Evaluation of a Standardized Extract Obtained from Cashew Apple (Anacardium occidentale L.) Bagasse in DSS-Induced Mouse Colitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Reagents
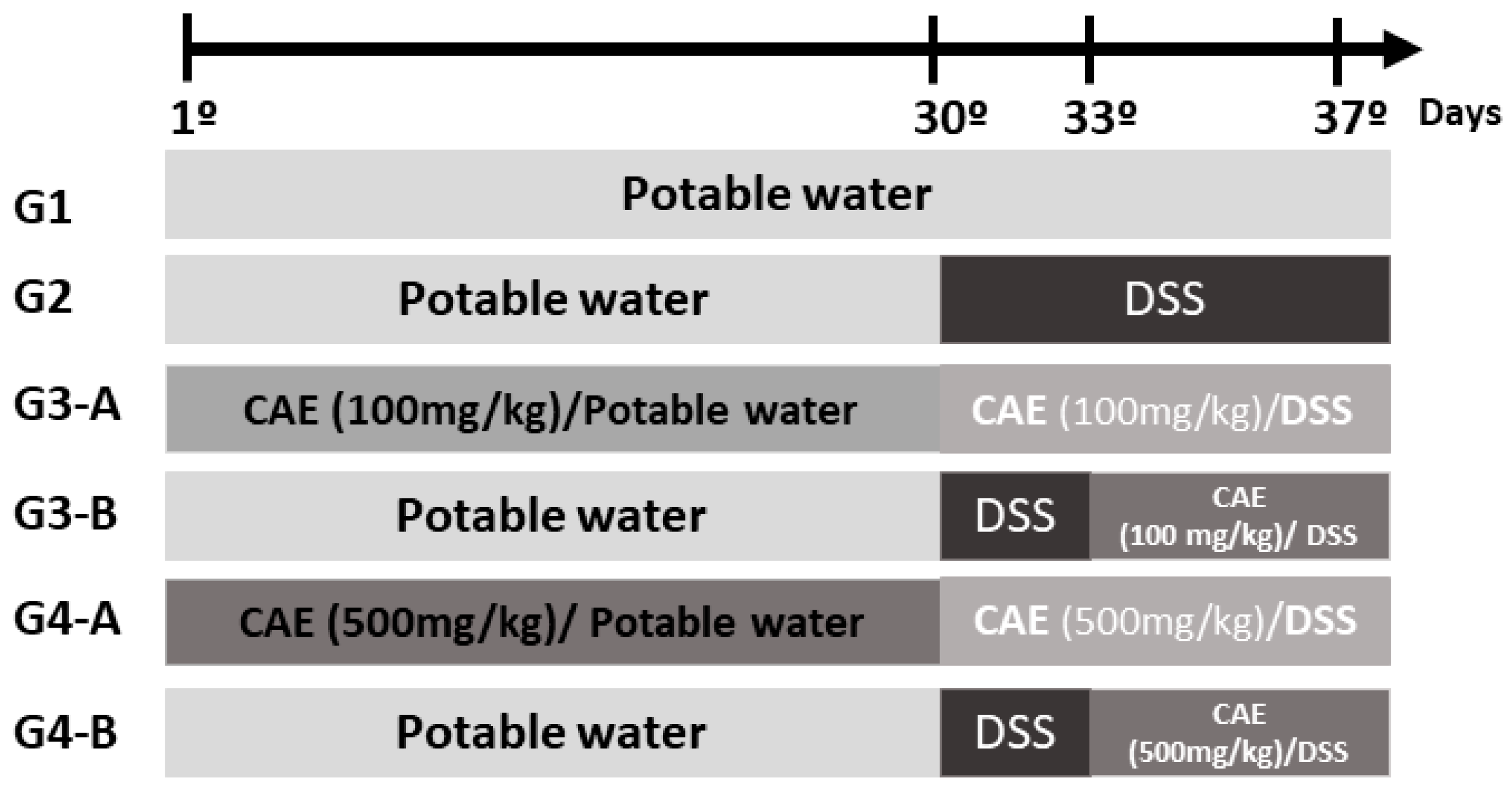
2.3. In Vivo Pharmacological Evaluation
2.3.1. Animals
2.3.2. Sample Preparation
2.3.3. Dextran Sulfate Sodium (DSS)-Induced Acute Colitis Model
2.4. Statistical Analysis
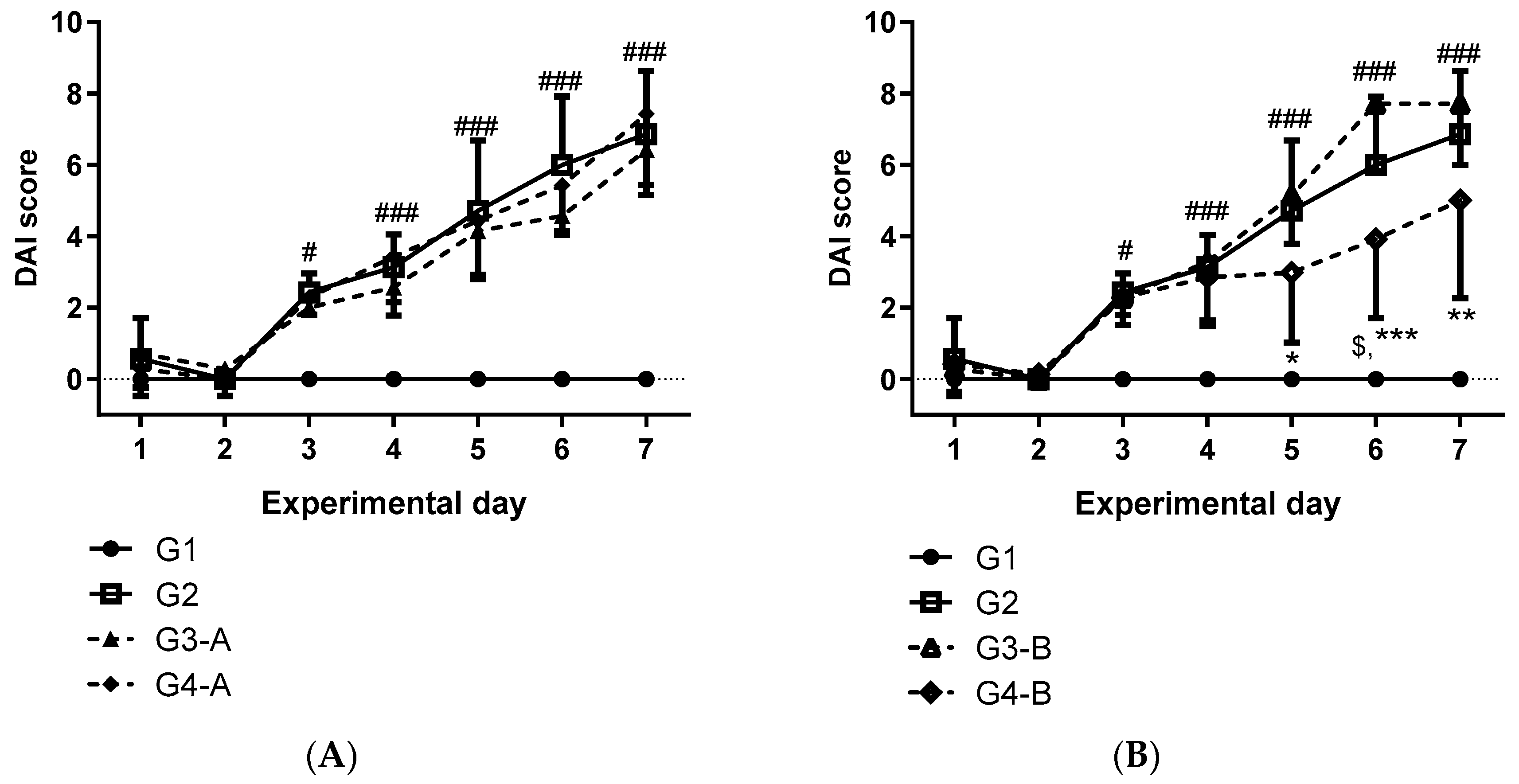
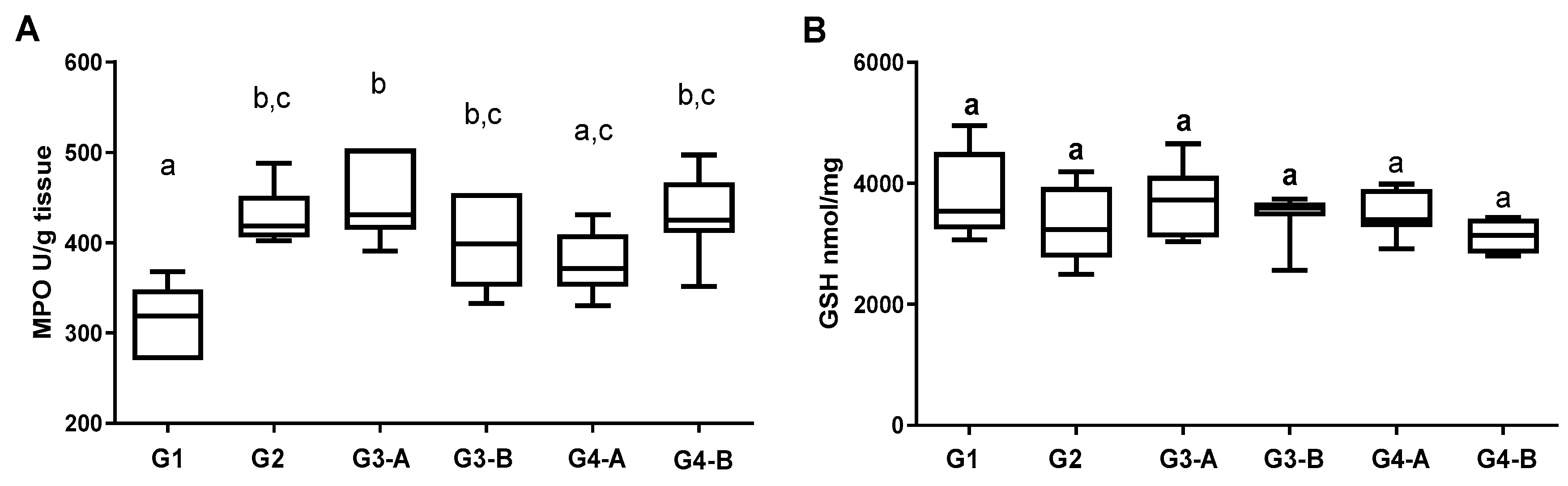
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, J.T. Pathophysiology of inflammatory bowel diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N. Impact of Diet on Risk of IBD. Crohns Colitis 360 2020, 2, otz054. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Sigall Boneh, R.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738. [Google Scholar] [CrossRef]
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The epidemiology of inflammatory bowel disease: East meets west. J. Gastroenterol. Hepatol. 2020, 35, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Long, G.H.; Tatro, A.R.; Oh, Y.S.; Reddy, S.R.; Ananthakrishnan, A.N. Analysis of safety, medical resource utilization, and treatment costs by drug class for management of inflammatory bowel disease in the United States based on insurance claims data. Adv. Ther. 2019, 36, 3079–3095. [Google Scholar] [CrossRef]
