman, P.J.; Lovejoy, J.; Greenway, F.; Volaufova, J.; Martin, C.K.; Arnett, C.; Ortego, L. Effects of Consuming Mycoprotein, Tofu or Chicken upon Subsequent Eating Behaviour, Hunger and Safety. Appetite 2006, 46, 41–48. [Google Scholar] [CrossRef]
- Finnigan, T.; Needham, L.; Abbott, C. Mycoprotein: A Healthy New Protein with a Low Environmental Impact. In Sustainable Protein Sources; Elsevier: Amsterdam, The Netherlands, 2017; pp. 305–325. [Google Scholar]
- Liu, S.-Q. Impact of Yeast and Bacteria on Beer Appearance and Flavour. In Woodhead Publishing Series in Food Science, Technology and Nutrition; Hill, A.E., Ed.; Woodhead Publishing: Oxford, UK, 2015; pp. 357–374. ISBN 978-1-78242-331-7. [Google Scholar] [CrossRef]
- Numfor, F.A.; Walter Jr, W.M.; Schwartz, S.J. Physicochemical Changes in Cassava Starch and Flour Associated With Fermentation: Effect on Textural Properties. Starch-Stärke 1995, 47, 86–91. [Google Scholar] [CrossRef]
- Jia, R.; Zhang, F.; Song, Y.; Lou, Y.; Zhao, A.; Liu, Y.; Peng, H.; Hui, Y.; Ren, R.; Wang, B. Physicochemical and Textural Characteristics and Volatile Compounds of Semihard Goat Cheese as Affected by Starter Cultures. J. Dairy Sci. 2021, 104, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Korcari, D.; Secchiero, R.; Laureati, M.; Marti, A.; Cardone, G.; Rabitti, N.S.; Ricci, G.; Fortina, M.G. Technological Properties, Shelf Life and Consumer Preference of Spelt-Based Sourdough Bread Using Novel, Selected Starter Cultures. LWT 2021, 151, 112097. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Pang, G.; Wang, S. Effect of Inoculation of Starter on Physicochemical Properties and Texture Characteristics of Fermented Beef Jerky. J. Food Process. Preserv. 2021, 45, e15744. [Google Scholar] [CrossRef]
- Steinkraus, K. Industrialization of Indigenous Fermented Foods, Revised and Expanded; CRC Press: Boca Raton, FL, USA, 2004; ISBN 0429215622. [Google Scholar]
- Elhalis, H.; Cox, J.; Zhao, J. Coffee Fermentation: Expedition from Traditional to Controlled Process and Perspectives for Industrialization. Appl. Food Res. 2023, 3, 100253. [Google Scholar] [CrossRef]
- Helmy, M.; Elhalis, H.; Yan, L.; Chow, Y.; Selvarajoo, K. Perspective: Multi-Omics and Machine Learning Help Unleash the Alternative Food Potential of Microalgae. Adv. Nutr. 2022, 14, 1–11. [Google Scholar] [CrossRef]
- Helmy, M.; Smith, D.; Selvarajoo, K. Systems Biology Approaches Integrated with Artificial Intelligence for Optimized Metabolic Engineering. Metab. Eng. Commun. 2020, 11, e00149. [Google Scholar] [CrossRef]
- Pérez-Miranda, S.; Cabirol, N.; George-Téllez, R.; Zamudio-Rivera, L.S.; Fernández, F.J. O-CAS, a Fast and Universal Method for Siderophore Detection. J. Microbiol. Methods 2007, 70, 127–131. [Google Scholar] [CrossRef]
- Kasana, R.C.; Salwan, R.; Yadav, S.K. Microbial Proteases: Detection, Production, and Genetic Improvement. Crit. Rev. Microbiol. 2011, 37, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Tang, J.; Zhang, Z.; Wu, Z.; Zhong, A.; Li, Z.; Wang, Y. Correlation between Flavor Compounds and Microorganisms of Chaling Natural Fermented Red Sufu. LWT 2022, 154, 112873. [Google Scholar] [CrossRef]
- Thomson, C.A.; Delaquis, P.J.; Mazza, G. Detection and Measurement of Microbial Lipase Activity: A Review. Crit. Rev. Food Sci. Nutr. 1999, 39, 165–187. [Google Scholar] [CrossRef]
- Pimentel, G.; Burton, K.J.; von Ah, U.; Bütikofer, U.; Pralong, F.P.; Vionnet, N.; Portmann, R.; Vergères, G. Metabolic Footprinting of Fermented Milk Consumption in Serum of Healthy Men. J. Nutr. 2018, 148, 851–860. [Google Scholar] [CrossRef]
