Comparison of Metabolites and Species Classification of Thirteen Zingiberaceae Spices Based on GC–MS and Multi-Spectral Fusion Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Sample Preprocessing for Spectra Acquisition
2.3. Near-Infrared (NIR) Spectra Acquisition
2.4. Mid-Infrared (MIR) Spectra Acquisition
2.5. Gas Chromatography–Mass Spectrometry (GC–MS) Conditions and Measurement
2.6. GC–MS Data Analysis
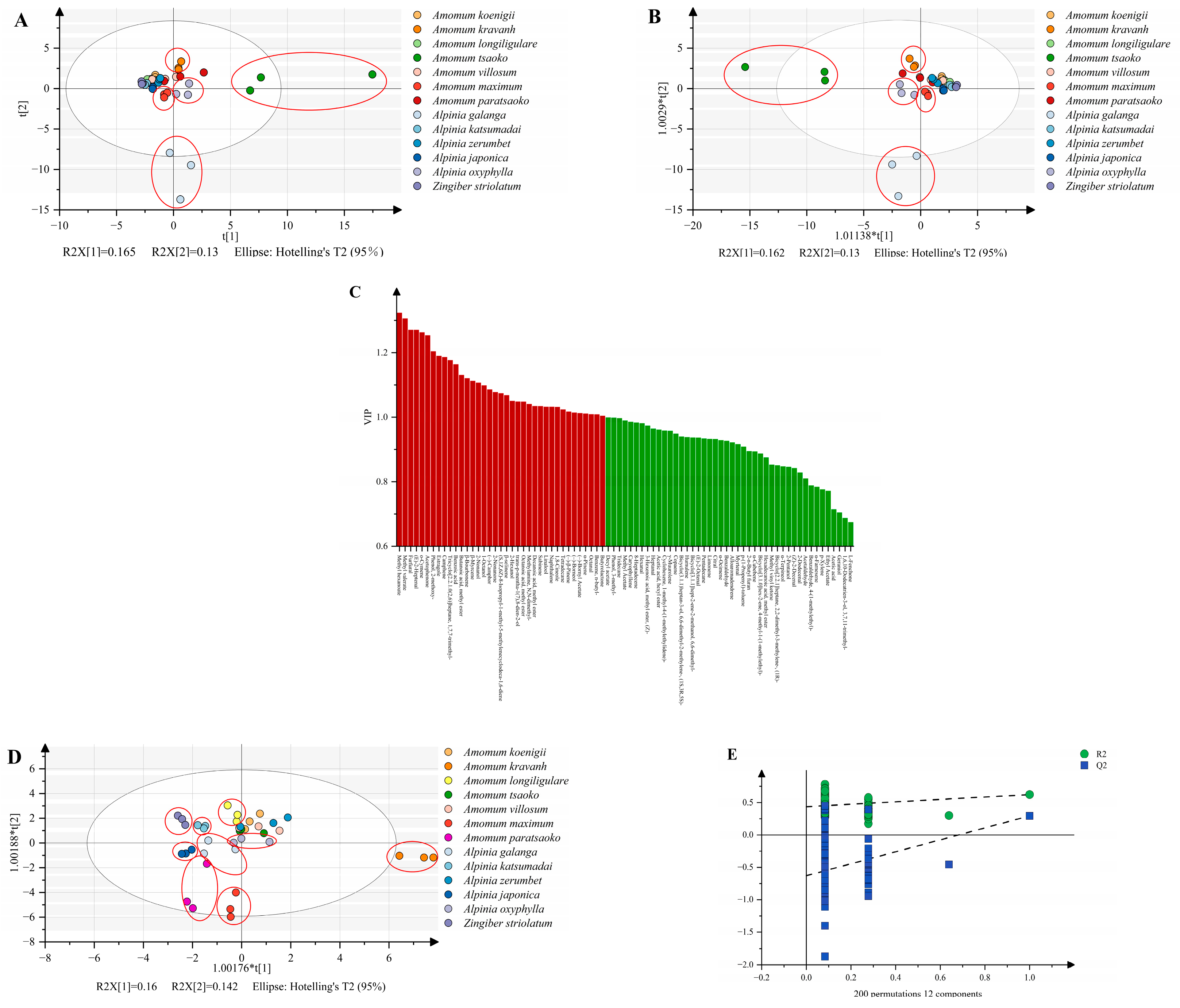
2.7. Multivariate Statistical Analysis for Spectra Data
3. Results and Discussion
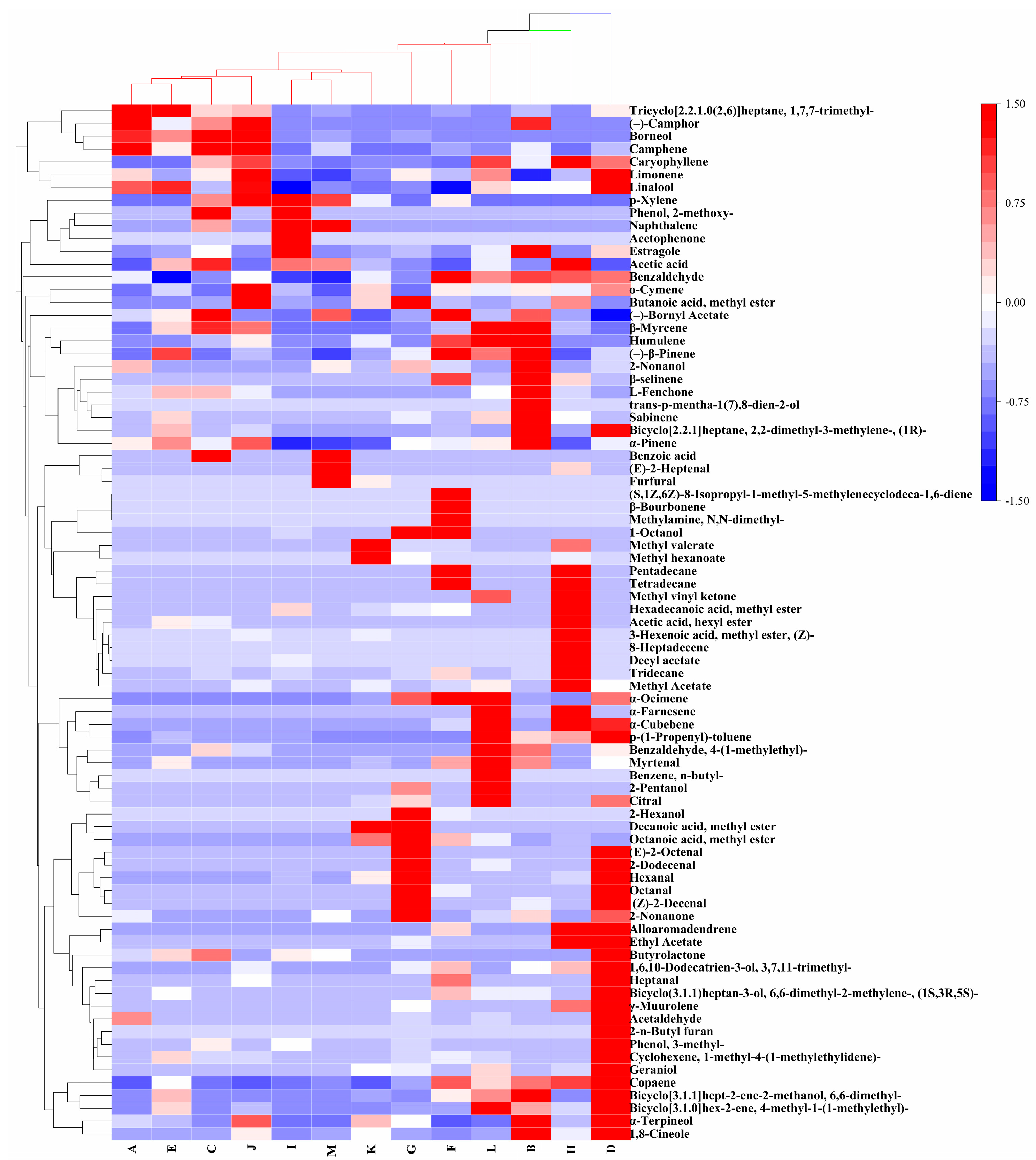
3.1. Analysis of Metabolites
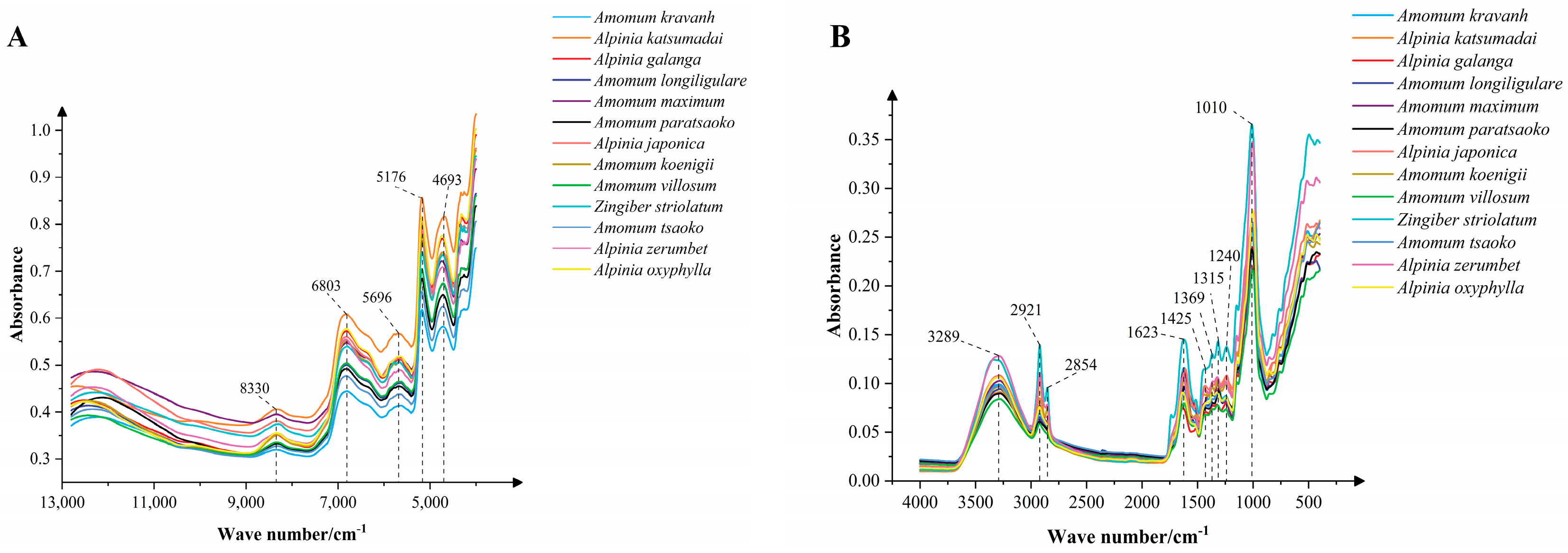
3.2. Spectroscopic Analysis
3.3. Comparison of Low-Level and Mid-Level Data Fusion Models
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kress, W.J.; Prince, L.M.; Williams, K.J. The phylogeny and a new classification of the gingers (Zingiberaceae): Evidence from molecular data. Am. J. Bot. 2002, 89, 1682–1696. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Li, P.; Ling, R.; Wang, Z.; Feng, X.; Liu, J.; Yang, Q.; Yan, J. Diversity of Volatile Compounds in Ten Varieties of Zingiberaceae. Molecules 2022, 27, 565. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.C.; Zhang, J.; Han, J.P.; Song, J.Y.; Yao, H.; Zhu, Y.J.; Li, J.C.; Wang, Z.Z.; Xiao, W.; Lin, Y.L.; et al. Testing the potential of proposed DNA barcodes for species identification of Zingiberaceae. J. Syst. Evol. 2011, 49, 261–266. [Google Scholar] [CrossRef]
- Erdil, İ. A review on biological activities of essential oils. KNOWLEDGE-Int. J. 2017, 20, 2171–2175. [Google Scholar]
- Wu, M.H.; Zhang, W.; Guo, P.; Zhao, Z.Z. Identification of seven Zingiberaceous species based on comparative anatomy of microscopic characteristics of seeds. Chin. Med. 2014, 9, 10. [Google Scholar] [CrossRef]
- Baharudin, M.K.A.; Hamid, S.A.; Susanti, D. Chemical composition and antibacterial activity of essential oils from three aromatic plants of the Zingiberaceae family in Malaysia. J. Phys. Sci. 2015, 26, 71. [Google Scholar]
