Bactericidal Efficacy and Mechanisms of Non-Electrolytic Slightly Acidic Hypochlorous Water on Pseudomonas fragi and Pseudomonas fluorescens
Abstract
:1. Introduction
2. Materials and Methods
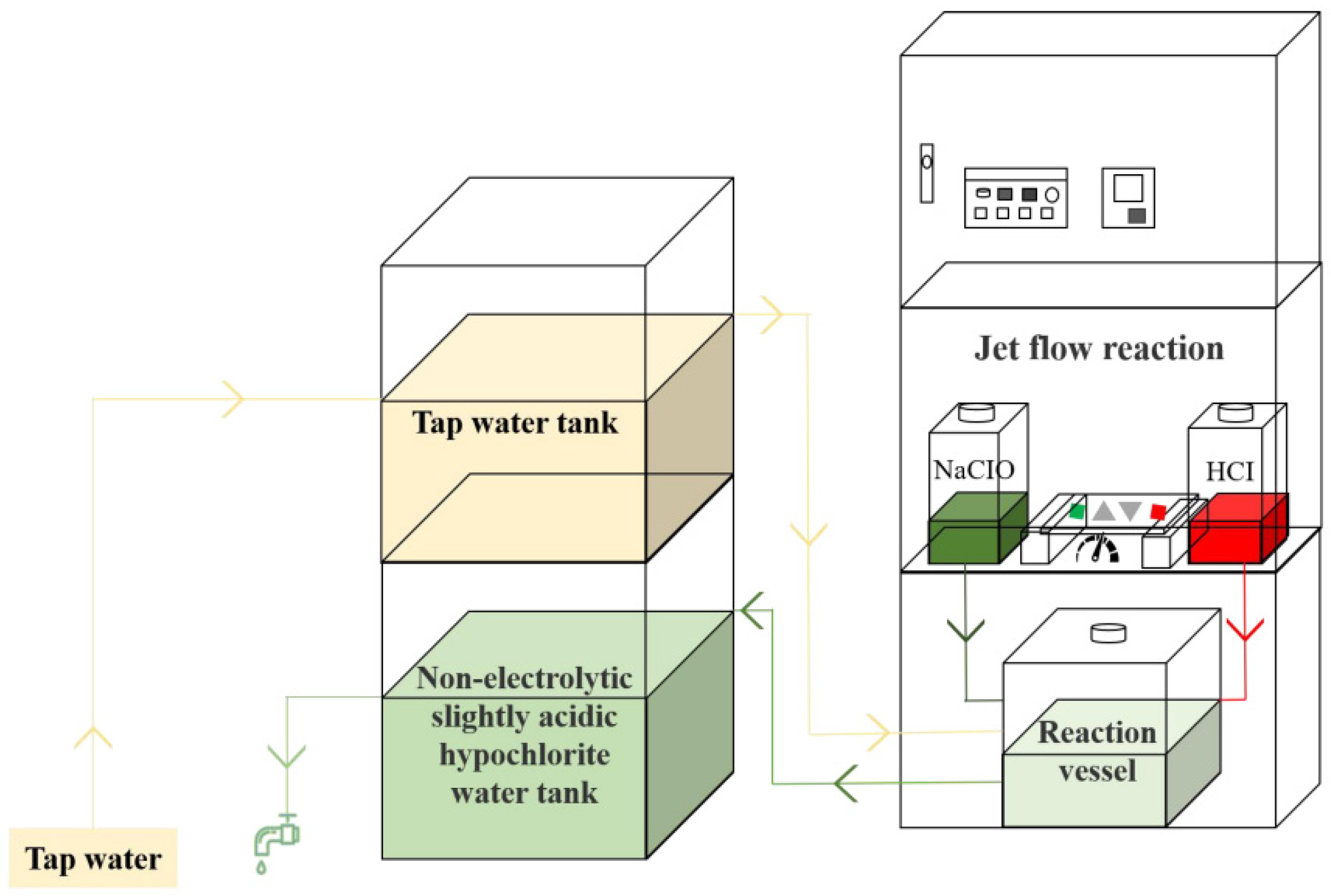
2.1. Material, Bacterial Strain, Culture, and Bacterial Suspension Methods
2.2. The Antibacterial Effect of NE-SAHW against P. fragi and P. fluorescens
2.3. Observation of Cell Membrane by SEM
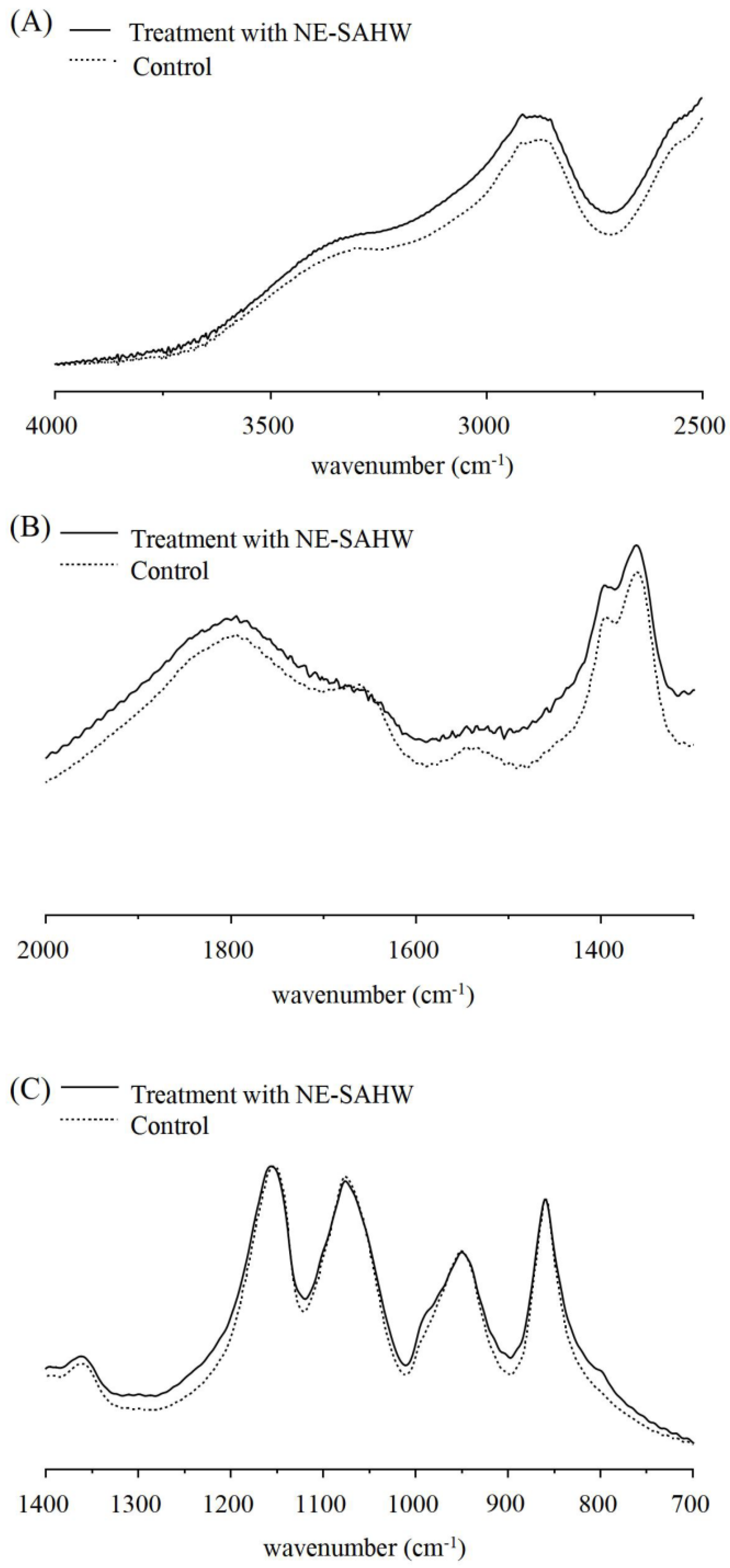
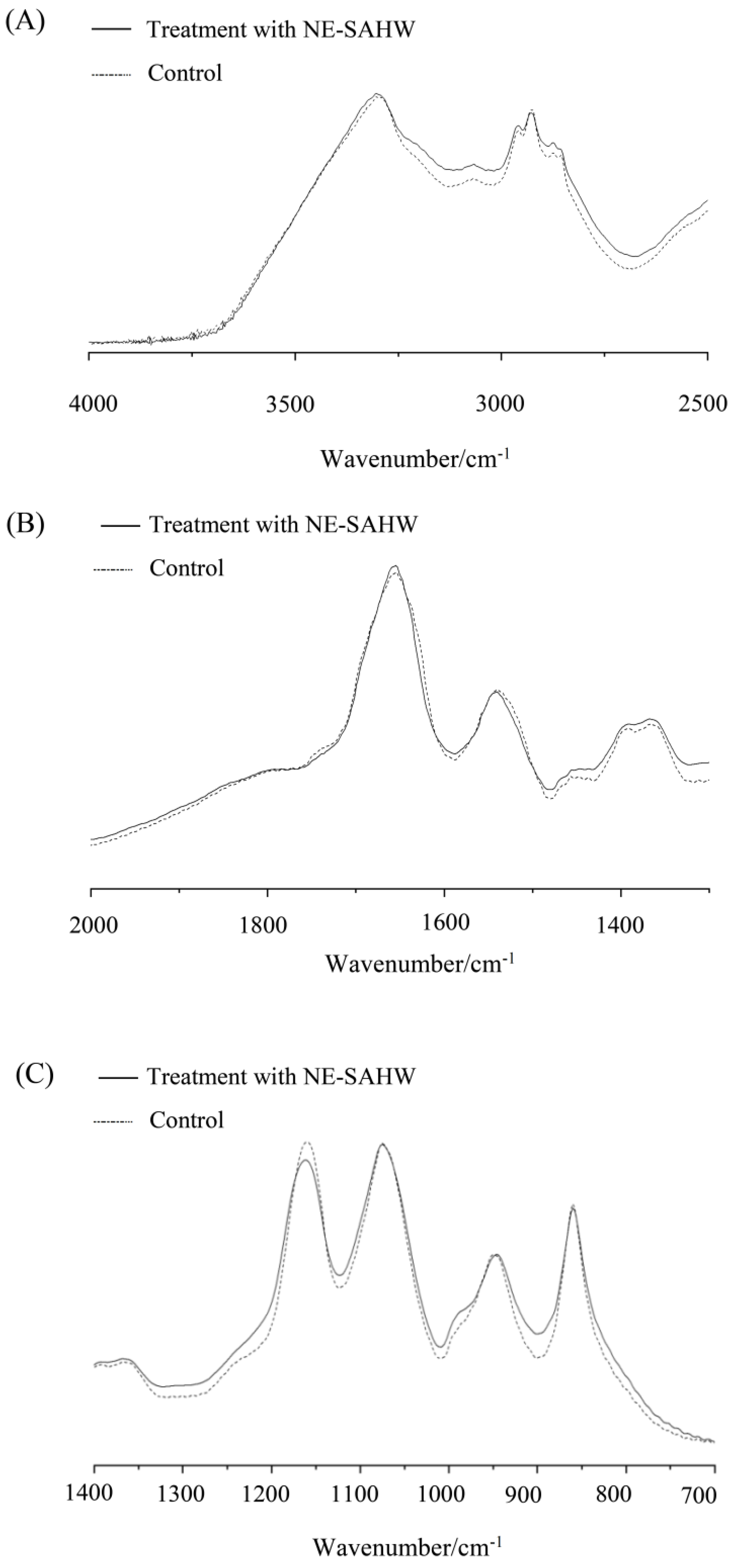
2.4. Observation of Cell Membrane by FTIR
