Influence of Some Hydrocolloids and Sterilization Conditions on the Physical Properties of Texture-Modified Foods Developed for the Swallow Training of Dysphagia Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation and Food Reference
2.3. Sample Measurement
2.3.1. Viscosity
2.3.2. Mechanical Properties
2.4. Sterilization Study
2.5. Microbiological Study
2.6. Color Measurement
2.7. Stability Study
2.8. Data Analysis
3. Results
3.1. Effects of Hydrocolloids on the Texture-Modified Foods
3.1.1. Physical Properties
3.1.2. Viscosity of the Sample before the Sterilization Process
3.2. Effects of Sterilization Conditions on the Texture-Modified Foods
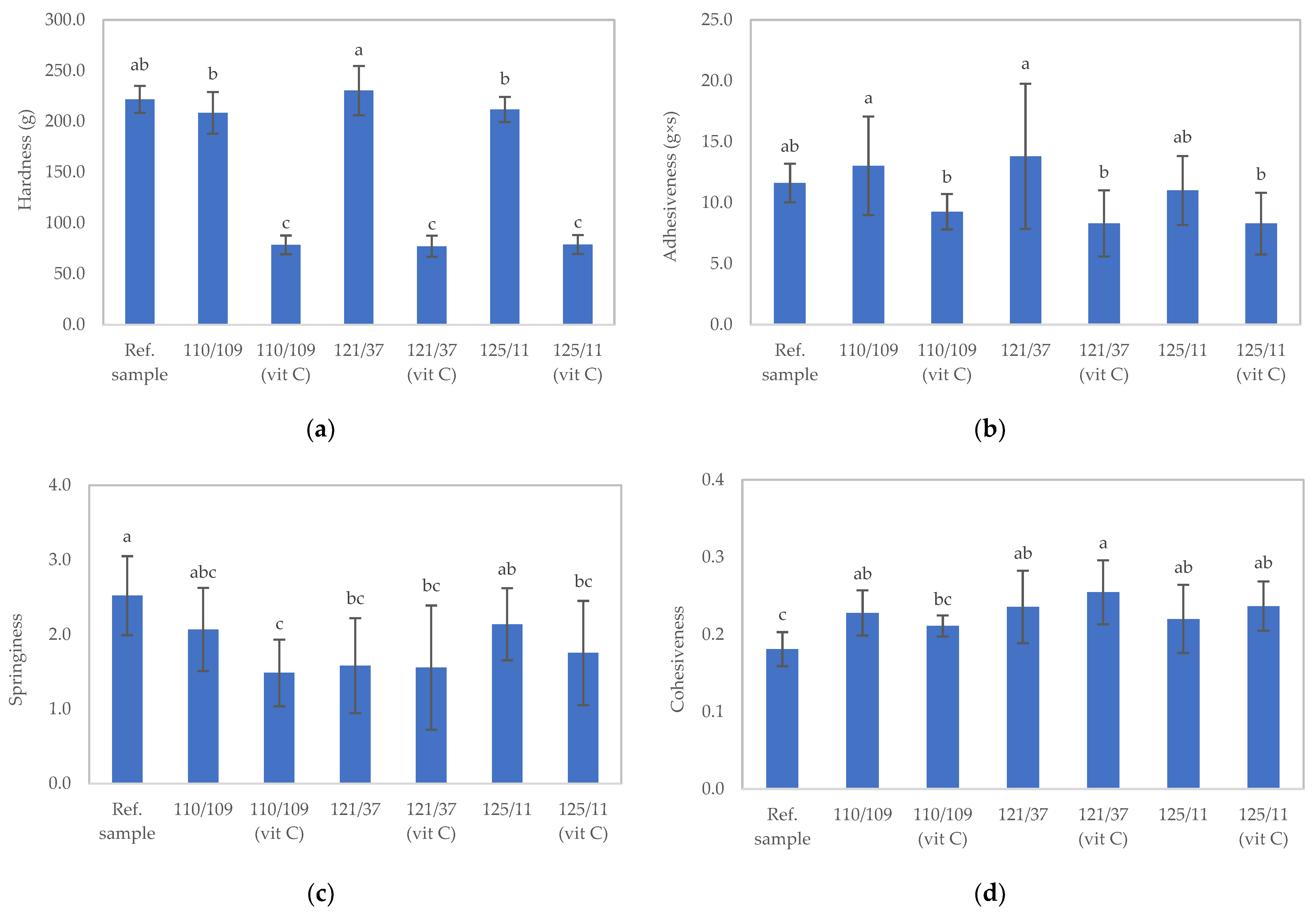
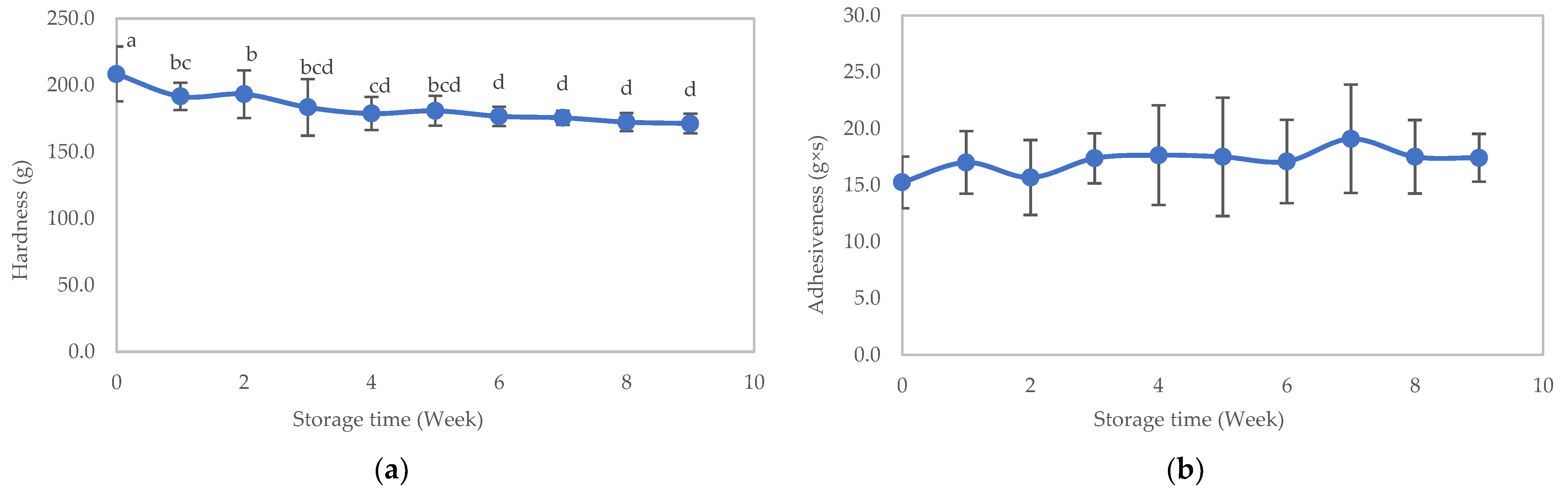
3.2.1. Physical Properties
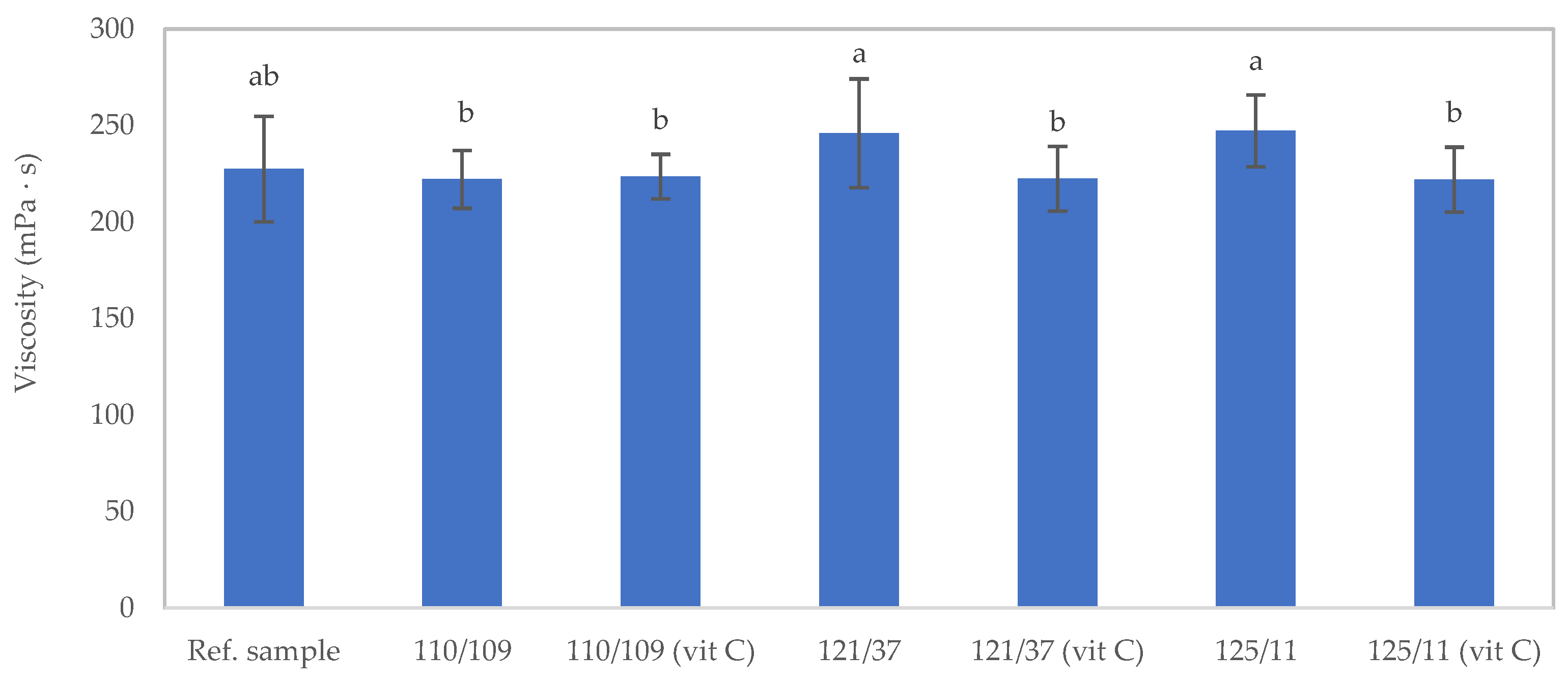
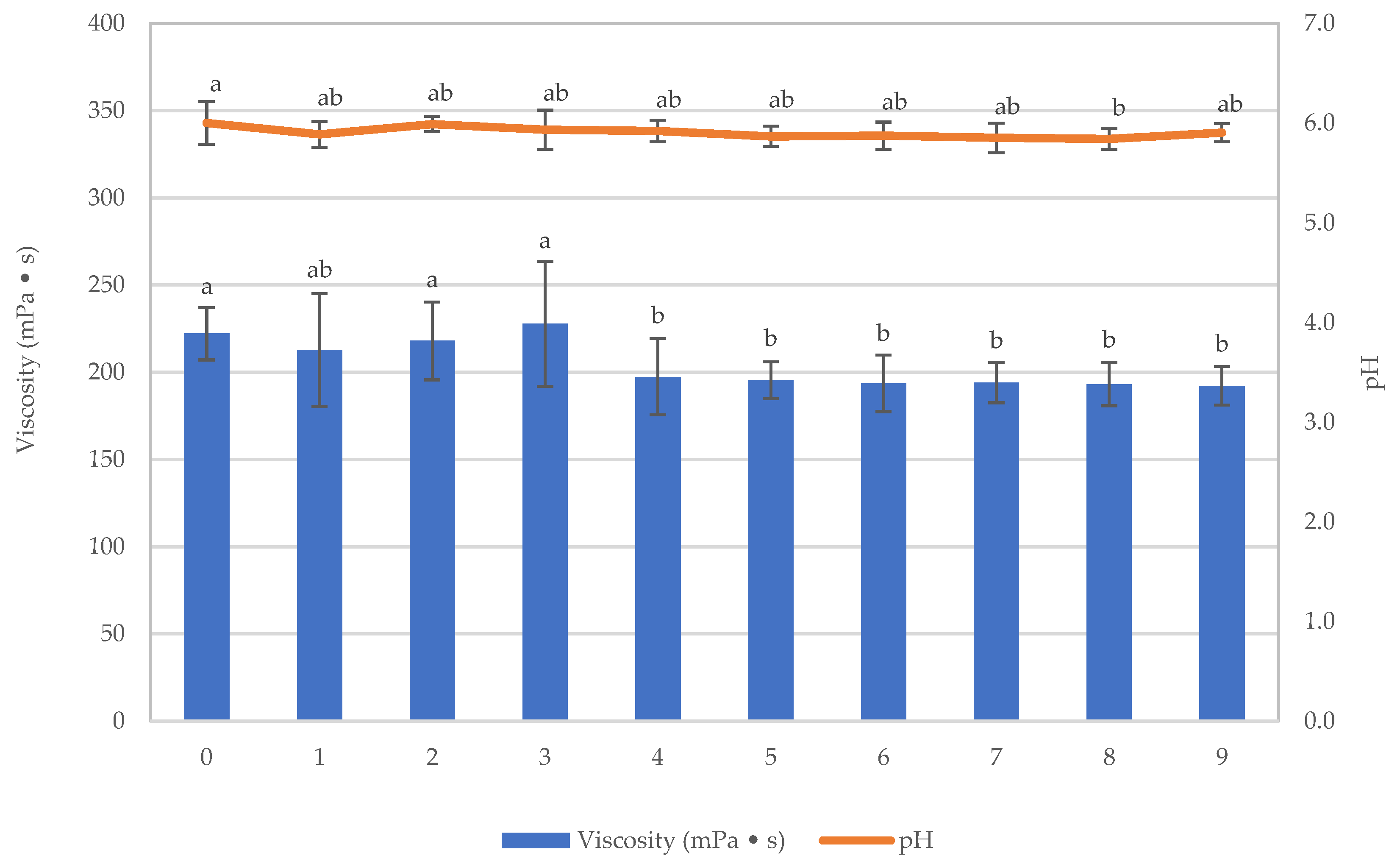
3.2.2. Viscosity of Samples after the Sterilization Process