- Ivanovic, M.; Makoter, K.; Islamcevic Razborsek, M. Comparative Study of Chemical Composition and Antioxidant Activity of Essential Oils and Crude Extracts of Four Characteristic Zingiberaceae Herbs. Plants 2021, 10, 501. [Google Scholar] [CrossRef]
- Tunnisa, F.; Nur Faridah, D.; Afriyanti, A.; Rosalina, D.; Ana Syabana, M.; Darmawan, N.; Dewi Yuliana, N. Antioxidant and antidiabetic compounds identification in several Indonesian underutilized Zingiberaceae spices using SPME-GC/MS-based volatilomics and in silico methods. Food Chem. X 2022, 14, 100285. [Google Scholar] [CrossRef]
- Putri, S.P.; Ikram, M.M.M.; Sato, A.; Dahlan, H.A.; Rahmawati, D.; Ohto, Y.; Fukusaki, E. Application of gas chromatography-mass spectrometry-based metabolomics in food science and technology. J. Biosci. Bioeng. 2022, 133, 425–435. [Google Scholar] [CrossRef]
- Suman, M.; Cavanna, D.; Sammarco, G.; Lambertini, F.; Loffi, C. Fighting food frauds exploiting chromatography-mass spectrometry technologies: Scenario comparison between solutions in scientific literature and real approaches in place in industrial facilities. TrAC Trends Anal. Chem. 2021, 142, 116305. [Google Scholar] [CrossRef]
- Valand, R.; Tanna, S.; Lawson, G.; Bengtstrom, L. A review of Fourier Transform Infrared (FTIR) spectroscopy used in food adulteration and authenticity investigations. Food Addit. Contam. Part A 2020, 37, 19–38. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Y.; Yao, C.L.; Guo, D.A. Quality assessment of herbal medicines based on chemical fingerprints combined with chemometrics approach: A review. J. Pharm. Biomed. Anal. 2020, 185, 113215. [Google Scholar] [CrossRef]
- Sandasi, M.; Kamatou, G.P.P.; Combrinck, S.; Viljoen, A.M. A chemotaxonomic assessment of four indigenous South African Lippia species using GC–MS and vibrational spectroscopy of the essential oils. Biochem. Syst. Ecol. 2013, 51, 142–152. [Google Scholar] [CrossRef]
