Inhibition Mechanism of Lactiplantibacillus plantarum on the Growth and Biogenic Amine Production in Morganella morganii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Growth Conditions
2.2. Preparation of Lpb. plantarum Cell-Free Supernatant (CFS)
2.3. Effect of Different Conditions on the Growth and BA Production of M. morganii
2.4. Transcriptome Sequencing Sample Preparation
2.5. RNA Extraction, cDNA Synthesis, Library Construction and Sequencing
2.6. RNA-Seq Data Analysis
2.7. Statistical Analysis
3. Results and Discussion
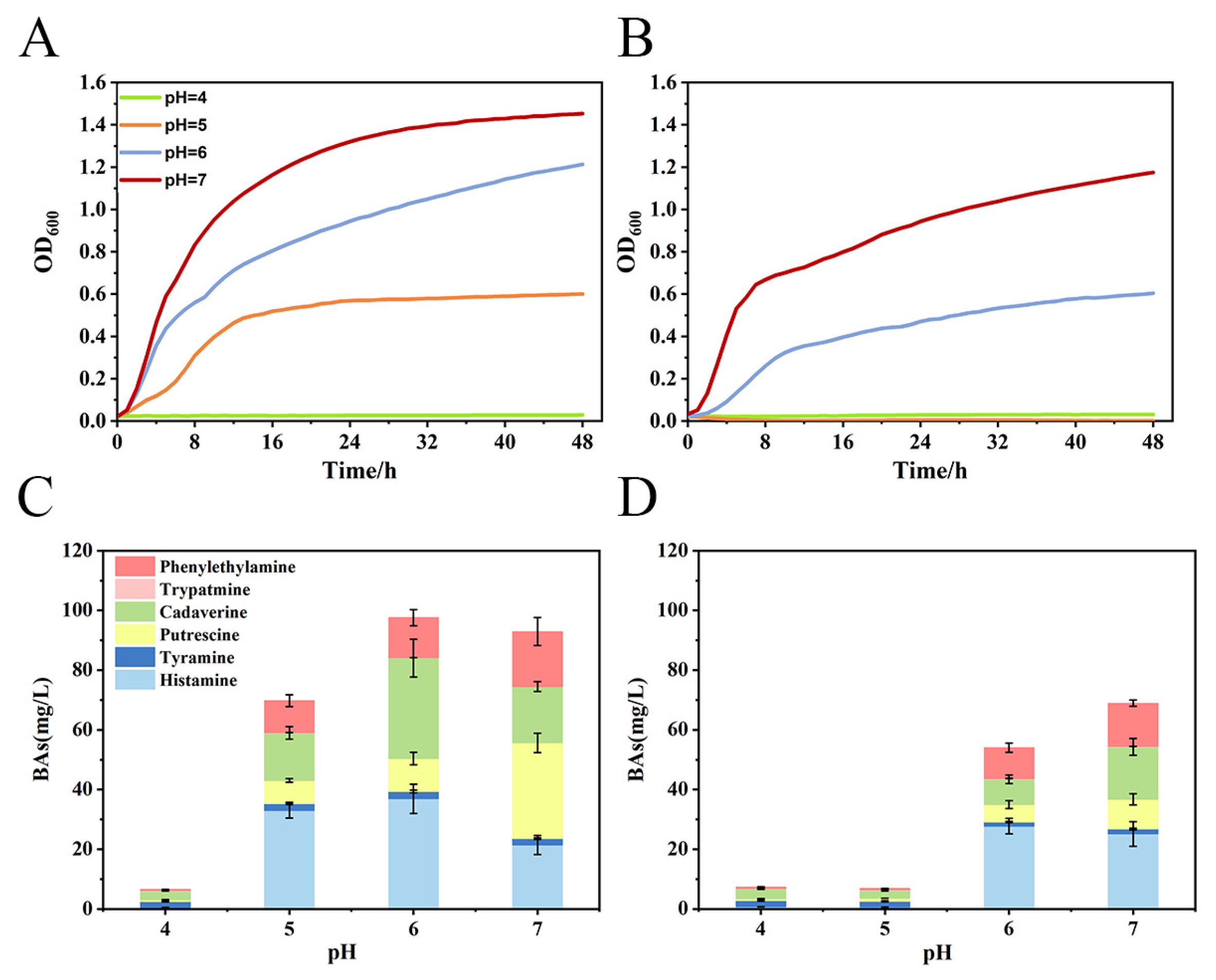
3.1. Effect of Lpb. plantarum CFS on M. morganii Growth and BA Production
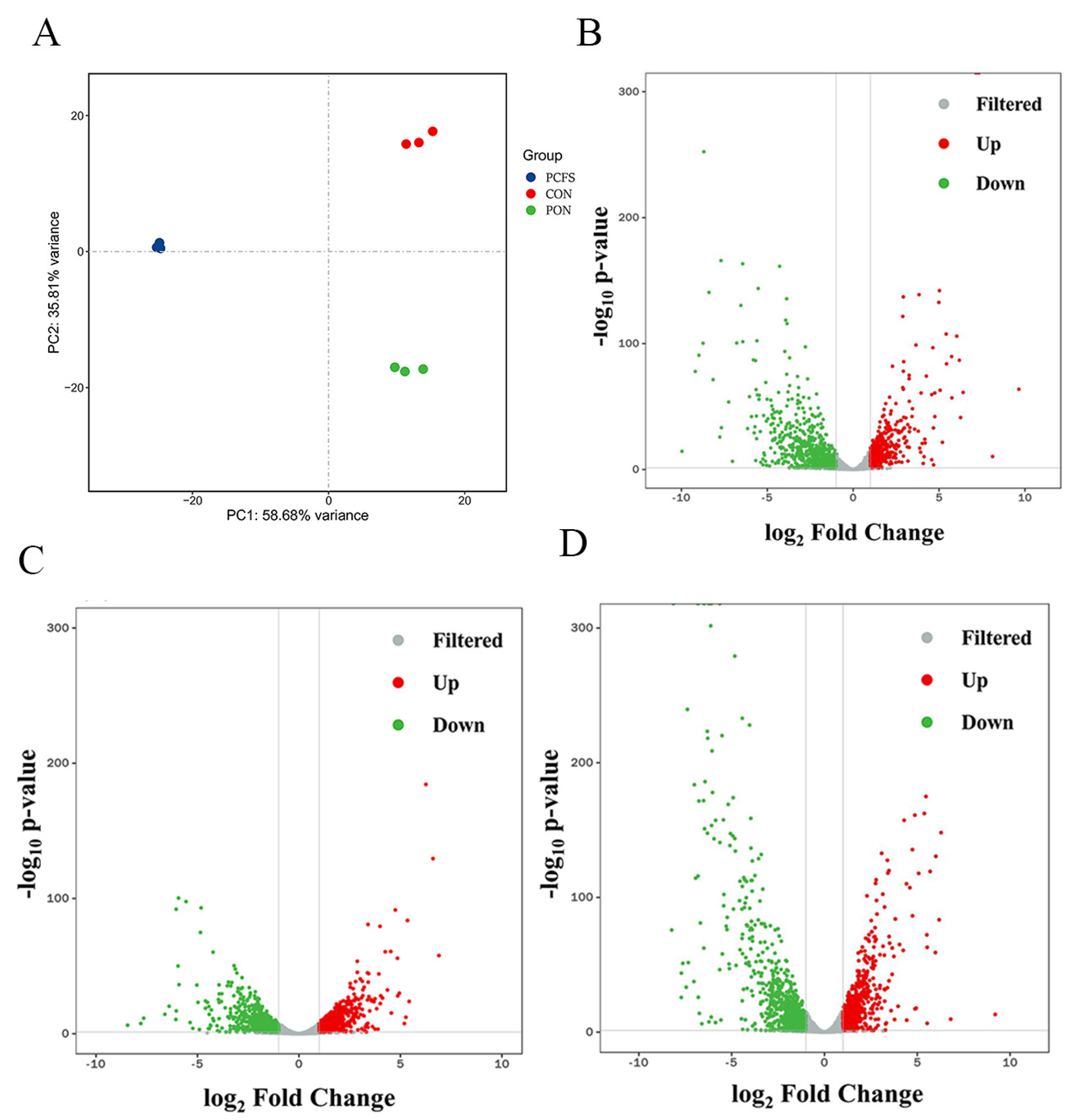
3.2. Overview of the Transcriptional Analysis and Differentially Expressed Genes
3.3. Functional Analysis and Classification of DEGs
3.4. Analysis of DEGs Associated with M. morganii BA Production Inhibition
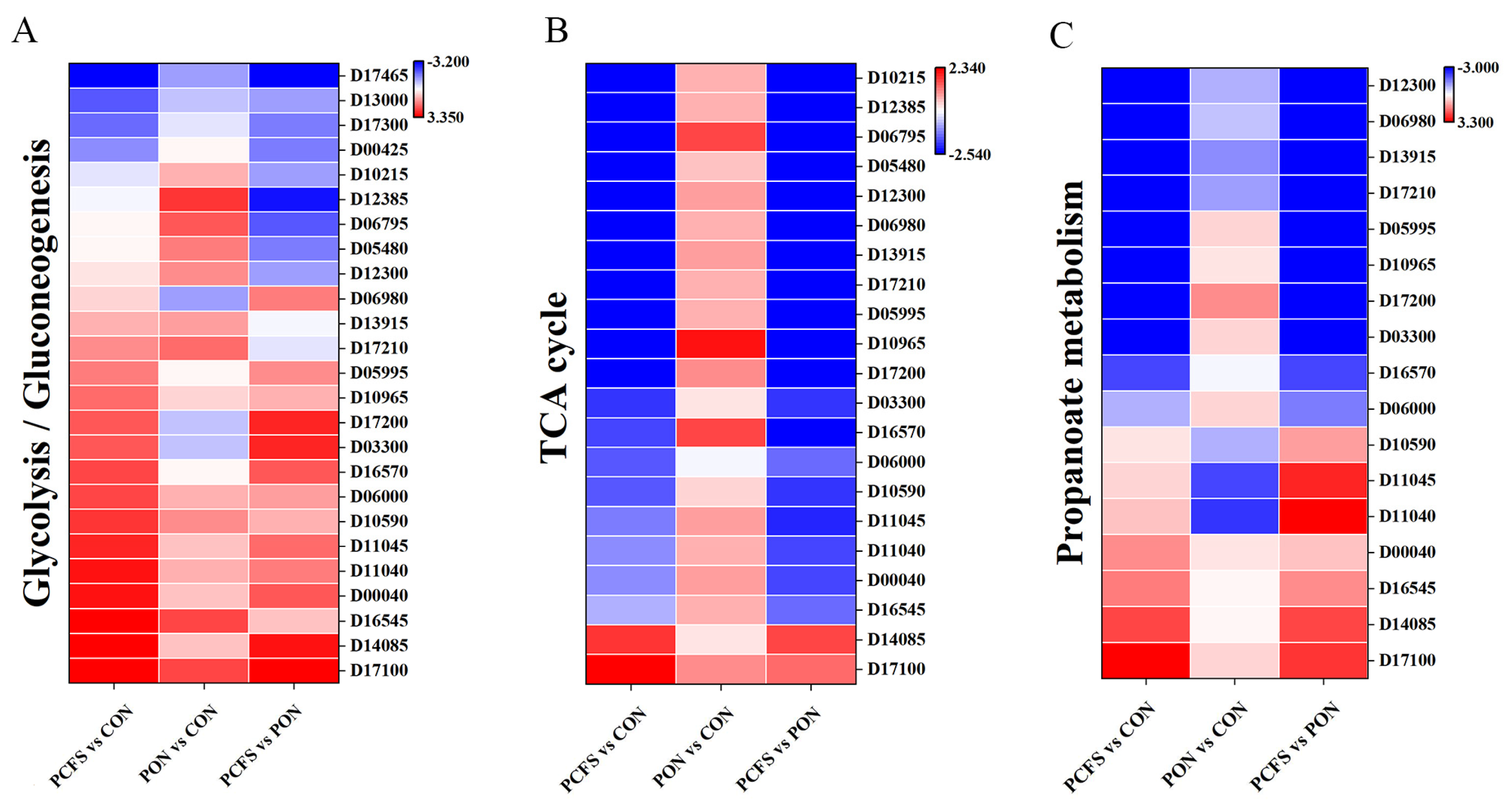
3.4.1. Carbohydrate Metabolic Pathways
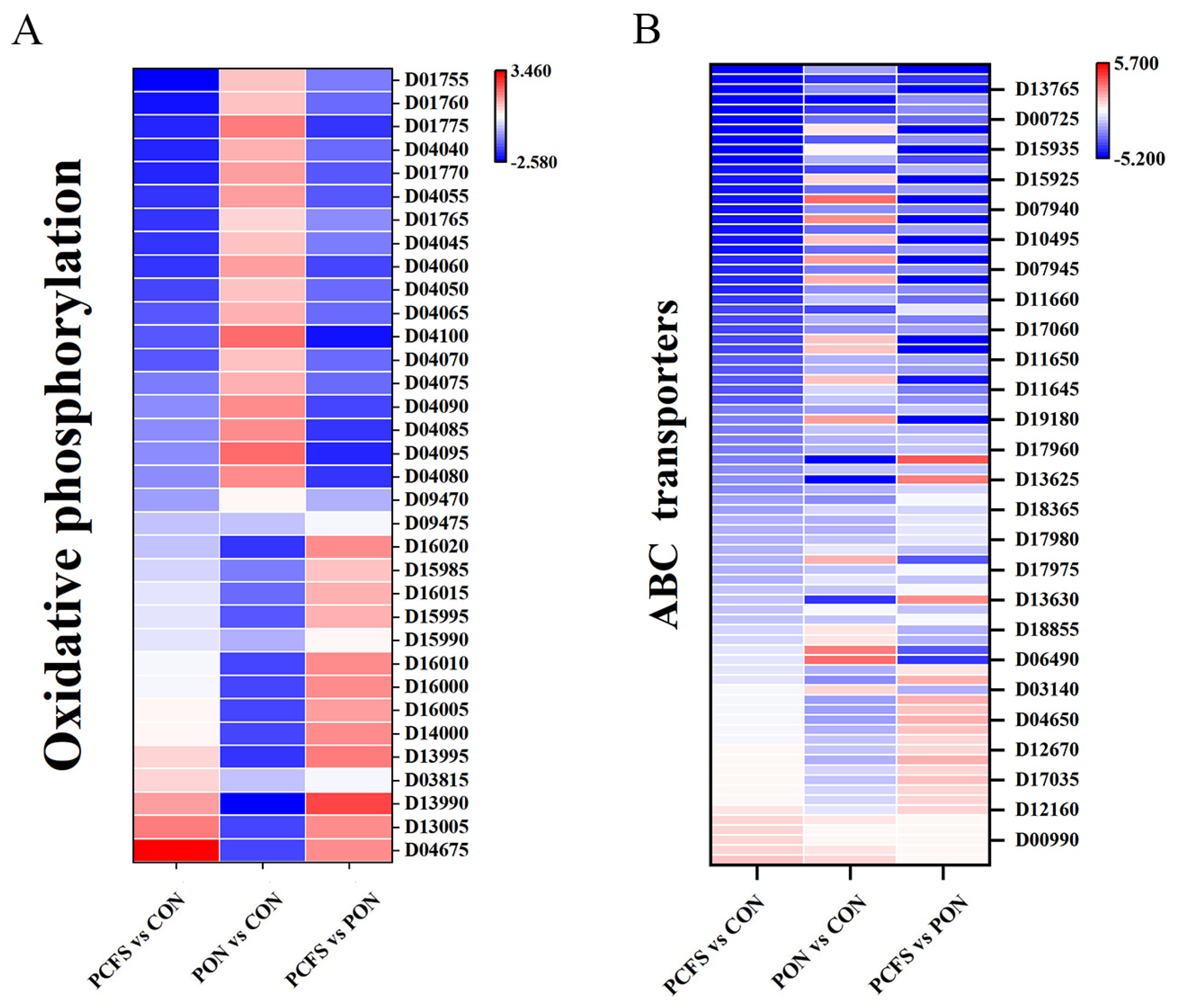
3.4.2. Energy Metabolic Pathways
3.4.3. Amino Acid Metabolic Pathways
3.4.4. Membrane Transporters-Related Pathways
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gardini, F.; Ozogul, Y.; Suzzi, G.; Tabanelli, G.; Ozogul, F. Technological Factors Affecting Biogenic Amine Content in Foods: A Review. Front. Microbiol. 2016, 7, 1218. [Google Scholar] [CrossRef] [PubMed]