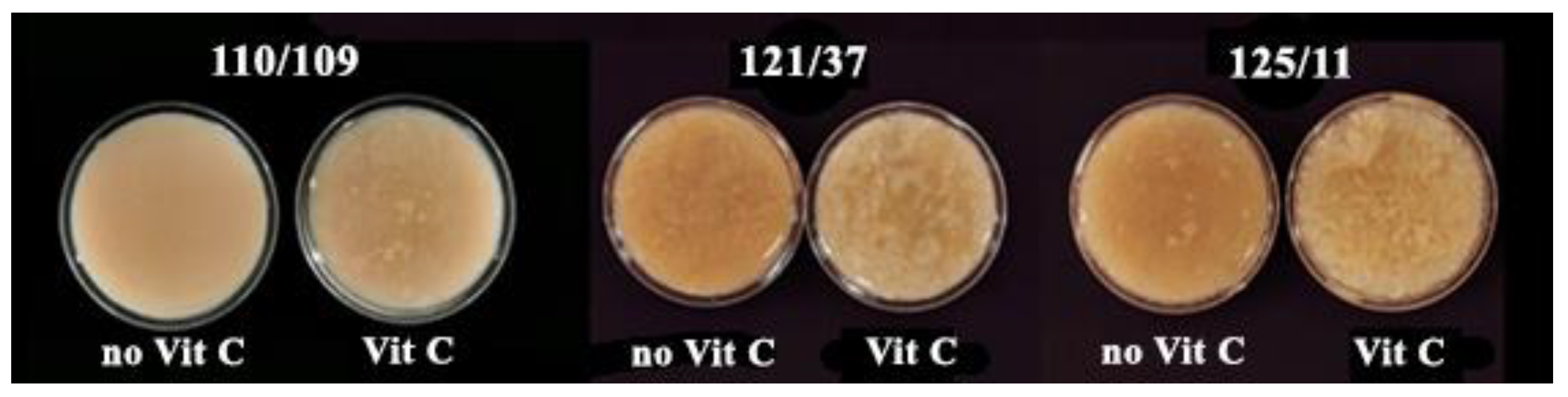
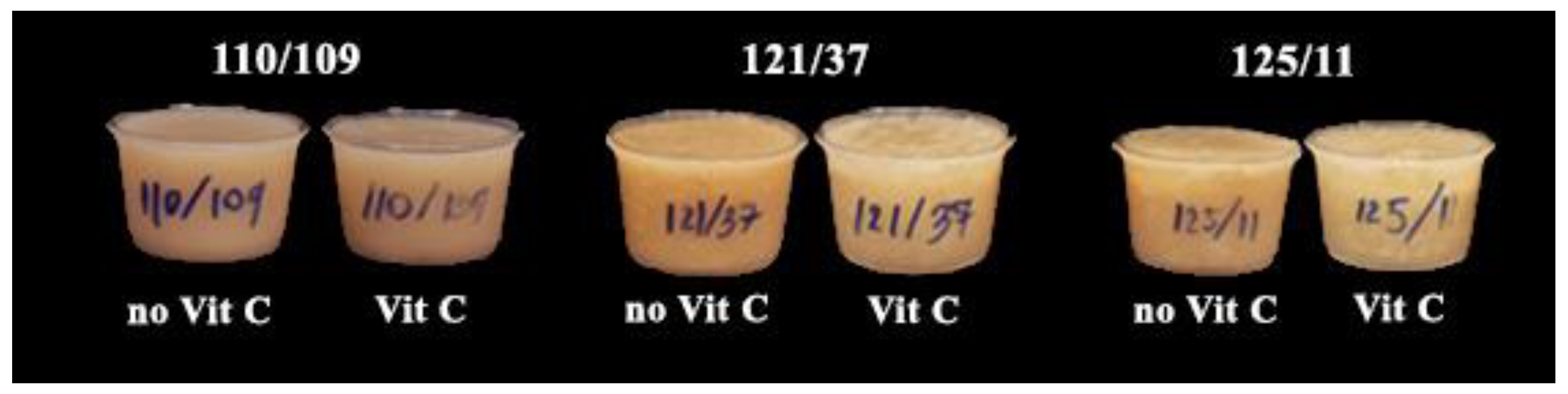
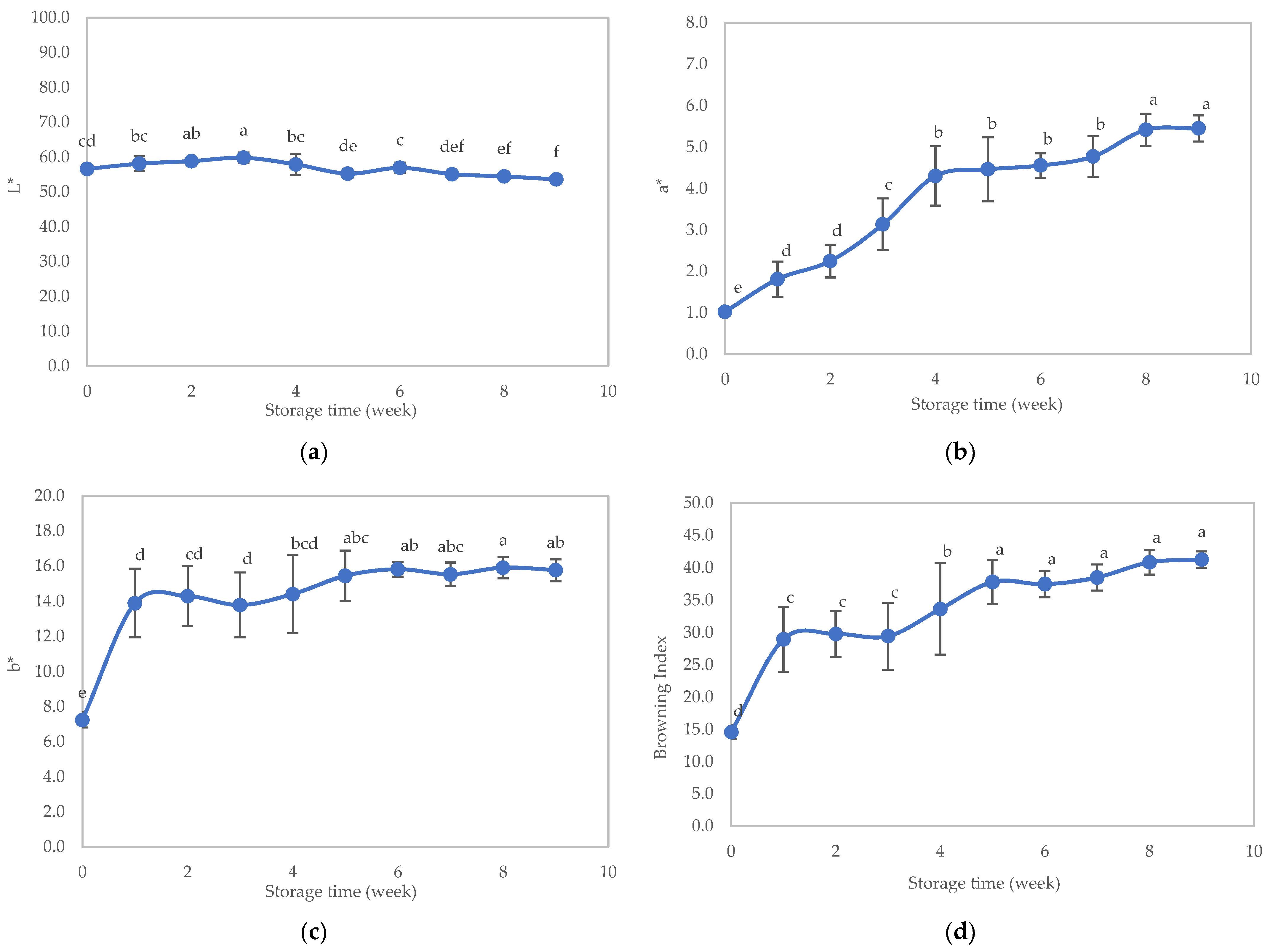
3.2.3. Appearance
3.2.4. Sterilization Efficiency
3.3. Stability Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDaniel, S.A.; Zimmer, Z. (Eds.) Global aging in twenty-century: An introduction. In Global Ageing in the Twenty-first Century; Ashgate Publishing Ltd.: Farnham, UK, 2013; pp. 1–12. [Google Scholar]
- Ou, Z.; Pan, J.; Tang, S.; Duan, D.; Yu, D.; Nong, H.; Wang, Z. Global Trends in the Incidence, Prevalence, and Years Lived With Disability of Parkinson’s Disease in 204 Countries/Territories From 1990 to 2019. Front. Public Healthy 2021, 9, 776847. [Google Scholar] [CrossRef]
- Kalf, J.G.; De Swart, B.J.M.; Bloem, B.R.; Munneke, M. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: A meta-analysis. Parkinsonism Relat. Disord. 2012, 18, 311–315. [Google Scholar] [CrossRef]