- Putri, S.P.; Irifune, T.; Yusianto; Fukusaki, E. GC/MS based metabolite profiling of Indonesian specialty coffee from different species and geographical origin. Metabolomics 2019, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.K.; Zhang, J.; Hayashi, K.; Liu, C. Identification of discriminating chemical compounds in banana species and their odor characterization using GC-MS, statistical, and clustering analysis. J. Food Sci. Technol. 2022, 59, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Kabbash, E.M.; Mediani, A.; Doll, S.; Esatbeyoglu, T.; Afifi, S.M. Comparative Metabolite Fingerprinting of Four Different Cinnamon Species Analyzed via UPLC-MS and GC-MS and Chemometric Tools. Molecules 2022, 27, 2935. [Google Scholar] [CrossRef] [PubMed]
- Van, H.T.; Dam, S.M.; Phan, U.T.X.; Nguyen, T.N.A.; Tran Nguyen, T.B.; Tran, T.L.; Luu, T.N.; Le, V.S.; An Huynh, N.T. Chemical Diversity of Essential Oils from Aerial Parts of Eight Species of Zingiberaceae Family from Vietnam. Acta Univ. Agric. Silvic. Mendel. Brun. 2022, 70, 273–281. [Google Scholar] [CrossRef]
- Pauzi, A.N. Discrimination of Zingiberaceae Medicinal Herbs Using Analytical Methods Combined with Chemometric Techniques. Ph.D. Thesis, Universiti Tun Hussein Onn Malaysia, Parit Raja, Malaysia, 2022. [Google Scholar]
- Wahyuni, D.S.C.; Wardianti, M.P.; Rinanto, Y.; Marliyana, S.D. Metabolite Profiling of Three Curcuma Species (Zingiberaceae) Based on H-NMR Spectroscopy. ALCHEMY J. Penelit. Kim. 2019, 15, 79–88. [Google Scholar] [CrossRef]
- Reale, S.; Biancolillo, A.; Foschi, M.; Di Donato, F.; Di Censo, E.; D’Archivio, A.A. Geographical discrimination of Italian carrot (Daucus carota L.) varieties: A comparison between ATR FT-IR fingerprinting and HS-SPME/GC-MS volatile profiling. Food Control 2023, 146, 109508. [Google Scholar] [CrossRef]
- Qin, H.; Wang, Y.; Yang, W.; Yang, S.; Zhang, J. Comparison of metabolites and variety authentication of Amomum tsao-ko and Amomum paratsao-ko using GC-MS and NIR spectroscopy. Sci. Rep. 2021, 11, 15200. [Google Scholar] [CrossRef]
- Jo, H.E.; Song, K.; Kim, J.-G.; Lee, C.H. Non-targeted metabolomic analysis for the comparative evaluation of volatile organic compounds in 20 globally representative cucumber lines. Front. Plant Sci. 2022, 13, 1028735. [Google Scholar] [CrossRef]
