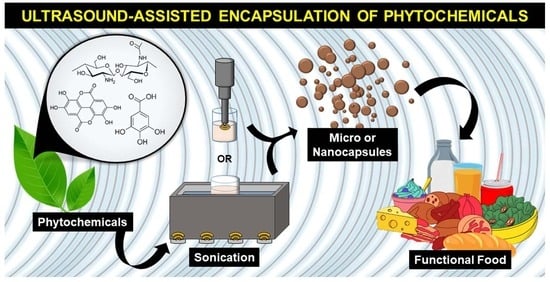
Ultrasound-Assisted Encapsulation of Phytochemicals for Food Applications: A Review
Abstract
:1. Introduction
2. Phytochemicals in Functional Food Products
3. Encapsulation Techniques for Food Applications
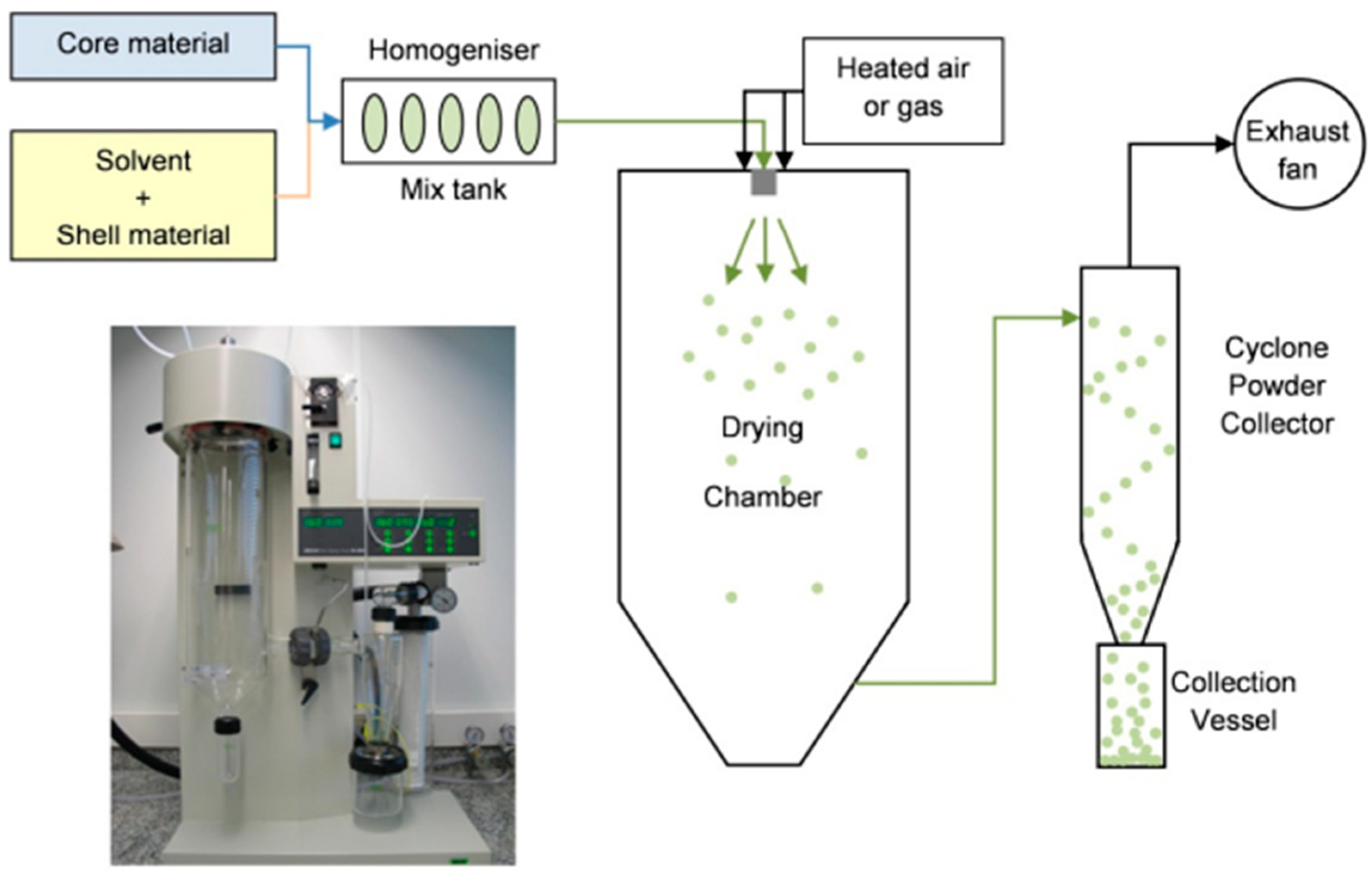
- Spray drying: This technique is one of the most widely used encapsulation systems for food applications. It has been used for over 60 years, being mostly applied for flavor encapsulation [35]. For spray drying applications, the core and wall material mixture (an aqueous solution for hydrophilic core materials, or an oil-in-water emulsion for hydrophobic core materials) is passed through a nozzle into a container in which hot air is circulated (atomization), causing droplets to disperse and dry evenly, resulting in solid particles with sizes ranging from 10 to 1000 µm [28,32]. A schematic representation of this process is displayed in Figure 2.
- Emulsification: This is a popular technique for the incorporation of hydrophobic phytochemicals into food products. Emulsification consists of the mixture of immiscible liquids through shear forces, resulting in capsules with a core–shell configuration. The liquid that becomes entrapped is referred to as the dispersive phase, while the liquid present in higher volume is defined as the continuous or bulk phase. When the dispersive phase is hydrophobic, an oil-in-water emulsion is formed, and when it is hydrophilic, a water-in-oil emulsion is obtained. However, emulsions usually provide low protection against the diffusion of the encapsulated substances due to the thin shell. Hence, several strategies have been used to increase capsule stability, such as double emulsification (e.g., water-in-oil-in-water emulsions, which have a dispersion–shell capsule configuration) and lyophilization (or other drying processes) of the prepared emulsion [8,32,35].
- Liposome entrapment: This technique uses liposomes (usually phospholipids) to encapsulate materials in a core–shell configuration [8,35]. Phospholipids usually form bilayer structures with a hydrophobic inner layer, formed by the phospholipid tails, and hydrophilic outer layers, formed by the polar heads. This results in a hydrophilic core, which is compatible with hydrophilic compounds, and a hydrophobic portion in between the core and the outer layer, which is compatible with both hydrophobic and amphiphilic substances [9]. Liposomes have the advantage of being resistant to stomach digestion, being able to carry phytochemicals that are more beneficial when released in the intestines [8,35].