2.5. Leakage of Bacterial Cellular Materials (Protein, Nucleic Acid, and K+)
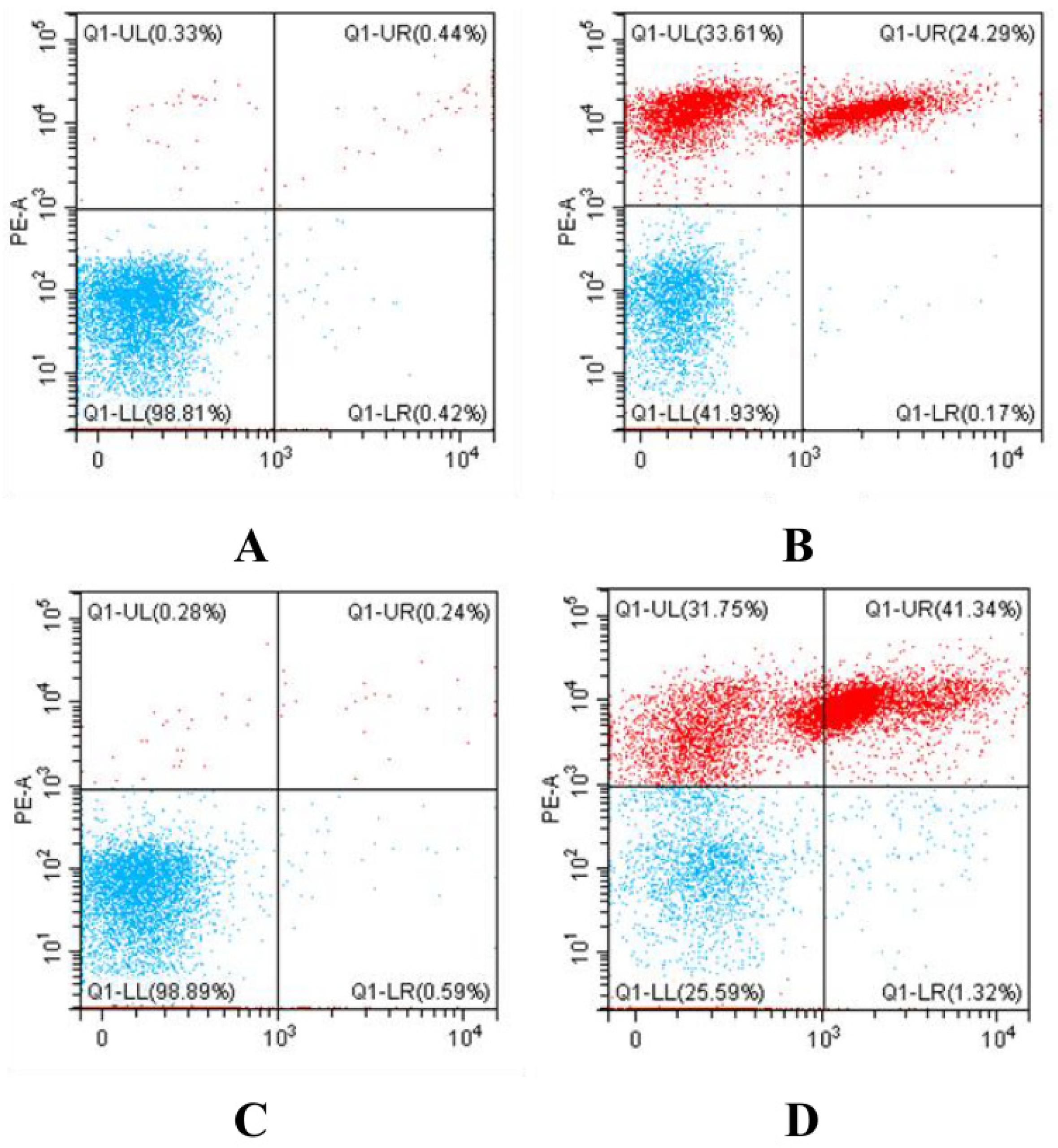
2.6. Determination of Bacterial Death by Flow Cytometry
3. Results and Discussion
3.1. Bactericidal Efficacy of NE-SAHW on the Strains of P. fragi and P. fluorescens
3.2. Effect of NE-SAHW on Cell Membrane of P. fragi and P. fluorescens by SEM
3.3. Effect of NE-SAHW on Intracellular Material Leakages of P. fragi and P. fluorescens
3.4. Effect of NE-SAHW on Molecular Composition Changes in Bacteria
3.5. Determination of Bacterial Death by Flow Cytometry