- Beyer, M.K.; Herlofson, K.; Arsland, D.; Larsen, J.P. Causes of Death in a Community-based study of Parkinson’s disease. Acta Neurol. Scand. 2001, 103, 7–11. [Google Scholar] [CrossRef]
- Fall, P.A.; Saleh, A.; Fredrickson, M.; Olsson, J.E.; Granérus, A.K. Survival time, mortality, and cause of death in elderly patients with Parkinson’s disease: A 9-year follow-up. Mov. Disord. 2003, 18, 1312–1316. [Google Scholar] [CrossRef]
- Cichero, J.A.Y.; Lam, P.T.; Chen, J.; Dantas, R.O.; Duivestein, J.; Hanson, B.; Kayashita, J.; Pillay, M.; Riquelme, L.F.; Steele, C.M.; et al. Release of updated International Dysphagia Diet Standardisation Initiative Framework (IDDSI 2.0). J. Texture Stud. 2020, 51, 195–196. [Google Scholar] [CrossRef]
- Robb, S.A.; Muntoni, F.; Simonds, A.K. Respiratory management of congenital myasthenic syndromes in childhood: Workshop 8th December 2009, UCL Institute of Neurology, London, UK. Neuromuscul. Disord. 2010, 20, 833–838. [Google Scholar] [CrossRef]