- Mijan, M.A.; Lim, B.O. Diets, functional foods, and nutraceuticals as alternative therapies for inflammatory bowel disease: Present status and future trends. World J. Gastroenterol. 2018, 24, 2673–2685. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Goulart, R.A.; Batista, G.L.D.S.A. Vitamin A and inflammatory bowel diseases: From cellular studies and animal models to human disease. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 25–35. [Google Scholar] [CrossRef]
- Currò, D.; Ianiro, G.; Pecere, S.; Bibbò, S.; Cammarota, G. Probiotics, fibre and herbal medicinal products for functional and inflammatory bowel disorders. Br. J. Pharmacol. 2017, 174, 1426–1449. [Google Scholar] [CrossRef]
- Larussa, T.; Imeneo, M.; Luzza, F. Potential role of nutraceutical compounds in inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 2483–2492. [Google Scholar] [CrossRef]
- Bohn, T. Bioactivity of Carotenoids—Chasms of knowledge. Int. J. Vitam. Nutr. Res. 2017, 87, 5–9. [Google Scholar] [CrossRef]
- Chacón-Ordóñez, T.; Carle, R.; Schweiggert, R. Bioaccessibility of carotenoids from plant and animal foods. J. Sci. Food Agric. 2019, 99, 3220–3239. [Google Scholar] [CrossRef] [PubMed]
- Głąbska, D.; Guzek, D.; Zakrzewska, P.; Włodarek, D.; Lech, G. Lycopene, lutein and zeaxanthin may reduce faecal blood, mucus and pus but not abdominal pain in individuals with ulcerative colitis. Nutrients 2016, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Masnadi Shirazi, K.; Nikniaz, Z.; Masnadi Shirazi, A.; Rohani, M. Vitamin A supplementation decreases disease activity index in patients with ulcerative colitis: A randomized controlled