- Coacervation: In coacervation, the capsules are usually formed via electrostatic forces. This technique is based on liquid–liquid separation into two phases: one that is polymer-rich (coacervate) and one that is polymer-poor. The coacervate is then deposited onto the core material, coating it and generating capsules with sizes that can range from 100 to 10,000 nm. Coacervation can be defined as simple (also known as ionic gelation), in which only one polymer is used, or complex, in which two or more types of polymers are used. It is also important to mention that this technique is highly sensitive towards specific parameters, such as pH and temperature [8,28,32,35].
- Freeze drying: Also known as lyophilization or cryodesiccation, this technique is based on the use of low temperatures and vacuums to freeze and sublimate solvent molecules from a solution containing the core and wall materials, generating solid capsules with an irregular shape. Although freeze drying is considered a time-consuming technique, it can be advantageous when dealing with heat-sensitive core materials [8,32].
4. Ultrasound Technology in Phytochemical Encapsulation
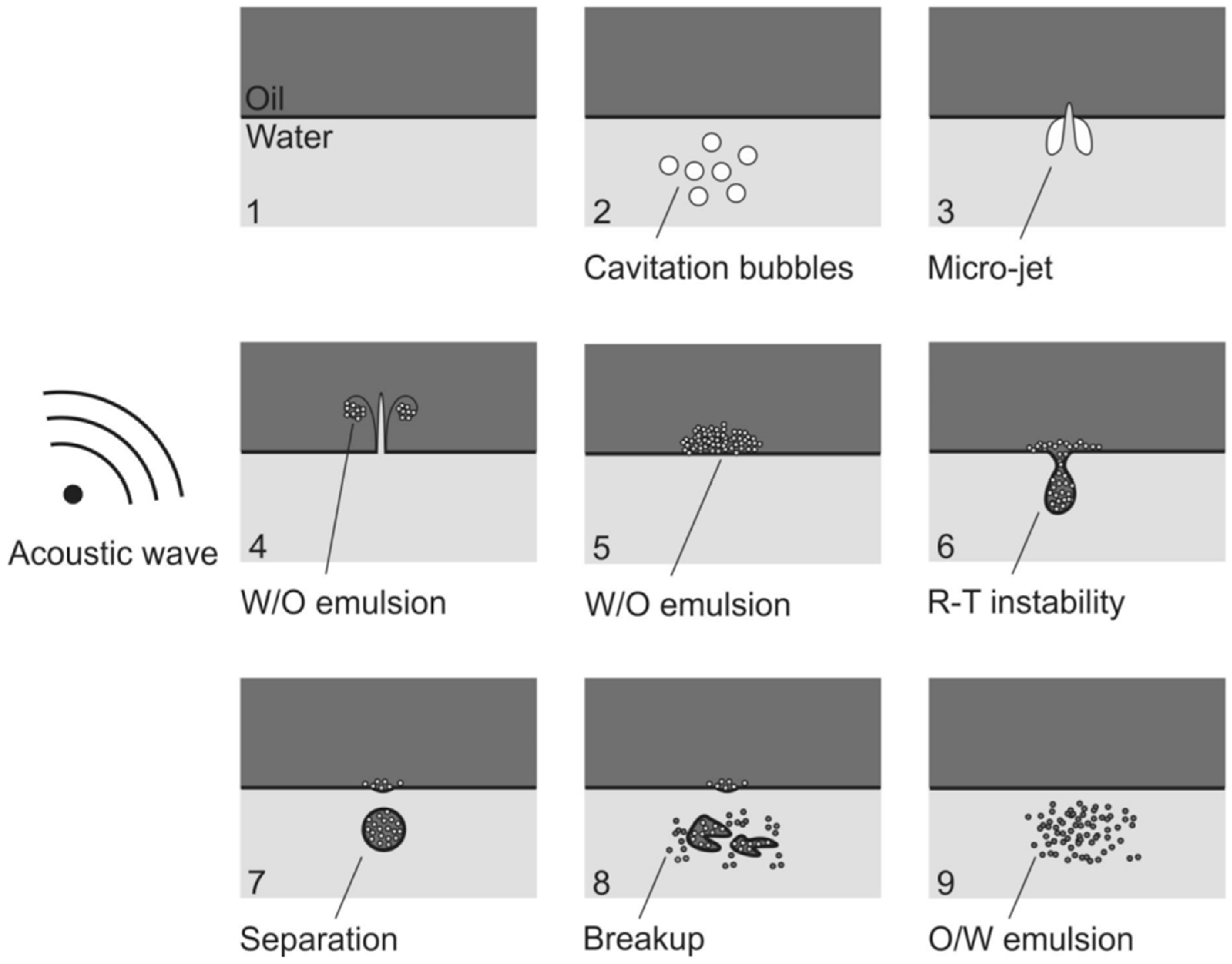
4.1. Ultrasonic Effects in a Liquid Medium
4.2. Specific Aspects of the Ultrasound-Assisted Encapsulation of Phytochemical Compounds
4.2.1. Ultrasonic Parameters
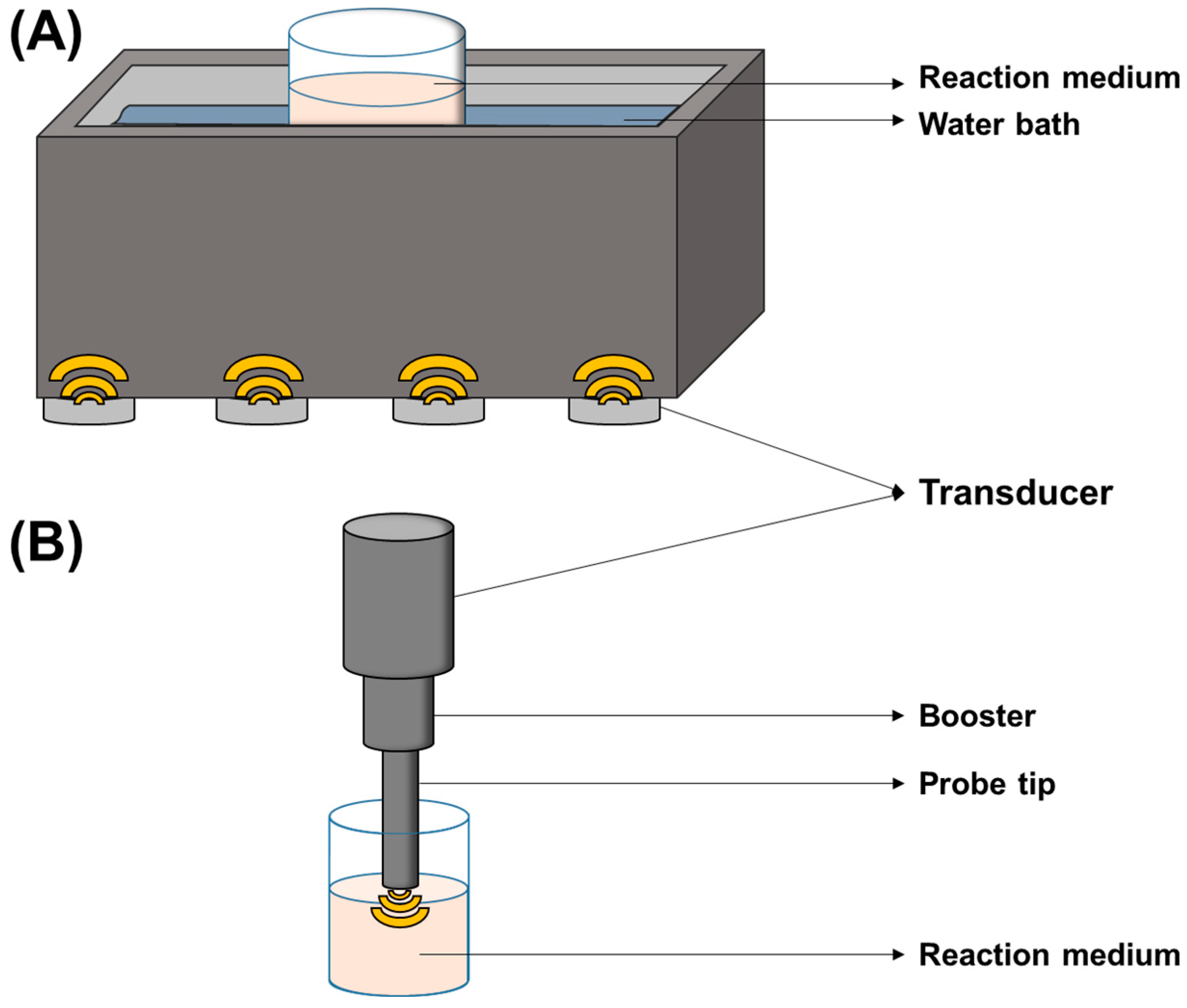
4.2.2. Reactor Configurations
4.2.3. Core and Shell Materials
4.2.4. Incorporation of Phytochemical Capsules into Food Products
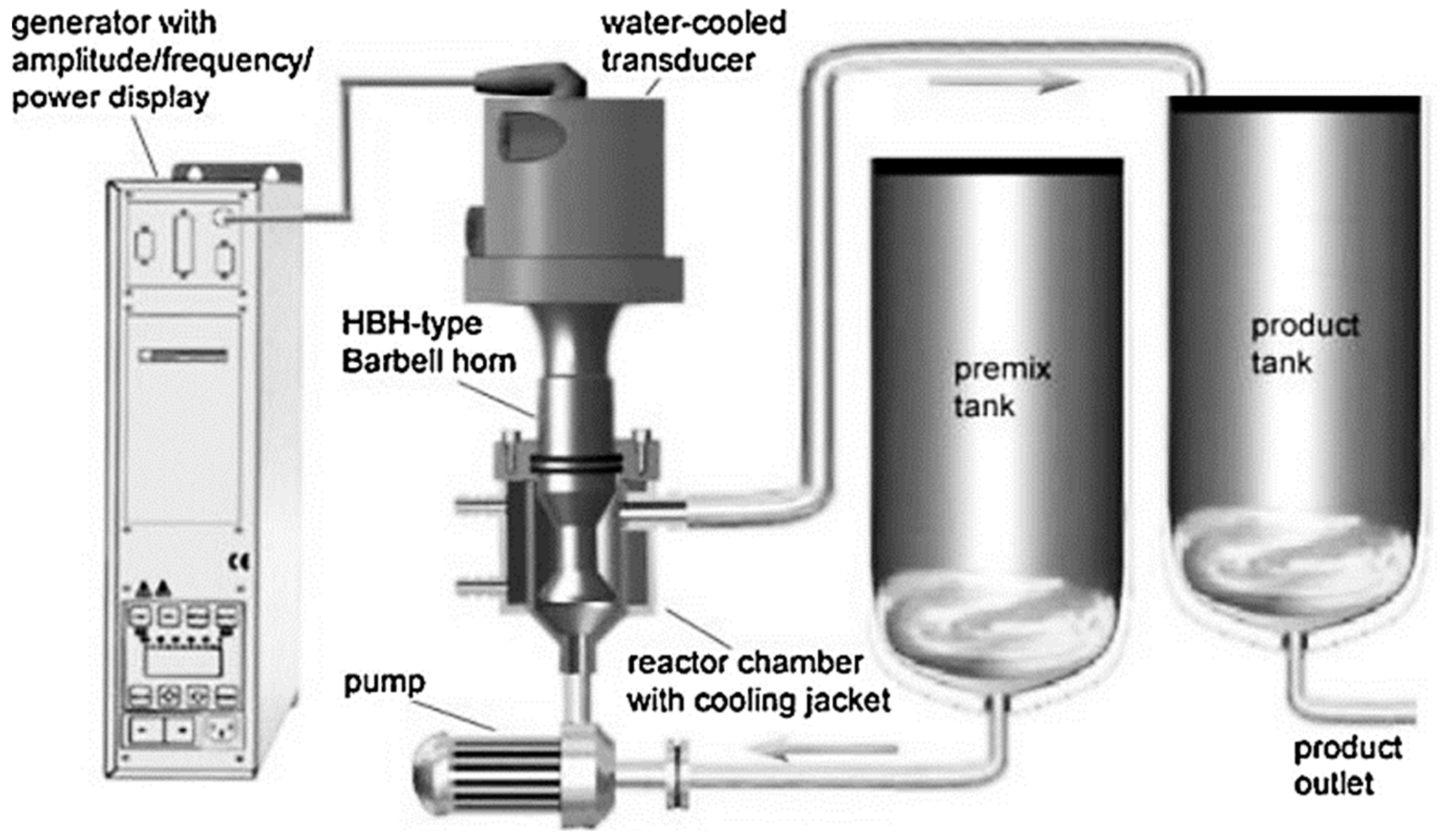
4.2.5. Possibility of Scaling Up
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, J.; Bai, W. Bioactive phytochemicals. Crit. Rev. Food Sci. Nutr. 2019, 59, 827–829. [Google Scholar] [CrossRef]
- Pattnaik, M.; Pandey, P.; Martin, G.J.O.; Mishra, H.N.; Ashokkumar, M. Innovative Technologies for Extraction and Microencapsulation of Bioactives from Plant-Based Food Waste and Their Applications in Functional Food Development. Foods 2021, 10, 279. [Google Scholar] [CrossRef]
- Domínguez, R.; Munekata, P.E.S.; Pateiro, M.; Maggiolino, A.; Bohrer, B.; Lorenzo, J.M. Red Beetroot. A Potential Source of Natural Additives for the Meat Industry. Appl. Sci. 2020, 10, 8340. [Google Scholar] [CrossRef]
- Domínguez, R.; Gullón, P.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Lorenzo, J.M. Tomato as Potential Source of Natural Additives for Meat Industry. A Review. Antioxidants 2020, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; He, X.; Zhao, D. Factors affecting phytochemical stability. In Handbook of Plant Food Phytochemicals; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 332–374. [Google Scholar]
- McClements, D.; Bai, L.; Chung, C. Recent Advances in the Utilization of Natural Emulsifiers to Form and Stabilize Emulsions. Annu. Rev. Food Sci. Technol. 2017, 8, 205–236. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Das, A.K.; Dhar, P.; Nanda, P.K.; Chatterjee, N. Nanoemulsion-Based Technologies for Delivering Natural Plant-Based Antimicrobials in Foods. Front. Sustain. Food Syst. 2021, 5, 64308. [Google Scholar] [CrossRef]
- Silva, B.V.; Barreira, J.C.M.; Oliveira, M.B.P.P. Natural phytochemicals and probiotics as bioactive ingredients for functional foods: Extraction, biochemistry and protected-delivery technologies. Trends Food Sci. Technol. 2016, 50, 144–158. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; McClements, D.J.; Lorenzo, J.M. Encapsulation of Bioactive Phytochemicals in Plant-Based Matrices and Application as Additives in Meat and Meat Products. Molecules 2021, 26, 3984. [Google Scholar] [CrossRef]
- Raddatz, G.C.; Fonseca, V.R.; Cichoski, A.J.; Zepka, L.Q.; Jacob-Lopes, E.; Campagnol, P.C.B.; Wagner, R.; Muller, E.I.; Flores, E.M.M.; Silva, C.B.; et al. Viability and stability evaluation of Lactobacillus casei LC03 co-encapsulated with red onion (Allium cepa L.) peel extract. LWT 2022, 153, 112434. [Google Scholar] [CrossRef]