- Camargo Prado, F.; De Dea Lindner, J.; Inaba, J.; Thomaz-Soccol, V.; Kaur Brar, S.; Soccol, C.R. Development and Evaluation of a Fermented Coconut Water Beverage with Potential Health Benefits. J. Funct. Foods 2015, 12, 489–497. [Google Scholar] [CrossRef]
- dos Santos Cruxen, C.E.; Funck, G.D.; Haubert, L.; da Silva Dannenberg, G.; de Lima Marques, J.; Chaves, F.C.; da Silva, W.P.; Fiorentini, Â.M.; Cruxen, C.E.d.S.; Funck, G.D.; et al. Selection of Native Bacterial Starter Culture in the Production of Fermented Meat Sausages: Application Potential, Safety Aspects, and Emerging Technologies. Food Res. Int. 2019, 122, 371–382. [Google Scholar] [CrossRef]
- Elhalis, H.; Cox, J.; Frank, D.; Zhao, J. Microbiological and Biochemical Performances of Six Yeast Species as Potential Starter Cultures for Wet Fermentation of Coffee Beans. LWT 2021, 137, 110430. [Google Scholar] [CrossRef]
- Pereira, G.V.d.M.; Miguel, M.G.d.C.P.; Ramos, C.L.; Schwan, R.F. Microbiological and Physicochemical Characterization of Small-Scale Cocoa Fermentations and Screening of Yeast and Bacterial Strains to Develop a Defined Starter Culture. Appl. Environ. Microbiol. 2012, 78, 5395–5405. [Google Scholar] [CrossRef]
- Fleet, G.H. Wine Yeasts for the Future. FEMS Yeast Res. 2008, 8, 979–995. [Google Scholar] [CrossRef] [PubMed]
- Soubeyrand, V.; Julien, A.; Sablayrolles, J.-M. Rehydration Protocols for Active Dry Wine Yeasts and the Search for Early Indicators of Yeast Activity. Am. J. Enol. Vitic. 2006, 57, 474–480. [Google Scholar] [CrossRef]
- Mogensen, G. Starter Cultures BT-Technology of Reduced-Additive Foods; Smith, J., Ed.; Springer: Boston, MA, USA, 1993; pp. 1–25. ISBN 978-1-4615-2115-0. [Google Scholar]
- Xiao, Z.; Lv, C.; Gao, C.; Qin, J.; Ma, C.; Liu, Z.; Liu, P.; Li, L.; Xu, P. A Novel Whole-Cell Biocatalyst with NAD+ Regeneration for Production of Chiral Chemicals. PLoS ONE 2010, 5, e8860. [Google Scholar] [CrossRef]
- Yu, L.; Pei, X.; Lei, T.; Wang, Y.; Feng, Y. Genome Shuffling Enhanced L-Lactic Acid Production by Improving Glucose Tolerance of Lactobacillus Rhamnosus. J. Biotechnol. 2008, 134, 154–159. [Google Scholar] [CrossRef]
- Shi, T.-Q.; Liu, G.-N.; Ji, R.-Y.; Shi, K.; Song, P.; Ren, L.-J.; Huang, H.; Ji, X.-J. CRISPR/Cas9-Based Genome Editing of the Filamentous Fungi: The State of the Art. Appl. Microbiol. Biotechnol. 2017, 101, 7435–7443. [Google Scholar] [CrossRef] [PubMed]
- Sarrocco, S.; Vannacci, G. Preharvest Application of Beneficial Fungi as a Strategy to Prevent Postharvest Mycotoxin Contamination: A Review. Crop. Prot. 2018, 110, 160–170. [Google Scholar] [CrossRef]
- Johansen, E. Future Access and Improvement of Industrial Lactic Acid Bacteria Cultures. Microb. Cell Fact. 2017, 16, 230. [Google Scholar] [CrossRef] [PubMed]
| Food | Nutritional Content (%) | Advantages | Disadvantages | References | ||
|---|---|---|---|---|---|---|
| Protein | Fat | PDCAAS 1 | ||||
| Meat | High protein content and protein digestibility High consumer acceptance and sensory quality Good source of iron and vitamin B12 | Resource-intensive production Animal-welfare concerns Red meat consumption linked to adverse health effects (e.g., cancer and cardiovascular disease) | ||||
| Chicken | 22.3–22.7 | 0.9–2.1 | 0.95 | [43,44,45] | ||
| Beef | 20.6–22.5 | 4.3–6.8 | 0.92 | [43,44,46] | ||
| Pork | 21.8 | 4.0 | - | [44,47] | ||
| Mutton | 20.2–21.6 | 4.6–8.0 | 0.99 | [43,44] | ||