clinical trial. Complement. Ther. Med. 2018, 41, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Almaraz-Sánchez, I.; Amaro-Reyes, A.; Acosta-Gallegos, J.A.; Mendoza-Sánchez, M. Processing agroindustry by-products for obtaining value-added products and reducing environmental impact. J. Chem. 2022, 2022, 3656932. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Singh, A.K.; Srivastava, R.K.; Gupta, V.K. Recent progress and future perspectives for zero agriculture waste technologies: Pineapple waste as a case study. Sustainability 2023, 15, 3575. [Google Scholar] [CrossRef]
- Eixenberger, D.; Carballo-Arce, A.F.; Vega-Baudrit, J.R.; Trimino-Vazquez, H.; Villegas-Pearanda, L.R.; Stbener, A.; Aguilar, F.; Mora-Villalobos, J.A.; Sandoval-Barrantes, M.; Bubenheim, P.; et al. Tropical agroindustrial biowaste revalorization through integrative biorefineries—Review part II: Pineapple, sugarcane and banana by-products in Costa Rica. Biomass Conv. Bioref. 2022; online first article. [Google Scholar] [CrossRef]
- Oliveira, N.N.; Mothé, C.G.; Mothé, M.G.; de Oliveira, L.G. Cashew nut and cashew apple: A scientific and technological monitoring worldwide review. J. Food Sci. Technol. 2020, 57, 12–21. [Google Scholar] [CrossRef]
- Ganesh, S.; Kannan, M.; Jawaharlal, M. Cashew industry—An outlook. Acta Hortic. 2015, 1080, 89–95. [Google Scholar] [CrossRef]