- Neuenfeldt, N.H.; Farias, C.A.A.; Mello, R.O.; Robalo, S.S.; Barin, J.S.; Silva, L.P.; Müller, E.I.; Flores, E.M.M.; Barcia, M.T.; Menezes, C.R. Effects of blueberry extract co-microencapsulation on the survival of Lactobacillus rhamnosus. LWT 2022, 155, 112886. [Google Scholar] [CrossRef]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Encapsulation of Active Ingredients in Food Industry by Spray-Drying and Nano Spray-Drying Technologies. Processes 2020, 8, 889. [Google Scholar] [CrossRef]
- Lu, W.; Kelly, A.L.; Miao, S. Emulsion-based encapsulation and delivery systems for polyphenols. Trends Food Sci. Technol. 2016, 47, 1–9. [Google Scholar] [CrossRef]
- Chatel, G. How sonochemistry contributes to green chemistry? Ultrason. Sonochemistry 2018, 40, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Leong, T.S.H.; Martina, G.J.O.; Ashokkumar, M. Ultrasonic food processing. In Alternatives to Conventional Food Processing, 2nd ed.; Proctor, A., Fortin, N., McKenna, B., Niemira, B.A., Spilimbergo, S., Srinivas, K., Jung, S., Ashokkumar, M., Moraru, C., Atungulu, G., Eds.; Royal Society of Chemistry: Croydon, UK, 2018; pp. 316–354. [Google Scholar]
- Cichoski, A.J.; Rampelotto, C.; Silva, M.S.; Moura, H.C.; Terra, N.N.; Wagner, R.; Menezes, C.R.; Flores, E.M.M.; Barin, J.S. Ultrasound-assisted post-packaging pasteurization of sausages. Innov. Food Sci. Emerg. Technol. 2015, 30, 132–137. [Google Scholar] [CrossRef]
- Munir, M.; Nadeem, M.; Qureshi, T.M.; Leong, T.S.H.; Gamlath, C.J.; Martin, G.J.O.; Ashokkumar, M. Effects of high pressure, microwave and ultrasound processing on proteins and enzyme activity in dairy systems—A review. Innov. Food Sci. Emerg. Technol. 2019, 57, 102192. [Google Scholar] [CrossRef]
- Ashokkumar, M. Applications of ultrasound in food and bioprocessing. Ultrason. Sonochemistry 2015, 25, 17–23. [Google Scholar] [CrossRef]
- Keven Silva, E.; Zabot, G.L.; Toledo Hijo, A.A.C.; Meireles, M.A.A. Chapter 13—Encapsulation of Bioactive Compounds Using Ultrasonic Technology. In Ultrasound: Advances for Food Processing and Preservation; Bermudez-Aguirre, D., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 323–350. [Google Scholar]
- Lee, S.-H.; Min, K.-J. Phytochemicals. In Encyclopedia of Biomedical Gerontology; Rattan, S.I.S., Ed.; Academic Press: Oxford, UK, 2020; pp. 35–47. [Google Scholar]
- Prakash, B.; Kumar, A.; Singh, P.P.; Songachan, L.S. 1—Antimicrobial and antioxidant properties of phytochemicals: Current status and future perspective. In Functional and Preservative Properties of Phytochemicals; Prakash, B., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–45. [Google Scholar]
- Nawaz, H. Phytochemical Composition: Antioxidant Potential and Biological Activities of Corn. In Corn—Production and Human Health in Changing Climate; Amanullah, D., Fahad, S., Eds.; IntechOpen: London, UK, 2018; pp. 49–68. [Google Scholar]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Kumar, A.; P, N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; K, S.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Šubarić, D.; Jokić, S. Food Industry By-Products as a Sources of Phytochemical Compounds. Foods 2022, 11, 1724. [Google Scholar] [CrossRef]
- Mantegna, S.; Binello, A.; Boffa, L.; Giorgis, M.; Cena, C.; Cravotto, G. A one-pot ultrasound-assisted water extraction/cyclodextrin encapsulation of resveratrol from Polygonum cuspidatum. Food Chem. 2012, 130, 746–750. [Google Scholar] [CrossRef]