- Jairath, G.; Singh, P.K.; Dabur, R.S.; Rani, M.; Chaudhari, M. Biogenic amines in meat and meat products and its public health significance: A review. J. Food Sci. Technol. Mysore 2015, 52, 6835–6846. [Google Scholar] [CrossRef]
- Bulushi, I.A.; Poole, S.; Deeth, H.C.; Dykes, G.A. Biogenic Amines in Fish: Roles in Intoxication, Spoilage, and Nitrosamine Formation—A Review. Crit. Rev. Food Sci. Nutr. 2009, 49, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.A.; Guo, W.R.; Zhai, Y.J.; Zhao, L.H.; Liu, T.; Yang, L.; Jin, Y.; Duan, Y. Screening and the ability of biogenic amine-degrading strains from traditional meat products in Inner Mongolia. LWT Food Sci. Technol. 2023, 176, 114533. [Google Scholar] [CrossRef]
- Zhai, H.; Yang, X.; Li, L.; Xia, G.; Cen, J.; Huang, H.; Hao, S. Biogenic amines in commercial fish and fish products sold in southern China. Food Control 2012, 25, 303–308. [Google Scholar] [CrossRef]
- Kim, S.H.; Barros-Velazquez, J.; Ben-Gigrey, B.; Eun, J.B.; Jun, S.H.; Wie, C.I.; An, H.J. Identification of the main bacteria contributing to histamine formation in seafood to ensure product safety. Food Sci. Biotechnol. 2003, 12, 451–460. [Google Scholar]
- Takahashi, H.; Sato, M.; Kimura, B.; Ishikawa, T.; Fujii, T. Evaluation of PCR-single-strand conformational polymorphism analysis for identification of gram-negative histamine-producing bacteria isolated from fish. J. Food Prot. 2007, 70, 1200–1205. [Google Scholar] [CrossRef]