- Salehi, B.; Gültekin-Özgüven, M.; Kırkın, C.; Özçelik, B.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Bezerra, C.F.; Silva, T.G.D.; Coutinho, H.D.M.; Amina, B.; et al. Anacardium plants: Chemical, nutritional composition and biotechnological applications. Biomolecules 2019, 9, 465. [Google Scholar] [CrossRef]
- Salehi, B.; Gültekin-Özgüven, M.; Kirkin, C.; Özçelik, B.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Bezerra, C.F.; da Silva, T.G.; Coutinho, H.D.M.; Amina, B.; et al. Antioxidant, antimicrobial, and anticancer effects of Anacardium plants: An ethnopharmacological perspective. Front. Endocrinol. 2020, 11, 295. [Google Scholar] [CrossRef]
- Araújo, W.F.; Rocha, L.M.; Araújo, I.M.S.; de Paula, G.A.; de Sousa, L.S.; Folha, M.F.; da Rocha Filho, L.B.; Araújo, R.V. Sustentabilidade em agroindústrias: Alternativas para evitar o desperdício de resíduos agroindustriais do pedúnculo de caju-uma revisão de literatura [Sustainability in agroindustrys: Alternatives to avoid waste of agro-industrial residue peduncle cashew—A literature review]. Braz. J. Dev. 2018, 4, 4546–4569. [Google Scholar]
- Rodrigues, T.H.; de Barros, E.M.; de Sá Brígido, J.; da Silva, W.M.; Rocha, M.V.; Gonçalves, L.R. The bioconversion of pretreated cashew apple bagasse into ethanol by SHF and SSF processes. Appl. Biochem. Biotechnol. 2016, 178, 1167–1183. [Google Scholar] [CrossRef]
- de Abreu, F.A.P.; Dornier, M.; Pallet, D.; Reynes, M.; Vaillant, F.; Furlani, F.C.T. Method for the Concentration and Purification of the Extract Obtained from Cashew Pseudofruit Waste and Product with a High Carotenoid Content WO 2013/159167 A1, 31 October 2013.