- Chen, X.-W.; Yin, W.-J.; Yang, D.-X.; Wan, Z.-L.; Ma, C.-G.; Yang, X.-Q. One-pot ultrasonic cavitational emulsification of phytosterols oleogel-based flavor emulsions and oil powder stabilized by natural saponin. Food Res. Int. 2021, 150, 110757. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoparticle-and Microparticle-Based Delivery Systems: Encapsulation, Protection and Release of Active Compounds; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Xiao, J. Recent advances on the stability of dietary polyphenols. eFood 2022, 3, e21. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Velderrain-Rodríguez, G.R.; Acevedo-Fani, A.; González-Aguilar, G.A.; Martín-Belloso, O. Encapsulation and stability of a phenolic-rich extract from mango peel within water-in-oil-in-water emulsions. J. Funct. Foods 2019, 56, 65–73. [Google Scholar] [CrossRef]
- Guía-García, J.L.; Charles-Rodríguez, A.V.; Reyes-Valdés, M.H.; Ramírez-Godina, F.; Robledo-Olivo, A.; García-Osuna, H.T.; Cerqueira, M.A.; Flores-López, M.L. Micro and nanoencapsulation of bioactive compounds for agri-food applications: A review. Ind. Crops Prod. 2022, 186, 115198. [Google Scholar] [CrossRef]
- Peixoto, F.B.; Aranha, A.C.R.; Nardino, D.A.; Defendi, R.O.; Suzuki, R.M. Extraction and encapsulation of bioactive compounds: A review. Food Process Eng. 2022, 45, e14167. [Google Scholar] [CrossRef]
- Gibbs, B.F.; Kermasha, S.; Alli, I.; Mulligan, C.N. Encapsulation in the food industry: A review. Int. J. Food Sci. Nutr. 1999, 50, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, V.; Paraskevopoulou, A.; Mantzouridou, F.; Lalou, S.; Pantić, M.; Bugarski, B.; Nedović, V. Encapsulation Technologies for Food Industry. In Emerging and Traditional Technologies for Safe, Healthy and Quality Food; Nedović, V., Raspor, P., Lević, J., Tumbas Šaponjac, V., Barbosa-Cánovas, G., Eds.; Springer: Cham, Switzerland, 2016; pp. 329–382. [Google Scholar]
- Raddatz, G.C.; Poletto, G.; Deus, C.d.; Codevilla, C.F.; Cichoski, A.J.; Jacob-Lopes, E.; Muller, E.I.; Flores, E.M.M.; Esmerino, E.A.; Menezes, C.R. Use of prebiotic sources to increase probiotic viability in pectin microparticles obtained by emulsification/internal gelation followed by freeze-drying. Food Res. Int. 2020, 130, 108902. [Google Scholar] [CrossRef]
- Raddatz, G.C.; Fonseca, B.S.; Poletto, G.; Jacob-Lopes, E.; Cichoski, A.J.; Muller, E.I.; Flores, E.M.M.; Silva, C.B.; Menezes, C.R. Influence of the prebiotics hi-maize, inulin and rice bran on the viability of pectin microparticles containing Lactobacillus acidophilus LA-5 obtained by internal gelation/emulsification. Powder Technol. 2020, 362, 409–415. [Google Scholar] [CrossRef]
- Bazana, M.; Silva, S.; Codevilla, C.; de Deus, C.; Lucas, B.; Ugalde, G.; Mazutti, M.; Flores, E.M.M.; Barin, J.; Silva, C.; et al. Development of nanoemulsions containing Physalis peruviana calyx extract: A study on stability and antioxidant capacity. Food Res. Int. 2019, 125, 108645. [Google Scholar] [CrossRef]
- Silva, T.M.; Deus, C.; Souza Fonseca, B.; Lopes, E.J.; Cichoski, A.J.; Esmerino, E.A.; Silva, C.B.; Muller, E.I.; Flores, E.M.M.; Menezes, C.R. The effect of enzymatic crosslinking on the viability of probiotic bacteria (Lactobacillus acidophilus) encapsulated by complex coacervation. Food Res. Int. 2019, 125, 108577. [Google Scholar] [CrossRef]
- Silva, T.M.; Lopes, E.J.; Codevilla, C.F.; Cichoski, A.J.; Flores, E.M.M.; Motta, M.H.; Silva, C.d.B.; Grosso, C.R.F.; Menezes, C.R. Development and characterization of microcapsules containing Bifidobacterium Bb-12 produced by complex coacervation followed by freeze drying. LWT 2018, 90, 412–417. [Google Scholar] [CrossRef]
- Chavarri, M.; Marañón, I.; Villarán, M. Encapsulation Technology to Protect Probiotic Bacteria. In Probiotics; Rigobelo, E., Ed.; IntechOpen: London, UK, 2012; pp. 501–540. [Google Scholar]
- Mason, T.J.; Lorimer, J.P. Applied Sonochemistry: The Uses of Power Ultrasound in Chemistry and Processing; Wiley-Vch: Weinheim, Germany, 2002; Volume 10. [Google Scholar]
- Baysal, T.; Demirdoven, A. Ultrasound in food technology. In Handbook on Applications of Ultrasound: Sonochemistry for Sustainability; Chen, D., Sharma, S.K., Mudhoo, A., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 163–182. [Google Scholar]