- Diao, W.R.; Zhang, L.L.; Feng, S.S.; Xu, J.G. Chemical Composition, Antibacterial Activity, and Mechanism of Action of the Essential Oil from Amomum kravanh. J. Food Prot. 2014, 77, 1740–1746. [Google Scholar] [CrossRef]
- Anh, T.; Ngoc, N.B.; Phuc, N.; Nhat, D.; Danh, P.; Bach, L. Essential oil from Amomum longiligulare TL Wu cultivated in Ninh Thuan province, Vietnam. Mater. Sci. Eng. 2020, 991, 012113. [Google Scholar]
- Cui, Q.; Wang, L.T.; Liu, J.Z.; Wang, H.M.; Guo, N.; Gu, C.B.; Fu, Y.J. Rapid extraction of Amomum tsao-ko essential oil and determination of its chemical composition, antioxidant and antimicrobial activities. J. Chromatogr. B 2017, 1061–1062, 364–371. [Google Scholar] [CrossRef]
- Ao, H.; Wang, J.; Chen, L.; Li, S.; Dai, C. Comparison of Volatile Oil between the Fruits of Amomum villosum Lour. and Amomum villosum Lour. var. xanthioides T. L. Wu et Senjen Based on GC-MS and Chemometric Techniques. Molecules 2019, 24, 1663. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.S.; You, C.X.; Liang, J.Y.; Zhang, W.J.; Yang, K.; Geng, Z.F.; Wang, C.F.; Du, S.S.; Lei, N. Essential Oil of Amomum maximum Roxb. and Its Bioactivities against Two Stored-Product Insects. J. Oleo Sci. 2015, 64, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Van, H.T.; Thang, T.D.; Luu, T.N.; Doan, V.D. An overview of the chemical composition and biological activities of essential oils from Alpinia genus (Zingiberaceae). RSC Adv. 2021, 11, 37767–37783. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Wong, S.K.; Chan, H.T. Alpinia zerumbet, a ginger plant with a multitude of medicinal properties: An update on its research findings. J. Chin. Pharm. Sci. 2017, 26, 775–788. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, Y.; Hu, X.; Hu, X.; Lv, W.; Lv, D.; Chen, J.; Wu, M.; Song, Q.; Shentu, J. Ethnopharmacological uses, phytochemistry, biological activities, and therapeutic applications of Alpinia oxyphylla Miquel: A review. J. Ethnopharmacol. 2018, 224, 149–168. [Google Scholar] [CrossRef]