| Meat alternatives | ||||||
| Tempeh (fermented whole soybean) | 61.9–56.9 | 8.4–23.9 | 0.92–0.99 | Good source of protein, low in saturated fat Good source of iron and fiber Free of cholesterol High digestibility More resource-efficient production than meat Low allergenicity, fermentation breaks down allergenic proteins | Lack sulfur-containing amino acids, including methionine and cysteine Lack of vitamin B12, except if vitamin B12-producing bacteria present during fermentation Low consumer acceptance Sensory quality is different from meat | [48,49,50] |
| Tofu (made from soymilk) | 11.3 | 7.84 | 0.56–0.70 | Rich in B vitamins, and low in sodium Net protein utilization (NPU) is estimated to be around 65%, making it comparable to chicken meat in terms of assimilation and digestion More resource efficient production than meat | Lack sulfur-containing amino acids Lower digestibility than meat Loss of nutritional and nutraceutical contents during processing Presence of anti-nutritive factors Lack flavors causing a low consumer acceptability | [51,52,53,54] |
| Seitan (made from wheat gluten) | 34.3 | 0.78 | 0. 23 | Consumption of 100 g provides 61.2–74.5% of recommended daily protein | Low in lysine | [53,55,56] |
| Its fibrous structure and high protein content make it an excellent meat substitute | Low digestibility | |||||
| Its sensory properties can be easily modulated by spices and flavors during manufacturing due to its neutral taste and aroma | Sensory quality is closer to meat than tempeh and tofu but still not a perfect real meat analogy | |||||
| Quorn (mycoprotein, made from Fusarium venenatum) | 9.4–11.5 | 2.6 | 0.91 | High protein digestibility, low in saturated fat Low antinutrient content More resource-efficient production than meat High fiber content Texture more like meat compared to plant proteins | Lower levels of iron and vitamin B12 than real meat May cause allergies and/or gastrointestinal symptoms Possible presence of mycotoxins after inoculating F. venenatum into rice culture | [57,58,59,60,61] |
| Texturized plant protein 2 Soybean isolates Wheat gluten Pea protein concentrates | 87.0 80.0 50.0–85.0 | <0.1 - <1 | ~1.0 0.26 0.73 | High protein content, low in saturated fat, free from cholesterol Fibrous structure and texture like meat Possible to blend protein sources to achieve a more complete amino acid profile | Deficient in micronutrients that are common in meat (e.g., vitamin B12 and iron) Considered as ultra-processed foods associated with adverse health effects Usually not clean label as additives are added to modulate the sensory properties (e.g., texture, color, and flavor). These additives may not diffuse in the product homogeneously, leading the worse sensory quality than meat | [62,63,64,65,66,67,68] |
| Target | Ingredients and Processes | Functions | Limitation of the Current Methods | References |
|---|---|---|---|---|
| Enhance product safety | Heat Add ascorbic acid, essential oils, curcumin, polyphenols, tocopherols, spices, carotenoids, and herbs | Minimize product contamination and food poisoning Improve product shelf-life and health | Survival of food spoilage and pathogens Resistance of anti-nutritional factors, such as saponins, alkaloids, phytates Failure to completely remove allergens such as soybean protein and gluten Some used additives are correlated to human diseases and public concerns Considered as ultra-processed products cause obesity and cancer | [70,77,89,91,92,112,122,127] |