- Gallego-Juárez, J.A. Basic Principles of Ultrasound. In Ultrasound in Food Processing: Recent Advances; Villamiel, M., Montilla, A., García-Pérez, J.V., Cárcel, J.A., Benedito, J., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 1–26. [Google Scholar]
- Huang, H.; Zhu, Y.; Fu, X.; Zou, Y.; Li, Q.; Luo, Z. Integrated natural deep eutectic solvent and pulse-ultrasonication for efficient extraction of crocins from gardenia fruits (Gardenia jasminoides Ellis) and its bioactivities. Food Chem. 2022, 380, 132216. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, M. Introductory text to sonochemistry. ChemTexts 2018, 4, 7. [Google Scholar] [CrossRef]
- Li, W.; Ashokkumar, M. Introduction to Ultrasound and Sonochemistry. Electrochem. Soc. Interface 2018, 27, 43. [Google Scholar] [CrossRef]
- Perdih, T.S.; Zupanc, M.; Dular, M. Revision of the mechanisms behind oil-water (O/W) emulsion preparation by ultrasound and cavitation. Ultrason. Sonochemistry 2019, 51, 298–304. [Google Scholar] [CrossRef]
- Wu, J. Acoustic Streaming and its Applications. Fluids 2018, 3, 108. [Google Scholar] [CrossRef]
- Ren, X.; Hou, T.; Liang, Q.; Zhang, X.; Hu, D.; Xu, B.; Chen, X.; Chalamaiah, M.; Ma, H. Effects of frequency ultrasound on the properties of zein-chitosan complex coacervation for resveratrol encapsulation. Food Chem. 2019, 279, 223–230. [Google Scholar] [CrossRef]
- Chotphruethipong, L.; Hutamekalin, P.; Sukketsiri, W.; Benjakul, S. Effects of sonication and ultrasound on properties and bioactivities of liposomes loaded with hydrolyzed collagen from defatted sea bass skin conjugated with epigallocatechin gallate. J. Food Biochem. 2021, 45, e13809. [Google Scholar] [CrossRef]
- Popović, B.M.; Blagojević, B.; Latković, D.; Četojević-Simin, D.; Kucharska, A.Z.; Parisi, F.; Lazzara, G. A one step enhanced extraction and encapsulation system of cornelian cherry (Cornus mas L.) polyphenols and iridoids with β-cyclodextrin. LWT 2021, 141, 110884. [Google Scholar] [CrossRef]
- Solano, M.Q.; Valenzuela, J.; Silva, C.; Lapa, B.C.; Cruz, A.H.; Cervantes, G.; Flores, D. Nanoencapsulation by ionic gelation of polyphenols from artichoke (Cynara scolymus L.) residues using ultrasound [pdf]. Acta Sci. Pol. Technol. Aliment. 2023, 22, 57–69. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Liu, Y.; Guo, Z.; Li, J. Improving effect of oleic acid-mediated sodium caseinate-based encapsulation in an ultrasound field on the thermal stability and bioaccessibility of quercetin. Ultrason. Sonochemistry 2022, 90, 106169. [Google Scholar] [CrossRef] [PubMed]
- Remanan, M.K.; Zhu, F. Encapsulation of rutin using quinoa and maize starch nanoparticles. Food Chem. 2021, 353, 128534. [Google Scholar] [CrossRef]
- Remanan, M.K.; Zhu, F. Encapsulation of ferulic acid in high internal phase Pickering emulsions stabilized using nonenyl succinic anhydride (NSA) and octenyl succinic anhydride (OSA) modified quinoa and maize starch nanoparticles. Food Chem. 2023, 429, 136748. [Google Scholar] [CrossRef]
- Sadiq, U.; Gill, H.; Chandrapala, J. Ultrasound-Assisted Encapsulation of Anthraquinones Extracted from Aloe-Vera Plant into Casein Micelles. Gels 2022, 8, 597. [Google Scholar] [CrossRef]
- Cimino, R.; Bhangu, S.K.; Baral, A.; Ashokkumar, M.; Cavalieri, F. Ultrasound-Assisted Microencapsulation of Soybean Oil and Vitamin D Using Bare Glycogen Nanoparticles. Molecules 2021, 26, 5157. [Google Scholar] [CrossRef]
- Elgegren, M.; Kim, S.; Cordova, D.; Silva, C.; Noro, J.; Cavaco-Paulo, A.; Nakamatsu, J. Ultrasound-Assisted Encapsulation of Sacha Inchi (Plukenetia volubilis Linneo.) Oil in Alginate-Chitosan Nanoparticles. Polymers 2019, 11, 1245. [Google Scholar] [CrossRef] [PubMed]
- Kentish, S.; Feng, H. Applications of power ultrasound in food processing. Annu. Rev. Food Sci. Technol. 2014, 5, 263–284. [Google Scholar] [CrossRef]
- Gogate, P.R.; Patil, P.N. Sonochemical Reactors. Top. Curr. Chem. 2016, 374, 61. [Google Scholar] [CrossRef]