- de Abreu, F.P.; Dornier, M.; Dionisio, A.P.; Carail, M.; Caris-Veyrat, C.; Dhuique-Mayer, C. Cashew apple (Anacardium occidentale L.) extract from by-product of juice processing: A focus on carotenoids. Food Chem. 2013, 138, 25–31. [Google Scholar] [CrossRef]
- Dionísio, A.P.; de Abreu, F.A.P.; de Brito, E.S.; Wurlitzer, N.J.; Ribeiro, P.R.V.; Goes, T.S.; Sousa, J.M.S.; Iunes, M.F.; de Lima, A.C.V.; Oiram Filho, F. Extrato concentrado de carotenoides obtido da fibra do pedúnculo de caju: Avaliação da toxicidade com uso do bioensaio de Artemia salina. Bol. Pesqui. E Desenvolv. Embrapa Agroindústria Trop. 2018, 162. Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1091917/extrato-concentrado-de-carotenoides-obtido-da-fibra-do-pedunculo-de-caju-avaliacao-da-toxicidade-com-uso-do-bioensaio-de-artemia-salina (accessed on 15 May 2023).
- Sousa, J.M.S.; de Abreu, F.A.P.; Ruiz, A.L.T.G.; da Silva, G.G.; Machado, S.L.; Garcia, C.P.G.; Filho, F.O.; Wurlitzer, N.J.; de Figueiredo, E.A.T.; Magalhães, F.E.A.; et al. Cashew apple (Anacardium occidentale L.) extract from a by-product of juice processing: Assessment of its toxicity, antiproliferative and antimicrobial activities. JFST 2021, 58, 764–776. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.G.; Della Torre, A.; Braga, L.E.O.; Bachiega, P.; Tinti, S.V.; de Carvalho, J.E.; Dionísio, A.P.; Ruiz, A.L.T.G. Yellow-colored extract from cashew byproduct—Nonclinical safety assessment. Regul. Toxicol. Pharmacol. 2020, 115, 104699. [Google Scholar] [CrossRef] [PubMed]
- Goulart da Silva, G.; de Oliveira Braga, L.E.; Souza de Oliveira, E.C.; Valério Tinti, S.; de Carvalho, J.E.; Goldoni Lazarini, J.; Rosalen, P.L.; Dionísio, A.P.; Ruiz, A.L.T.G. Cashew apple byproduct: Gastroprotective effects of standardized extract. J. Ethnopharmacol. 2021, 269, 113744. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Braga, L.E.; da Silva, G.G.; de Oliveira Sousa, I.M.; de Oliveira, E.C.S.; Jorge, M.P.; Monteiro, K.M.; Sedano, T.C.; Foglio, M.A.; Ruiz, A.L.T.G. Gastrointestinal effects of Mentha aquatica L. essential oil. Inflammopharmacology 2022, 30, 2127–2137. [Google Scholar] [CrossRef]
- Witaicenis, A.; de Oliveira, E.C.S.; Tanimoto, A.; Zorzella-Pezavento, S.F.G.; de Oliveira, S.L.; Sartori, A.; Di Stasi, L.C. 4-methylesculetin, a coumarin derivative, ameliorates dextran sulfate sodium-induced intestinal inflammation. Chem. Biol. Interact. 2018, 280, 59–63. [Google Scholar] [CrossRef]
- Jhansyrani, T.; Sujatha, D.; Bharathi, K.; Prasad, K.V.S.R.G. Ethanolic extract of cashew apple inhibits lipid metabolism and ameliorates obesity in atherogenic diet-induced obese rats. Asian Pac. J. Trop. Biomed. 2019, 9, 405–414. [Google Scholar]
- Beejmohun, V.; Mignon, C.; Mazollier, A.; Peytavy-Izard, M.; Pallet, D.; Dornier, M.; Chapal, N. Cashew apple extract inhibition of fat storage and insulin resistance in the diet-induced obesity mouse model. J. Nutr. Sci. 2015, 4, e38. [Google Scholar] [CrossRef]
- Prasertsri, P.; Roengrit, T.; Kanpetta, Y.; Tong-Un, T.; Muchimapura, S.; Wattanathorn, J.; Leelayuwat, N. Cashew apple juice supplementation enhanced fat utilization during high-intensity exercise in trained and untrained men. J. Int. Soc. Sports Nutr. 2013, 10, 13. [Google Scholar] [CrossRef]