- Fang, C.; Fernie, A.R.; Luo, J. Exploring the Diversity of Plant Metabolism. Trends Plant Sci. 2019, 24, 83–98. [Google Scholar] [CrossRef]
- Cai, Z.M.; Peng, J.Q.; Chen, Y.; Tao, L.; Zhang, Y.Y.; Fu, L.Y.; Long, Q.D.; Shen, X.C. 1,8-Cineole: A review of source, biological activities, and application. J. Asian Nat. Prod. Res. 2021, 23, 938–954. [Google Scholar] [CrossRef]
- An, Q.; Ren, J.N.; Li, X.; Fan, G.; Qu, S.S.; Song, Y.; Li, Y.; Pan, S.Y. Recent updates on bioactive properties of linalool. Food Funct. 2021, 12, 10370–10389. [Google Scholar] [CrossRef] [PubMed]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef] [PubMed]
- Hachlafi, N.E.L.; Aanniz, T.; Menyiy, N.E.; Baaboua, A.E.; Omari, N.E.; Balahbib, A.; Shariati, M.A.; Zengin, G.; Fikri-Benbrahim, K.; Bouyahya, A. In Vitro and in Vivo Biological Investigations of Camphene and Its Mechanism Insights: A Review. Food Rev. Int. 2021, 39, 1799–1826. [Google Scholar] [CrossRef]
- Farrés, M.; Platikanov, S.; Tsakovski, S.; Tauler, R. Comparison of the variable importance in projection (VIP) and of the selectivity ratio (SR) methods for variable selection and interpretation. J. Chemom. 2015, 29, 528–536. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, S.; Wang, Y.; Zhang, J. Discrimination of the fruits of Amomum tsao-ko according to geographical origin by 2DCOS image with RGB and Resnet image analysis techniques. Microchem. J. 2021, 169, 106545. [Google Scholar] [CrossRef]
- Rueda, P.M.; Comino, F.; Aranda, V.; Ayora-Canada, M.J.; Dominguez-Vidal, A. Understanding the compositional changes of organic matter in torrefied olive mill pomace compost using infrared spectroscopy and chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 293, 122450. [Google Scholar] [CrossRef]
- Yue, J.Q.; Li, Z.M.; Zuo, Z.T.; Zhao, Y.L.; Zhang, J.; Wang, Y.Z. Study on the identification and evaluation of growth years for Paris polyphylla var. yunnanensis using deep learning combined with 2DCOS. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 261, 120033. [Google Scholar] [CrossRef]