- Kentish, S.; Ashokkumar, M. The Physical and Chemical Effects of Ultrasound. In Ultrasound Technologies for Food and Bioprocessing; Feng, H., Barbosa-Canovas, G., Weiss, J., Eds.; Springer: New York, NY, USA, 2011; pp. 1–12. [Google Scholar]
- Chen, H.; Jia, Y.; Guo, Q. Chapter 6—Polysaccharides and polysaccharide complexes as potential sources of antidiabetic compounds: A review. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 67, pp. 199–220. [Google Scholar]
- Song, J.; Yu, Y.; Chen, M.; Ren, Z.; Chen, L.; Fu, C.; Ma, Z.F.; Li, Z. Advancement of Protein- and Polysaccharide-Based Biopolymers for Anthocyanin Encapsulation. Front. Nutr. 2022, 9, 938829. [Google Scholar] [CrossRef]
- Miguel, G.A.; Jacobsen, C.; Prieto, C.; Kempen, P.J.; Lagaron, J.M.; Chronakis, I.S.; García-Moreno, P.J. Oxidative stability and physical properties of mayonnaise fortified with zein electrosprayed capsules loaded with fish oil. J. Food Eng. 2019, 263, 348–358. [Google Scholar] [CrossRef]
- Zhu, H.; Mettu, S.; Cavalieri, F.; Ashokkumar, M. Ultrasonic microencapsulation of oil-soluble vitamins by hen egg white and green tea for fortification of food. Food Chem. 2021, 353, 129432. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, Y.; Qu, Q.; Xiong, R.; Tang, H.; Huang, C. Encapsulated Microstructures of Beneficial Functional Lipids and Their Applications in Foods and Biomedicines. J. Agric. Food Chem. 2022, 70, 8165–8187. [Google Scholar] [CrossRef] [PubMed]
- Alzorqi, I.; Manickam, S. Ultrasonic Process Intensification for the Efficient Extraction of Nutritionally Active Ingredients of Polysaccharides from Bioresources. In Handbook of Ultrasonics and Sonochemistry; Springer: Singapore, 2016; pp. 1271–1286. [Google Scholar]
- Leibtag, S.; Peshkovsky, A. Cannabis extract nanoemulsions produced by high-intensity ultrasound: Formulation development and scale-up. J. Drug Deliv. Sci. Technol. 2020, 60, 101953. [Google Scholar] [CrossRef]
- Peshkovsky, A.S.; Bystryak, S. Continuous-flow production of a pharmaceutical nanoemulsion by high-amplitude ultrasound: Process scale-up. Chem. Eng. Process. Process Intensif. 2014, 82, 132–136. [Google Scholar] [CrossRef]

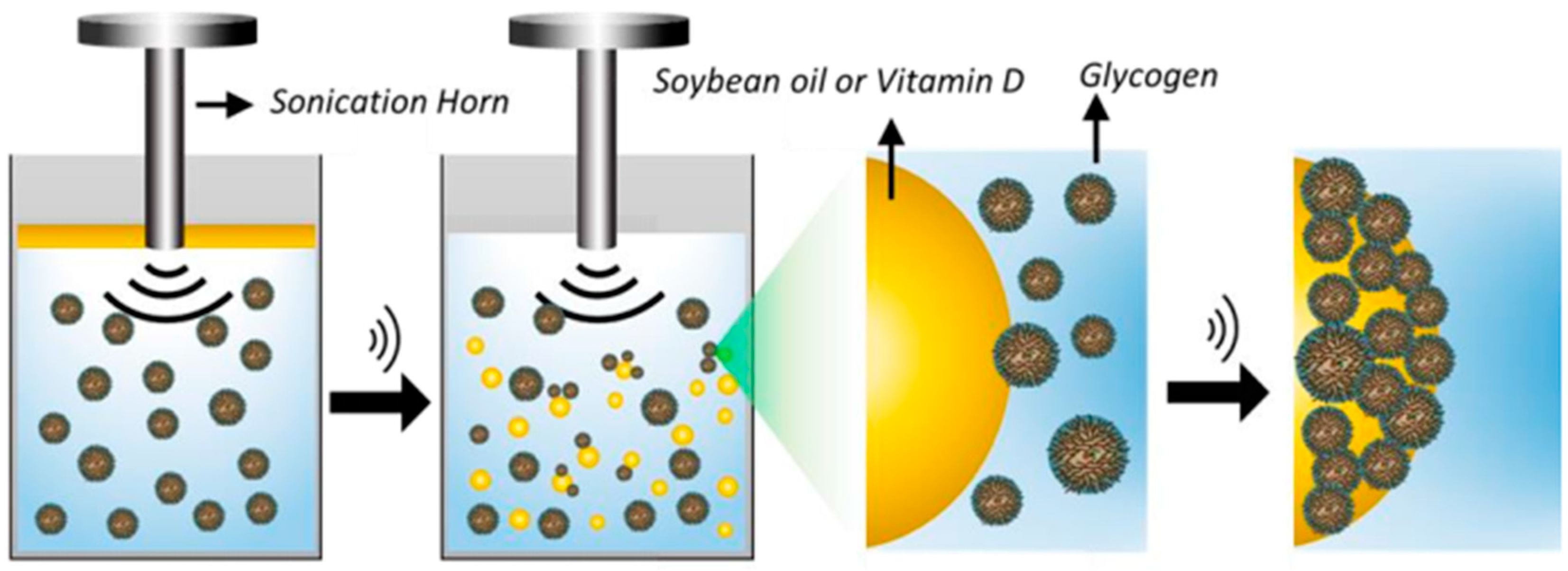
| Core Composition | Shell Composition | Encapsulation Technique | Experimental Conditions | Remarks | Reference |
|---|---|---|---|---|---|
| Polyphenols, iridoids, anthocyanins, and tannins (from cornelian cherry) | β-cyclodextrin | Emulsification followed by freeze drying | Type of US reactor: bath US frequency: 37 kHz US power: 550 W US amplitude: n.i. Time: 20 min Temperature: 30 °C Observations: 50 mL of solvent per gram of plant. Solvent consisted of a 1.5% β-cyclodextrin solution in 50% ethanol (w w−1) | Extraction and encapsulation of phytochemicals were performed in the same procedure. An encapsulation efficiency of 65.62% was achieved. | [52] |
| Resveratrol | Zein–chitosan | Coacervation | Type of US reactor: bath US frequency: single- (28 or 40 kHz), dual- (28/40 kHz), or multi- (20/28/40 kHz) frequency mode US power: 300 W US amplitude: n.i. Time: 40 min (pulsed mode: 10 s on/3 s off) Temperature: 25 ± 2 °C (a water bath was used) Observations: Different zein: chitosan ratios were evaluated (from 1:1 to 1:2.5). A sample volume of 3 L was used. The samples were kept away from light during sonication. Capsules were freeze dried after synthesis. | Regarding complex ratios, the 1:2.5 zein: chitosan mixture presented the highest encapsulation efficiency (51.4%). Regarding US frequency, the 20/40 kHz treatment presented the highest encapsulation efficiency (65.2%), which was 32% higher than the control. The US treatment significantly decreased the capsule size and polydispersity index. | [50] |