- Balcombe, J.P.; Barnard, N.D.; Sandusky, C. Laboratory routines cause animal stress. Contemp. Top. Lab. Anim. Sci. 2004, 43, 42–51. [Google Scholar]
- Harris, R.B. Chronic and acute effects of stress on energy balance: Are there appropriate animal models? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R250–R265. [Google Scholar] [CrossRef]
- Melgar, S.; Karlsson, A.; Michaëlsson, E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: Correlation between symptoms and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G1328–G1338. [Google Scholar] [CrossRef]
- Bruewer, M.; Luegering, A.; Kucharzik, T.; Parkos, C.A.; Madara, J.L.; Hopkins, A.M.; Nusrat, A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J. Immunol. 2003, 171, 6164–6172. [Google Scholar] [CrossRef]
- Parker, H.; Winterbourn, C.C. Reactive oxidants and myeloperoxidase and their involvement in neutrophil extracellular traps. Front. Immunol. 2013, 3, 424. [Google Scholar] [CrossRef]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Kilby, K.; Mathias, H.; Boisvenue, L.; Heisler, C.; Jones, J.L. Micronutrient absorption and related outcomes in people with inflammatory bowel disease: A review. Nutrients 2019, 11, 1388. [Google Scholar] [CrossRef]
- Mourad, F.H.; Barada, K.A.; Saade, N.E. Impairment of small intestinal function in ulcerative colitis: Role of enteric innervation. J. Crohns Colitis 2017, 11, 369–377. [Google Scholar] [CrossRef]
- Rubin, L.P.; Ross, A.C.; Stephensen, C.B.; Bohn, T.; Tanumihardjo, S.A. Metabolic effects of inflammation on vitamin A and carotenoids in humans and animal models. Adv. Nutr. 2017, 8, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.M.; Silva, K.A.; Araujo, H.; Vieira, I.G.; Alves, D.R.; Fontenelle, R.O.; Silva, A.M. Anacardic acid constituents from cashew nut shell liquid: NMR characterization and the effect of unsaturation on its biological activities. Pharmaceuticals 2017, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Huang, J.; Zheng, Y.; Sun, Q. Histone acetyltransferase inhibitors: An overview in synthesis, structure-activity relationship and molecular mechanism. Eur. J. Med. Chem. 2019, 178, 259–286. [Google Scholar] [CrossRef]
- Link, A.; Balaguer, F.; Goel, A. Cancer chemoprevention by dietary polyphenols: Promising role for epigenetics. Biochem. Pharmacol. 2010, 80, 1771–1792. [Google Scholar] [CrossRef] [PubMed]
- Bai, A.H.; Wu, W.K.; Xu, L.; Wong, S.H.; Go, M.Y.; Chan, A.W.; Harbord, M.; Zhang, S.; Chen, M.; Wu, J.C.; et al. Dysregulated lysine acetyltransferase 2B promotes inflammatory bowel disease pathogenesis through transcriptional repression of interleukin-10. J. Crohns Colitis 2016, 10, 726–734. [Google Scholar] [CrossRef]
- Siracusa, R.; Fusco, R.; Peritore, A.F.; Cordaro, M.; D’Amico, R.; Genovese, T.; Gugliandolo, E.; Crupi, R.; Smeriglio, A.; Mandalari, G.; et al. The antioxidant and anti-inflammatory properties of Anacardium occidentale L. cashew nuts in a mouse model of colitis. Nutrients 2020, 12, 834. [Google Scholar] [CrossRef]


| Chemical Constituents | CAE 1 | |