- Yue, J.Q.; Huang, H.Y.; Wang, Y.Z. Extended application of deep learning combined with 2DCOS: Study on origin identification in the medicinal plant of Paris polyphylla var. yunnanensis. Phytochem. Anal. 2022, 33, 136–150. [Google Scholar] [CrossRef]
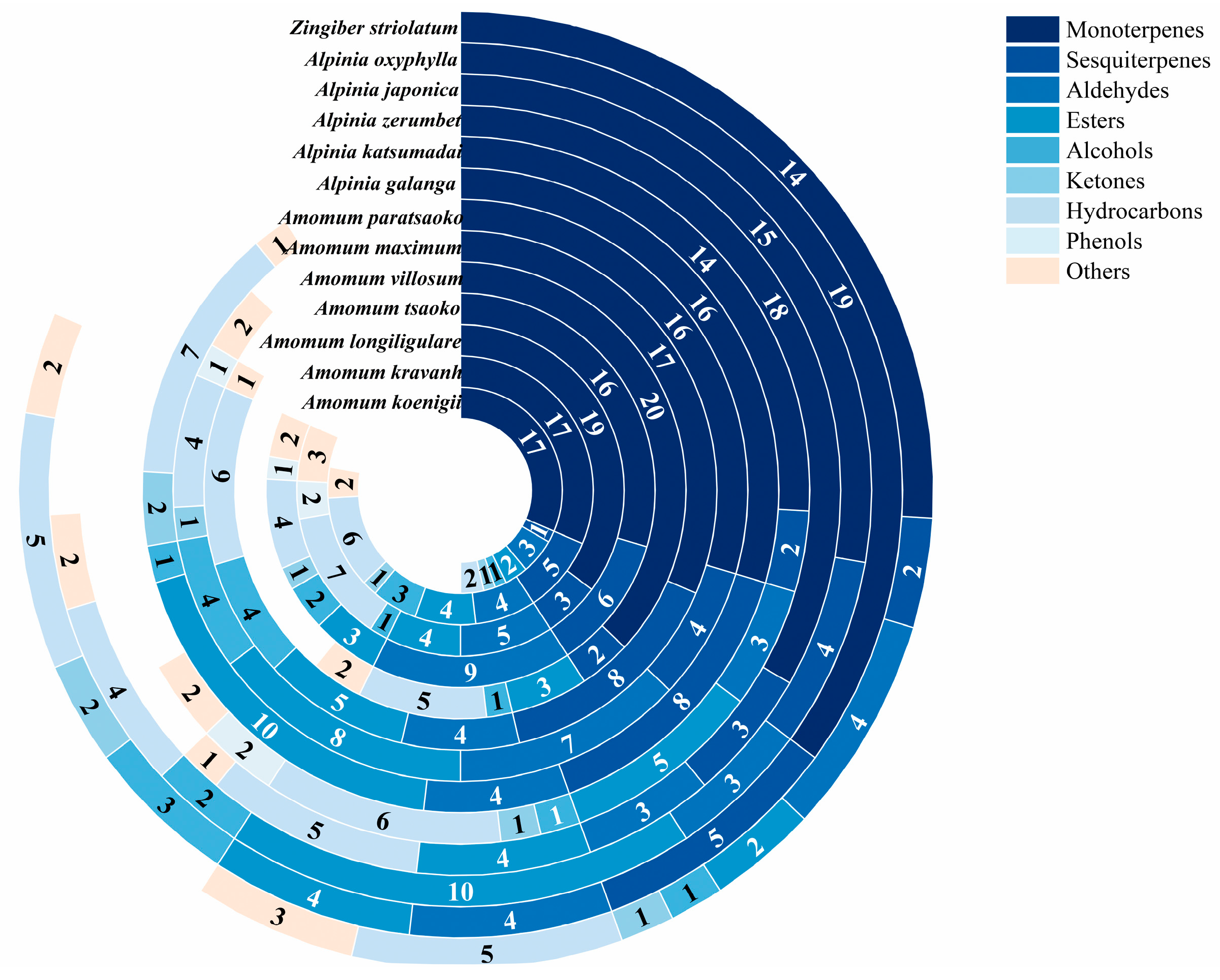


| Name | Main Volatile Components | Compound Classification | Relative Content * (%) |
|---|---|---|---|
| Amomum koenigii | Linalool | Monoterpenes | 1.9096 ± 0.1795 |
| Camphene | Monoterpenes | 1.5867 ± 0.5335 | |
| (–)-Camphor | Monoterpenes | 1.1778 ± 0.2981 | |
| Borneol | Monoterpenes | 1.0792 ± 0.4300 | |
| Limonene | Monoterpenes | 1.0783 ± 0.7768 | |
| α-Pinene | Monoterpenes | 0.9384 ± 0.1091 | |
| Amomum kravanh | 1,8-Cineole | Monoterpenes | 8.6057 ± 2.3572 |
| β-Selinene | Sesquiterpenes | 3.3511 ± 0.2210 | |
| α-Pinene | Monoterpenes | 2.6347 ± 0.4049 | |
| (–)-β-Pinene | Monoterpenes | 2.3827 ± 1.7027 | |
| Sabinene | Monoterpenes | 2.0574 ± 0.2369 | |
| β-Myrcene | Monoterpenes | 1.6901 ± 1.2053 | |
| Amomum longiligulare | Borneol | Monoterpenes | 1.4594 ± 1.1597 |
| Camphene | Monoterpenes | 1.4351 ± 0.3747 | |
| β-Myrcene | Monoterpenes | 1.2737 ± 0.9165 | |
| Limonene | Monoterpenes | 0.9416 ± 1.0558 | |
| Linalool | Monoterpenes | 0.8842 ± 0.2345 | |
| Caryophyllene | Sesquiterpenes | 0.8372 ± 0.4591 | |
| Amomum tsaoko | 1,8-Cineole | Monoterpenes | 8.6216 ± 1.8458 |
| γ-Muurolene | Sesquiterpenes | 3.0686 ± 1.3184 | |
| Limonene | Monoterpenes | 2.4858 ± 1.9670 | |
| Linalool | Monoterpenes | 2.4640 ± 1.2840 | |
| Copaene | Sesquiterpenes | 2.2274 ± 0.6573 | |
| Geraniol | Monoterpenes | 1.5468 ± 1.6610 | |
| Amomum villosum | Linalool | Monoterpenes | 2.0851 ± 0.6833 |
| (–)-β-Pinene | Monoterpenes | 1.7271 ± 1.2663 | |
| α-Pinene | Monoterpenes | 1.3186 ± 0.3246 | |
| Borneol | Monoterpenes | 0.7760 ± 1.0974 | |
| β-Myrcene | Monoterpenes | 0.7023 ± 0.9932 | |
| Copaene | Monoterpenes | 0.6476 ± 0.9159 | |