- Yang, Q.; Meng, J.; Zhang, W.; Liu, L.; He, L.; Deng, L.; Zeng, X.; Ye, C. Effects of Amino Acid Decarboxylase Genes and pH on the Amine Formation of Enteric Bacteria From Chinese Traditional Fermented Fish (Suan Yu). Front. Microbiol. 2020, 11, 1130. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, N.; Battista, N.; Prete, R.; Corsetti, A. Health-Promoting Role of Lactiplantibacillus plantarum Isolated from Fermented Foods. Microorganisms 2021, 9, 349. [Google Scholar] [CrossRef]
- Martin, I.; Rodriguez, A.; Delgado, J.; Cordoba, J.J. Strategies for Biocontrol of Listeria monocytogenes Using Lactic Acid Bacteria and Their Metabolites in Ready-to-Eat Meat- and Dairy-Ripened Products. Foods 2022, 11, 542. [Google Scholar] [CrossRef]
- Wang, Y.L.; Pei, H.J.; Liu, Y.X.; Huang, X.H.; Deng, L.; Lan, Q.J.; Chen, S.J.; He, L.; Liu, A.P.; Ao, X.L.; et al. Inhibitory mechanism of cell-free supernatants of Lactobacillus plantarum on Proteus mirabilis and influence of the expression of histamine synthesis-related genes. Food Control 2021, 125, 107982. [Google Scholar] [CrossRef]
- Kiousi, D.E.; Efstathiou, C.; Tzampazlis, V.; Plessas, S.; Panopoulou, M.; Koffa, M.; Galanis, A. Genetic and phenotypic assessment of the antimicrobial activity of three potential probiotic lactobacilli against human enteropathogenic bacteria. Front. Cell. Infect. Microbiol. 2023, 13, 1127256. [Google Scholar] [CrossRef] [PubMed]