| Improve product nutrition | Blend proteins, carbohydrates, and oils Fortification and encapsulation for micronutrients, including minerals, and vitamins | Qualify as good sources of protein, energy, and fiber Increases the concentration and bioavailability of essential nutrients overcome their deficiencies | The extensiveness of processes and functional ingredients and additives make it an expensive purchase Damage heat-labile nutrients during processing Presence of phytates reduces bioavailability of essential minerals | [74,75,76,112,119] |
| Fermentation by | Contributions to | References |
|---|---|---|
| Bacillus subtilis/Bacillus velezensis/ Ligilactobacillus salivarius/Weissella spp./Leuconostoc spp./Lactiplantibacillus plantarum Lactobacillus casei/ Pichia anomala/Saccharomyces cerevisiae/ Neurospora crassa/Monascus purpureus/Aspergillus oryzae/ Rhizopus oligosporus | Improves digestibility (breakdown of polysaccharides, proteins, and lipids) | [134,135,136,164,165,166,167] |
| Weissella spp./Leuconostoc spp. L. plantarum/ L. casei | Decreases trypsin inhibitors, phytates, tannins, and convicine | [137,139,140] |
| Kluyveromyces marxianus/ Lindnera saturnus | Decreases phytic acid and trypsin inhibitors | [141,142] |
| A. oryzae | Reduces trypsin inhibitors and phytic acid | [16,143] |
| Rhizopus spp./N. crassa | Reduces glycinin, β-conglycinin, trypsin inhibitors, and oligosaccharides | [144,145] |
| L. casei/Lacticaseibacillus helveticus/ Enterococcus faecalis/ B. subtilis/A. oryzae | Reduces allergenicity | [89,156,157,158,159] |
| Bifidobacterium species | Increases protein concentration | [171] |
| L. plantarum/ L. acidophilus | Increases the levels of methionine, tryptophan, and lysine Competes and reduces the growth of spoilage and pathogenic microorganisms | [153,172,173] |
| B. subtilis/B. velezensis/L. plantarum/ P. anomala/S. cerevisiae/N. crassa/M. purpureus/A. oryzae/R. oligosporus | Increases phenolics, flavonoids, antioxidants, and antimicrobials Enhances digestibility Decreases allergenicity | [168,175,176,177] |
| L. acidophilus/L. delbrueckii/L. salivarius/C. butyricum/S. boulardii | Probiotics health benefits | [222,223] |
| Aspergillus spp./Penicillium spp./Fusarium spp. | Secretes mycotoxins (carcinogens) Decreases immunity | [201] |
| B. subtilis/B. amyloliquefaciens Lactobacillus spp./Enterococcus spp./Lactococcus spp./Leuconostoc spp./Streptococcus spp. | Forms biogenic amines | [210,212,213] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhalis, H.; See, X.Y.; Osen, R.; Chin, X.H.; Chow, Y. Significance of Fermentation in Plant-Based Meat Analogs: A Critical Review of Nutrition, and Safety-Related Aspects. Foods 2023, 12, 3222. https://doi.org/10.3390/foods12173222
Elhalis H, See XY, Osen R, Chin XH, Chow Y. Significance of Fermentation in Plant-Based Meat Analogs: A Critical Review of Nutrition, and Safety-Related Aspects. Foods. 2023; 12(17):3222. https://doi.org/10.3390/foods12173222
Chicago/Turabian StyleElhalis, Hosam, Xin Yi See, Raffael Osen, Xin Hui Chin, and Yvonne Chow. 2023. "Significance of Fermentation in Plant-Based Meat Analogs: A Critical Review of Nutrition, and Safety-Related Aspects" Foods 12, no. 17: 3222. https://doi.org/10.3390/foods12173222
APA StyleElhalis, H., See, X. Y., Osen, R., Chin, X. H., & Chow, Y. (2023). Significance of Fermentation in Plant-Based Meat Analogs: A Critical Review of Nutrition, and Safety-Related Aspects. Foods, 12(17), 3222. https://doi.org/10.3390/foods12173222