| Amomum maximum | (–)-β-Pinene | Monoterpenes | 2.4277 ± 0.1931 |
| β-Selinene | Sesquiterpenes | 1.4536 ± 0.1007 | |
| Copaene | Sesquiterpenes | 1.2216 ± 0.0431 | |
| β-Bourbonene | Sesquiterpenes | 1.2136 ± 0.1201 | |
| α-Ocimene | Monoterpenes | 1.1048 ± 0.7753 | |
| Humulene | Sesquiterpenes | 0.9789 ± 0.6923 | |
| Amomum paratsaoko | (Z)-2-Decenal | Aldehydes | 1.4423 ± 0.5351 |
| Octanal | Aldehydes | 1.1835 ± 0.6240 | |
| (E)-2-Octenal | Aldehydes | 1.0320 ± 0.4831 | |
| Limonene | Monoterpenes | 0.9243 ± 0.2577 | |
| α-Pinene | Monoterpenes | 0.8682 ± 0.0838 | |
| α-Ocimene | Monoterpenes | 0.7899 ± 0.1907 | |
| Alpinia galanga | Caryophyllene | Sesquiterpenes | 2.1802 ± 1.5630 |
| Tridecane | Hydrocarbons | 1.9142 ± 0.3532 | |
| Decyl acetate | Esters | 1.5508 ± 0.5689 | |
| Copaene | Sesquiterpenes | 1.3953 ± 0.1010 | |
| Alloaromadendrene | Sesquiterpenes | 1.3661 ± 0.0971 | |
| 1,8-Cineole | Monoterpenes | 1.3611 ± 0.4756 | |
| Alpinia katsumadai | Estragole | Monoterpenes | 1.3972 ± 0.3235 |
| Acetic acid | Acids | 0.2495 ± 0.1786 | |
| (–)-β-Pinene | Monoterpenes | 0.2406 ± 0.1470 | |
| o-Cymene | Hydrocarbons | 0.2366 ± 0.1923 | |
| 1,8-Cineole | Monoterpenes | 0.1577 ± 0.0265 | |
| Limonene | Monoterpenes | 0.1514 ± 0.0243 | |
| Alpinia zerumbet | 1,8-Cineole | Monoterpenes | 2.4631 ± 0.9457 |
| Linalool | Monoterpenes | 2.2433 ± 0.2904 | |
| Limonene | Monoterpenes | 1.9982 ± 1.1538 | |
| o-Cymene | Hydrocarbons | 1.7801 ± 1.2708 | |
| α-Pinene | Monoterpenes | 1.4746 ± 0.2429 | |
| Borneol | Monoterpenes | 1.4343 ± 1.0396 | |
| Alpinia japonica | 1,8-Cineole | Monoterpenes | 1.8123 ± 0.1452 |
| Methyl hexanoate | Esters | 0.7849 ± 0.1505 | |
| Linalool | Monoterpenes | 0.6186 ± 0.1141 | |
| o-Cymene | Hydrocarbons | 0.5333 ± 0.0981 | |
| α-Terpineol | Monoterpenes | 0.4864 ± 0.0370 | |
| (–)-β-Pinene | Monoterpenes | 0.4055 ± 0.0520 | |
| Alpinia oxyphylla | Citral | Monoterpenes | 3.1651 ± 2.6423 |
| Humulene | Sesquiterpenes | 1.6426 ± 1.1672 | |
| (–)-β-Pinene | Monoterpenes | 1.5048 ± 0.7049 | |
| α-Ocimene | Monoterpenes | 1.4872 ± 0.8404 | |