- Kuley, E.; Kuscu, M.M.; Durmus, M.; Ucar, Y. Inhibitory activity of Co-microencapsulation of cell free supernatant from Lactobacillus plantarum with propolis extracts towards fish spoilage bacteria. LWT Food Sci. Technol. 2021, 146, 111433. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Lin, X.; Liang, H.; Zhang, S.; Ji, C. Lactobacillus strains inhibit biogenic amine formation in salted mackerel (Scomberomorus niphonius). LWT Food Sci. Technol. 2022, 155, 112851. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, J.; Wang, S.; Lin, X.; Liang, H.; Li, S.; Yu, C.; Dong, X.; Ji, C. Developing and Validating a UPLC-MS Method with a StageTip-Based Extraction for the Biogenic Amines Analysis in Fish. J. Food Sci. 2019, 84, 1138–1144. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Tjaden, B. De novo assembly of bacterial transcriptomes from RNA-seq data. Genome Biol. 2015, 16, 1–10. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef]
- Ryser, L.T.; Arias-Roth, E.; Berthoud, H.; Delbes-Paus, C.; Chassard, C.; Bruggmann, R.; Irmler, S. Cadaverine, putrescine, and histamine formation of Morganella morganii in raclette-type cheese. Int. Dairy J. 2022, 129, 105362. [Google Scholar] [CrossRef]