|---|---|---|
| mg/100 g | Relative Amount (%) | |
| Anacardic acids | ||
| C15:3 | 94.2 ± 0.6 | - |
| C15:2 | 108.4 ± 0.1 | - |
| C15:1 | 214.8 ± 0.2 | - |
| Phenolic compound (total) 2 | 37.49 ± 0.64 | - |
| Carotenoids | ||
| Total 3 | 96.28 ± 0.15 | |
| Non-provitamin A 4 | - | 76.9 |
| Provitamin A 4 | - | 22.4 |
| Chemical Constituents | CAE 1 | |
|---|---|---|
| 100 mg/kg | 500 mg/kg | |
| Anacardic acids | ||
| C15:3 | 1.9 | 9.4 |
| C15:2 | 2.2 | 11 |
| C15:1 | 4.3 | 21.5 |
| Phenolic compound (total) 2 | 0.75 | 3.75 |
| Carotenoids | ||
| Total 3 | 1.93 | 9.63 |
| Non-provitamin A 4 | 1.48 | 7.40 |
| Provitamin A 4 | 0.43 | 2.16 |
| Experimental Week | Parameters | Groups | |||||
|---|---|---|---|---|---|---|---|
| G1 | G2 | G3-A | G4-A | G3-B | G4-B | ||
| Basal | Weight | 19.90 ± 0.43 | 20.12 ± 0.25 | 21.08 ± 0.43 | 20.10 ± 0.56 | 20.21 ± 0.36 | 20.24 ± 0.41 |
| 1st | Weight | 20.58 ± 0.52 | 20.74 ± 0.25 | 21.41 ± 0.34 | 20.87 ± 0.55 | 20.39 ± 0.34 | 20.91 ± 0.4 |
| ∆ | 3.4 ± 1.8 a | 3.1 ± 2.5 a | 1.7 ± 3.4 a | 0.9 ± 1.3 a | 4.0 ± 4.8 a | 3.3 ± 1.8 a | |
| 2nd | Weight | 20.69 ± 0.43 | 20.54 ± 0.22 | 20.54 ± 0.22 | 20.52 ± 0.54 | 19.98 ± 0.44 | 20.84 ± 0.46 |
| ∆ | 4.0 ± 1.2 a | 2.1 ± 2.2 a | 1.2 ± 2.4 a | −1.1 ± 1.7 b,c | 2.1 ± 2.3 a,c | 2.9 ± 3.3 a | |
| 3rd | Weight | 21.23 ± 0.51 | 21.46 ± 0.19 | 21.60 ± 0.30 | 21.10 ± 0.42 | 20.47 ± 0.41 | 21.39 ± 0.28 |
| ∆ | 6.6 ± 1.5 a | 6.7 ± 2.7 a | 2.6 ± 3.4 a,c | 1.4 ± 4.4 b,c | 5.2 ± 3.6 a,c | 5.8 ± 3.8 a | |
| 4th | Weight | 21.10 ± 0.38 | 21.42 ± 0.2 | 21.29 ± 0.41 | 21.11 ± 0.34 | 20.51 ± 0.42 | 21.42 ± 0.20 |
| ∆ | 6.0 ± 1.5 a | 6.5 ± 2.5 a | 1.1 ± 3.9 b | 1.5 ± 2.9 b,c | 5.2 ± 3.6 a,c | 6.0 ± 3.9 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, G.G.; Braga, L.E.d.O.; de Oliveira, E.C.S.; de Carvalho, J.E.; Lazarini, J.G.; Rosalen, P.L.; Dionísio, A.P.; Ruiz, A.L.T.G. Evaluation of a Standardized Extract Obtained from Cashew Apple (Anacardium occidentale L.) Bagasse in DSS-Induced Mouse Colitis. Foods 2023, 12, 3318. https://doi.org/10.3390/foods12173318
da Silva GG, Braga LEdO, de Oliveira ECS, de Carvalho JE, Lazarini JG, Rosalen PL, Dionísio AP, Ruiz ALTG. Evaluation of a Standardized Extract Obtained from Cashew Apple (Anacardium occidentale L.) Bagasse in DSS-Induced Mouse Colitis. Foods. 2023; 12(17):3318. https://doi.org/10.3390/foods12173318
Chicago/Turabian Styleda Silva, Gisele Goulart, Lucia Elaine de Oliveira Braga, Ellen Cristina Souza de Oliveira, João Ernesto de Carvalho, Josy Goldoni Lazarini, Pedro Luiz Rosalen, Ana Paula Dionísio, and Ana Lucia Tasca Gois Ruiz. 2023. "Evaluation of a Standardized Extract Obtained from Cashew Apple (Anacardium occidentale L.) Bagasse in DSS-Induced Mouse Colitis" Foods 12, no. 17: 3318. https://doi.org/10.3390/foods12173318
APA Styleda Silva, G. G., Braga, L. E. d. O., de Oliveira, E. C. S., de Carvalho, J. E., Lazarini, J. G., Rosalen, P. L., Dionísio, A. P., & Ruiz, A. L. T. G. (2023). Evaluation of a Standardized Extract Obtained from Cashew Apple (Anacardium occidentale L.) Bagasse in DSS-Induced Mouse Colitis. Foods, 12(17), 3318. https://doi.org/10.3390/foods12173318