| Linalool | Monoterpenes | 1.4704 ± 0.1282 | |
| β-Myrcene | Monoterpenes | 1.4372 ± 0.1290 | |
| Zingiber striolatum | Linalool | Monoterpenes | 0.6799 ± 0.3538 |
| (–)-Bornyl acetate | Monoterpenes | 0.5004 ± 0.0663 | |
| Camphene | Monoterpenes | 0.3450 ± 0.1443 | |
| Acetic acid | Acids | 0.2345 ± 0.0430 | |
| 1,8-Cineole | Monoterpenes | 0.2029 ± 0.0813 | |
| Copaene | Sesquiterpenes | 0.1585 ± 0.0688 |
| Model | Data Fusion Strategy | LV | R2Y | Q2 | RMSEE | RMSECV | RMSEP | Accuracy of Training Set (%) | Accuracy of Test Set (%) |
|---|---|---|---|---|---|---|---|---|---|
| Model I | Low-level data fusion | 23 | 0.921 | 0.847 | 0.0789 | 0.1366 | 0.0859 | 100 | 100 |
| Model II | Mid-level data fusion (VIP > 1) | 22 | 0.907 | 0.833 | 0.0836 | 0.1264 | 0.0794 | 100 | 100 |
| Model III | Mid-level data fusion (latent variables) | 12 | 0.952 | 0.913 | 0.0121 | 0.0450 | 0.0122 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, H.; Yang, T.; Yang, W.; Yang, M.; Wang, Y.; Zhang, J. Comparison of Metabolites and Species Classification of Thirteen Zingiberaceae Spices Based on GC–MS and Multi-Spectral Fusion Technology. Foods 2023, 12, 3714. https://doi.org/10.3390/foods12203714
Wen H, Yang T, Yang W, Yang M, Wang Y, Zhang J. Comparison of Metabolites and Species Classification of Thirteen Zingiberaceae Spices Based on GC–MS and Multi-Spectral Fusion Technology. Foods. 2023; 12(20):3714. https://doi.org/10.3390/foods12203714
Chicago/Turabian StyleWen, Hui, Tianmei Yang, Weize Yang, Meiquan Yang, Yuanzhong Wang, and Jinyu Zhang. 2023. "Comparison of Metabolites and Species Classification of Thirteen Zingiberaceae Spices Based on GC–MS and Multi-Spectral Fusion Technology" Foods 12, no. 20: 3714. https://doi.org/10.3390/foods12203714
APA StyleWen, H., Yang, T., Yang, W., Yang, M., Wang, Y., & Zhang, J. (2023). Comparison of Metabolites and Species Classification of Thirteen Zingiberaceae Spices Based on GC–MS and Multi-Spectral Fusion Technology. Foods, 12(20), 3714. https://doi.org/10.3390/foods12203714