| Hydrolyzed collagen (from sea bass skin) conjugated with 3% epigallocatechin gallate | Phospholipids (soy lecithin: cholesterol, 4:1) | Liposome entrapment | Type of US reactor: bath or probe US frequency: n.i. (bath) and 20 ± 0.05 kHz (probe) US power: n.i. (bath) and 750 W (probe) US amplitude: n.i. (bath) and 20 or 40% (probe); Time: 30 min (bath) and 2, 5, 10, or 15 min (probe; pulsed mode: 1 s on/ 10 s off) Temperature: 25 ± 5 °C (an ice water bath was used) Observations: 35 mL of 1% core material was added to the phospholipid film, which was then sonicated using bath or probe US. | Probe US resulted in a lower encapsulation efficiency than bath US, which was more pronounced with longer times and higher amplitude. Probe US resulted in a smaller capsule size, lower polydispersity index, and higher negative surface charge. A higher encapsulation efficiency and antioxidant activity, as well as a lower capsule size after storage (28 days) were also observed. US had no effect on the release rate of core material. Probe US resulted in a higher in vitro bioavailability. | [51] |
| Phenolic compounds (from mango peel extract) | Sunflower oil (continuous phase of first emulsion) and distilled water (continuous phase of second emulsion) | Double emulsification (water-in-oil-in-water) | Type of US reactor: probe (22 mm sonotrode) US frequency: 24 kHz US power: 400 W US amplitude: 40, 60, 80 or 100 µm (first emulsion); 50 µm (second emulsion) Time: 1, 2, or 3 min (first emulsion); 1.5 min (second emulsion) Temperature: n.i. Observations: first emulsion medium—extract in 0.1 mol L−1 NaCl (22%), corn oil (70%), polyglycerol polyricinoleate (5%), and glycerol (3%). Second emulsion medium—first emulsion (25%) and 2% of hydrophilic surfactant in 0.1 mol L−1 NaCl (25%). Pre-emulsification was performed via mechanical agitation (6000 rpm for 8 min for the first emulsion and 3000 rpm for 4 min for the second emulsion). | The US treatment using 100 µm of amplitude for 3 min (first emulsion step) resulted in the lowest capsule size (0.38 µm). The highest encapsulation efficiency was obtained using Tween 20 as the surfactant for the second emulsion (98.65 ± 1.14%). The highest encapsulation stability after storage (21 days) was obtained using Tween 80 as the surfactant for the second emulsion. | [31] |
| Phenolic compounds | Chitosan | Simple coacervation followed by freeze drying | Type of US reactor: bath US frequency: 42 kHz US power: n.i. US amplitude: 70% Time: 4.79 min Temperature: room temperature Observations: The optimum conditions for nanoencapsulation were a mixture of 0.28% chitosan and 0.29% sodium tripolyphosphate in the ratio of 5:1 and pH of 4.9. The obtained capsules were freeze dried. | A power supply of 230 V was used. The nanoencapsulation efficiency was 69.9 ± 0.67%, with a particle size between 72.3 and 460.7 nm, along with a polydispersity index of 0.458. A ζ potential of +15.73 mV was obtained. | [53] |
| Quercetin | Oleic acid and sodium caseinate | Emulsification | Type of US reactor: probe US frequency: n.i. US power: 300 W US amplitude: n.i. Time: 5 min Temperature: room temperature Observations: A 5 mg mL−1 quercetin solution in ethanol was used. Oleic acid and sodium caseinate mixtures were evaluated in the ratios of 1:40, 1:15, 1:8, or 1:4 (m m−1). After sonication, the mixture was kept in the dark for 30 min. | A polydispersity index below 0.3 and a ζ potential higher than 30 mV were obtained. The bioaccessibility of quercetin increased from 25% to more than 60% after encapsulation. | [54] |
| Rutin | Quinoa and maize starch | Emulsification followed by freeze drying | Type of US reactor: probe US frequency: 20 kHz US power: n.i. US amplitude: n.i. Time: 30 min (in 3 min intervals) Temperature: 80 °C Observations: A solution of 1.5% rutin in ethanol was dripped into 1.5% starch suspensions containing 0.1 mol L−1 NaOH at 80 °C (in the ratio of 1:10) over 30 min with continuous stirring prior to sonication. | Capsule sizes between 107 and 222 nm, encapsulation efficiencies of 67.4 and 63.1%, and ζ potentials of −18.0 and −18.6 mv, were obtained for the quinoa and maize starch capsules, respectively. Rutin’s bioavailability was significantly higher in quinoa capsules. | [55] |