- Müller, D.M.; Carrasco, M.S.; Tonarelli, G.G.; Simonetta, A.C. Characterization and purification of a new bacteriocin with a broad inhibitory spectrum produced by Lactobacillus plantarum lp31 strain isolated from dry-fermented sausage. J. Appl. Microbiol. 2009, 106, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Lund, P.; Tramonti, A.; De Biase, D. Coping with low pH: Molecular strategies in neutralophilic bacteria. FEMS Microbiol. Rev. 2014, 38, 1091–1125. [Google Scholar] [CrossRef] [PubMed]
- Passerini, D.; Coddeville, M.; Le Bourgeois, P.; Loubiere, P.; Ritzenthaler, P.; Fontagne-Faucher, C.; Daveran-Mingot, M.-L.; Cocaign-Bousquet, M. The Carbohydrate Metabolism Signature of Lactococcus lactis Strain A12 Reveals Its Sourdough Ecosystem Origin. Appl. Environ. Microbiol. 2013, 79, 5844–5852. [Google Scholar] [CrossRef]
- Yin, X.; Li, J.; Shin, H.-d.; Du, G.; Liu, L.; Chen, J. Metabolic engineering in the biotechnological production of organic acids in the tricarboxylic acid cycle of microorganisms: Advances and prospects. Biotechnol. Adv. 2015, 33, 830–841. [Google Scholar] [CrossRef]
- Zheng, C.; Yu, Z.; Du, C.; Gong, Y.; Yin, W.; Li, X.; Li, Z.; Roemling, U.; Chou, S.-H.; He, J. 2-Methylcitrate cycle: A well-regulated controller of Bacillus sporulation. Environ. Microbiol. 2020, 22, 1125–1140. [Google Scholar] [CrossRef] [PubMed]
- Rocco, C.J.; Escalante-Semerena, J.C. In Salmonella enterica, 2-Methylcitrate Blocks Gluconeogenesis. J. Bacteriol. 2010, 192, 771–778. [Google Scholar] [CrossRef]