- Tasiri, P.; Suttisansanee, U.; Hudthagosol, C.; Somboonpanyakul, P. Development of riceberry rice vegan jelly contains high protein and high energy for the elderly with dysphagia. Agric. Sci. J. 2015, 46, 369–372. [Google Scholar]
- Strother, H.; Moss, R.; McSweeney, M.B. Comparison of 3D printed and molded carrots produced with gelatin, guar gum and xanthan gum. J. Texture Stud. 2020, 51, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Pematilleke, N.; Kaur, M.; Adhikari, B.; Torley, P.J. Investigation of the effects of addition of carboxy methyl cellulose (CMC) and tapioca starch (TS) on the beef patties targeted to the needs of people with dysphagia: A mixture design approach. Meat Sci. 2022, 191, 108868. [Google Scholar] [CrossRef]
- Xing, X.; Chitrakar, B.; Hati, S.; Xie, S.; Li, H.; Li, C.; Liu, Z.; Mo, H. Development of black fungus-based 3D printed foods as dysphagia diet: Effect of gums incorporation. Food Hydrocoll. 2022, 123, 107173. [Google Scholar] [CrossRef]
- Kim, D.S.; Iida, F. Texture Characteristics of Sea Buckthorn (Hippophae rhamnoides) Jelly for the Elderly Based on the Gelling Agent. Foods 2022, 11, 1892. [Google Scholar] [CrossRef]
- Min, C.; Yang, Q.; Pu, H.; Cao, Y.; Ma, W.; Kuang, J.; Huang, J.; Xiong, Y.L. Textural characterization of calcium salts-induced mung bean starch-flaxseed protein composite gels as dysphagia food. Food Res. Int. 2023, 164, 112355. [Google Scholar] [CrossRef]
- Wang, X.; Rong, L.; Shen, M.; Yu, Q.; Chen, Y.; Li, J.; Xie, J. Rheology, Texture and Swallowing Characteristics of a Texture-Modified Dysphagia Food Prepared Using Common Supplementary Materials. Foods 2023, 12, 2287. [Google Scholar] [CrossRef]
- McCullough, G.H.; Pelletier, C.A.; Steele, C.M. National dysphagia diet: What to swallow? ASHA Leader 2003, 8, 16–27. [Google Scholar] [CrossRef]
- Fernandez, P.S.; Gomez, F.J.; Ocio, M.J.; Rodrigo, M.; Sanchez, T.; Martinez, A. D-Values of Bacillus stearothermophilus spores as a function of pH and recovery medium acidulant. J. Food Prot. 1995, 58, 628–632. [Google Scholar] [CrossRef]
- Maturin, L.; Peeler, J.T. Chapter 3. Aerobic Plate Count. In Food and Drug Administration (FDA), Bacteriological Analytical Manual Online, 8th ed.; Silver Spring: Berlin, Germany, 2001. [Google Scholar]
- Palou, E.; López-Malo, A.; Barbosa-Cánovas, G.V.; Welti-Chanes, J.; Swanson, B.G. Polyphenoloxidase activity and color of blanched and high hydrostatic pressure treated banana puree. J. Food Sci. 1999, 64, 42–45. [Google Scholar] [CrossRef]
- Official Methods of Analysis of the Association of Official’s Analytical Chemists, 17th ed.; Association of Official Analytical Chemists (AOAC): Arlington, VA, USA, 2003.