| Ferulic acid | Modified quinoa and maize starch | Sonoprecipitation followed by freeze drying | Type of US reactor: probe US frequency: 20 kHz US power: 750 W US amplitude: 80% Time: 10 min Temperature: 25 (an ice water bath was used) Observations: 1.5% starch suspensions in 0.1 mol L−1 NaOH were preheated at 80 °C and stirred for 30 min prior to sonication. Afterwards, starch nanoparticles were precipitated through the addition of ethanol in the ratio of 1:2, centrifuged, and freeze dried. | Particle sizes were 153 and 221 nm for quinoa and maize starch capsules, respectively. The polydispersity index was lower than 0.4. Zeta potentials for quinoa and maize starch were 24.2 and 16.2 mV, respectively. Quinoa-based capsules presented a higher encapsulation efficiency. | [56] |
| Phytosterols | Flavor oil | Emulsification followed by spray drying | Type of US reactor: probe US frequency: 20 kHz US power: 200, 280, and 360 W US amplitude: n.i. Time: 5 min (pulsed mode: 5 s on/ 5 s off) Temperature: n.i. (an ice water bath was used) Observations: the reaction medium consisted of flavor oil (20%), tea saponin (3%), phytosterols (4%), and aqueous buffer (pH 7). Emulsions were spray dried. | The higher treatment time led to a smaller capsule size. | [27] |
| Anthraquinones (aloin, aloe-emodin, and rhein) extracted from aloe vera | Casein micelles | Emulsification | Type of US reactor: probe US frequency: 20 kHz US power: 500 W (input power); 39.74 W (output power) US amplitude: 50% US intensity/density: n.i. Time: 15 min (pulsed mode: 30 s on/ 30 s off) Temperature: 25 °C (ice tube) Observations: no observations. | The particle size and ζ potential of capsules ranged from 171 to 179 nm and −23 to −17 mV, respectively. Encapsulation efficiencies ranged from 98 to 100%. | [57] |
| Soybean oil and vitamin D | Glycogens (from rabbit and bovine liver, oyster, or sweet corn) | Emulsification | Type of US reactor: probe US frequency: 20 kHz US power: 160 W US amplitude: n.i. Time: 45 s Temperature: room temperature Observations: the medium consisted of 10 mg mL−1 of different glycogens and 50 µL of the oil phase. The capsules were isolated via flotation and washing with ultrapure water. | Microcapsules with sizes varying from 0.3 to 8 µm were obtained. | [58] |
| Sacha inchi oil | Alginate and chitosan | Emulsification followed by coacervation | Type of US reactor: probe US frequency: n.i. US power: n.i. US amplitude: n.i. Time: 3 min Temperature: 7 to 10 °C Observations: Samples containing a surfactant, the core, and the shell materials were sonicated. Then, coacervation was performed using alginate, followed by coating with chitosan. | The obtained nanoparticles presented a higher loading efficiency and stability in comparison to other vegetable oils. | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cauduro, V.H.; Cui, J.; Flores, E.M.M.; Ashokkumar, M. Ultrasound-Assisted Encapsulation of Phytochemicals for Food Applications: A Review. Foods 2023, 12, 3859. https://doi.org/10.3390/foods12203859
Cauduro VH, Cui J, Flores EMM, Ashokkumar M. Ultrasound-Assisted Encapsulation of Phytochemicals for Food Applications: A Review. Foods. 2023; 12(20):3859. https://doi.org/10.3390/foods12203859
Chicago/Turabian StyleCauduro, Vitoria Hagemann, Jiwei Cui, Erico Marlon Moraes Flores, and Muthupandian Ashokkumar. 2023. "Ultrasound-Assisted Encapsulation of Phytochemicals for Food Applications: A Review" Foods 12, no. 20: 3859. https://doi.org/10.3390/foods12203859
APA StyleCauduro, V. H., Cui, J., Flores, E. M. M., & Ashokkumar, M. (2023). Ultrasound-Assisted Encapsulation of Phytochemicals for Food Applications: A Review. Foods, 12(20), 3859. https://doi.org/10.3390/foods12203859