- Sun, D.; Li, H.; Song, D.; Zhang, L.; Zhao, X.; Xu, X. Genome, transcriptome and fermentation analyses of Lactobacillus plantarum LY-78 provide new insights into the mechanism of phenyllactate biosynthesis in lactic acid bacteria. Biochem. Biophys. Res. Commun. 2019, 519, 351–357. [Google Scholar] [CrossRef]
- Li, J.; Zhou, L.; Feng, W.; Cheng, H.; Muhammad, A.I.; Ye, X.; Zhi, Z. Comparison of Biogenic Amines in Chinese Commercial Soy Sauces. Molecules 2019, 24, 1522. [Google Scholar] [CrossRef]
- Arena, M.E.; de Nadra, M.C.M. Biogenic amine production by Lactobacillus. J. Appl. Microbiol. 2001, 90, 158–162. [Google Scholar] [CrossRef]
- Iwadate, Y.; Ramezanifard, R.; Golubeva, Y.A.; Fenlon, L.A.; Slauch, J.M. PaeA (YtfL) protects from cadaverine and putrescine stress in Salmonella Typhimurium and E. coli. Mol. Microbiol. 2021, 115, 1379–1394. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Yan, Z.; Si, F.; Zhou, Y.; Fu, W.; Chen, B. ATP-Binding Cassette (ABC) Transporter Genes Involved in Pyrethroid Resistance in the Malaria Vector Anopheles sinensis: Genome-Wide Identification, Characteristics, Phylogenetics, and Expression Profile. Int. J. Mol. Sci. 2019, 20, 1409. [Google Scholar] [CrossRef] [PubMed]
- Tischler, A.D.; Leistikow, R.L.; Ramakrishnan, P.; Voskuil, M.I.; McKinney, J.D. Mycobacterium tuberculosis Phosphate Uptake System Component PstA2 Is Not Required for Gene Regulation or Virulence. PLoS ONE 2016, 11, e0161467. [Google Scholar] [CrossRef] [PubMed]