- IBM SPSS Statistics for Windows, Version 22.0.; SEM software; IBM: Armonk, NY, USA, 2013.
- Balakrishnan, G.; Silva, J.V.C.; Nicolai, T.; Chassenieux, C.; Bovay, C.; Buczkowski, J.; Schmitt, C. Specific effect of calcium ions on thermal gelation of aqueous micellar casein suspensions. Colloids Surf. B. 2018, 163, 218–224. [Google Scholar] [CrossRef]
- Tang, M.; Zhu, Y.; Li, D.; Adhikari, B.; Wang, L. Rheological, thermal, and microstructural properties of casein/κ-carrageenan mixed systems. LWT Food Sci. Technol. 2019, 113, 108296. [Google Scholar] [CrossRef]
- Langendorff, V.; Cuvelier, G.; Launay, B.; Michon, C.; Parker, A.; Kruif, C.G.D. Casein Micelle/Iota Carrageenan Interactions in Milk Influence of Temperature. Food Hydrocoll. 1999, 13, 211–218. [Google Scholar] [CrossRef]
- Enriquez, L.G.; Flick, G.J. Marine colloids. In Food Emulsifiers: Chemistry, Technology, Functional Properties and Applications; Charalambous, G., Doxastakis, G., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; pp. 235–334. [Google Scholar]
- Armisen, R.; Thomas, W.R.; Poppe, J.; Urlacher, B.; Noble, O. Agar, Carrageenan, Gelatin, Konjac gum, and Xanthan gum. In Thickening and Gelling Agents for Food, 2nd ed.; Imeson, A.P., Ed.; Springer: New York, NY, USA, 1997; pp. 1–21; 45–59; 144–168; 169–179; 284–311. [Google Scholar]
- Spiegel, C. Mathematical Modeling of Heat Transfer to Determine Cooling Drum Temperatures and Gelatin Material Properties for Soft gel Manufacturing. 2014. Available online: https://biopharma-asia.com/technical-papers/mathematical-modeling-heat-transfer-determine-cooling-drum-temperatures-gelatin-material-properties-softgel-manufacturing-colleen-spiegel-ph-d/ (accessed on 10 December 2018).
- Pirsa, S.; Hafezi, K. Hydrocolloids: Structure, preparation method, and application in food industry. Food Chem. 2023, 399, 133967. [Google Scholar] [CrossRef]
- Yemenicioglu, A.; Farris, S.; Turkyilmaz, M.; Gulec, S. A review of current and future food applications of natural hydrocolloids. Int. J. Food Sci. Technol. 2020, 55, 1389–1406. [Google Scholar] [CrossRef]
- Sun, T.; Tao, H.; Xie, j.; Zhang, S.; Xu, X. Degradation and Antioxidant Activity of κ-Carrageenans. J. Appl. Polym. 2009, 117, 194–199. [Google Scholar] [CrossRef]
- Sow, L.C.; Chong, J.M.; Liao, Q.X.; Yang, H. Effects of κ-Carrageenan on the Structure and Rheological Properties of Fish Gelatin. J. Food Eng. 2018, 239, 92–103. [Google Scholar] [CrossRef]
- Yuan, D.; Xu, Y.; Wang, C.; Li, Y.; Li, F.; Zhou, Y.; Chen, J.; Jin, Z.; Zhang, H.; Jiang, Y. Comparison of anti-browning ability and characteristics of the fractionated Maillard reaction products with different polarities. J. Food Sci. Technol. 2015, 52, 7163–7172. [Google Scholar] [CrossRef]
- Morris, E.R.; Rees, D.A.; Robinson, G. Cation-specific aggregation of carrageenan helices: Domain model of polymer gel structure. J. Mol. Biol. 1980, 138, 349–362. [Google Scholar] [CrossRef]