- Hudek, L.; Premachandra, D.; Webster, W.A.J.; Brau, L. Role of Phosphate Transport System Component PstB1 in Phosphate Internalization by Nostoc punctiforme. Appl. Environ. Microbiol. 2016, 82, 6344–6356. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Zhang, Y.; Lin, X.; Zhang, S.; Chen, Y.; Ji, C. Inhibition Mechanism of Lactiplantibacillus plantarum on the Growth and Biogenic Amine Production in Morganella morganii. Foods 2023, 12, 3625. https://doi.org/10.3390/foods12193625
Sun Z, Zhang Y, Lin X, Zhang S, Chen Y, Ji C. Inhibition Mechanism of Lactiplantibacillus plantarum on the Growth and Biogenic Amine Production in Morganella morganii. Foods. 2023; 12(19):3625. https://doi.org/10.3390/foods12193625
Chicago/Turabian StyleSun, Zhenxiao, Yi Zhang, Xinping Lin, Sufang Zhang, Yingxi Chen, and Chaofan Ji. 2023. "Inhibition Mechanism of Lactiplantibacillus plantarum on the Growth and Biogenic Amine Production in Morganella morganii" Foods 12, no. 19: 3625. https://doi.org/10.3390/foods12193625
APA StyleSun, Z., Zhang, Y., Lin, X., Zhang, S., Chen, Y., & Ji, C. (2023). Inhibition Mechanism of Lactiplantibacillus plantarum on the Growth and Biogenic Amine Production in Morganella morganii. Foods, 12(19), 3625. https://doi.org/10.3390/foods12193625