- Spagnuoloa, P.A.; Dalgleisha, D.G.; Goffa, H.D.; Morris, E.R. Kappa-carrageenan interactions in systems containing casein micelles and polysaccharide stabilizers. Food Hydrocoll. 2005, 19, 371–377. [Google Scholar] [CrossRef]



| Types of Hydrocolloids | Level of Addition | ||
|---|---|---|---|
| 1% | 3% | 5% | |
| Agar | A1 | A3 | A5 |
| κ-Carrageenan | C1 | C3 | C5 |
| Gelatin | G1 | G3 | G5 |
| Konjac Glucomannan | K1 | K3 | K5 |
| Xanthan gum | X1 | X3 | X5 |
| Sample | Hardness (g) | Adhesiveness (g·s) | Springiness | Cohesiveness |
|---|---|---|---|---|
| Richese® (Ref. sample) | 238.29 ± 21.72 c | 3.28 ± 1.19 e | 0.9 ± 0.41 g | 0.29 ± 0.04 d |
| A1 | 45.57 ± 2.37 e | 44.86 ± 21.89 d | 6.33 ± 0.36 b | 0.35 ± 0.03 c |
| A3 | 77.83 ± 2.88 e | 145.09 ± 10.58 b | 1.18 ± 0.10 fg | 0.28 ± 0.02 d |
| A5 | 211.47 ± 3.08 cd | 232.88 ± 35.83 a | 8.15 ± 0.81 a | 0.38 ± 0.03 bc |
| C1 | 221.72 ± 13.36 cd | 11.63 ± 1.58 e | 2.52 ± 0.53 d | 0.18 ± 0.02 f |
| C3 | 329.15 ± 35.19 b | 46.29 ± 14.04 d | 3.02 ± 0.73 c | 0.23 ± 0.02 e |
| C5 | 814.74 ± 169.45 a | 50.09 ± 13.43 d | 1.83 ± 0.17 e | 0.24 ± 0.02 e |
| G1 | NA | NA | NA | NA |
| G3 | 71.63 ± 11.93 e | 49.30 ± 5.95 d | 1.47 ± 0.44 ef | 0.35 ± 0.41 c |
| G5 | 163.85 ± 15.69 d | 114.29 ± 29.07 c | 1.31 ± 0.32 fg | 0.40 ± 0.41 b |
| K1 | NA | NA | NA | NA |
| K3 | NA | NA | NA | NA |
| K5 | 54.87 ± 0.72 e | 160.59 ± 13.67 b | 1.14 ± 0.16 fg | 0.71 ± 0.04 a |
| X1 | NA | NA | NA | NA |
| X3 | NA | NA | NA | NA |
| X5 | NA | NA | NA | NA |
| Physical Characteristics of Gel Formation and Syneresis | Sample |
|---|---|
| Soft gel with syneresis | A1, G1 |
| Soft gel without syneresis (target texture characteristics) | K5, C1 |
| Hard gel with syneresis | G3, G5 |
| Hard gel without syneresis | A3, A5, C3, C5 |
| Viscous solution (no gel formed) | K1, K3, X1, X3, X5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limampai, T.; Impaprasert, R.; Suntornsuk, W. Influence of Some Hydrocolloids and Sterilization Conditions on the Physical Properties of Texture-Modified Foods Developed for the Swallow Training of Dysphagia Patients. Foods 2023, 12, 3676. https://doi.org/10.3390/foods12193676
Limampai T, Impaprasert R, Suntornsuk W. Influence of Some Hydrocolloids and Sterilization Conditions on the Physical Properties of Texture-Modified Foods Developed for the Swallow Training of Dysphagia Patients. Foods. 2023; 12(19):3676. https://doi.org/10.3390/foods12193676
Chicago/Turabian StyleLimampai, Thitiwat, Rarisara Impaprasert, and Worapot Suntornsuk. 2023. "Influence of Some Hydrocolloids and Sterilization Conditions on the Physical Properties of Texture-Modified Foods Developed for the Swallow Training of Dysphagia Patients" Foods 12, no. 19: 3676. https://doi.org/10.3390/foods12193676
APA StyleLimampai, T., Impaprasert, R., & Suntornsuk, W. (2023). Influence of Some Hydrocolloids and Sterilization Conditions on the Physical Properties of Texture-Modified Foods Developed for the Swallow Training of Dysphagia Patients. Foods, 12(19), 3676. https://doi.org/10.3390/foods12193676