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brautigam, K.R.; Jorissen, J.; Priefer, C. The extent of food waste generation across EU-27: Different calculation meth-ods and the reliability of their results. Waste Manag. Res. 2014, 32, 683–694. [Google Scholar] [CrossRef]
- Franzetti, L.; Scarpellini, M. Characterisation of Pseudomonas spp. isolated from foods. Ann. Microbiol. 2007, 57, 39–47. [Google Scholar] [CrossRef]
- Mohareb, F.; Iriondo, M.; Doulgeraki, A.I.; Van Hoek, A.; Aarts, H.; Cauchi, M.; Nychas, G.-J.E. Identification of meat spoilage gene biomarkers in Pseudomonas putida using gene profiling. Food Control 2015, 57, 152–160. [Google Scholar] [CrossRef]
- Aswathanarayan, J.B.; Vittal, R.R. Attachment and biofilm formation of Pseudomonas fluorescens PSD4 isolated from a dairy processing line. Food Sci. Biotechnol. 2014, 23, 1903–1910. [Google Scholar] [CrossRef]
- Hu, H.; Cai, L.; Dong, Y.; Wang, H.; Xu, X.; Zhou, G. Modeling the degradation of acidic electrolyzed water and its ability to disinfect a dual-species biofilm. LWT-Food Sci. Technol. 2019, 104, 159–164. [Google Scholar] [CrossRef]
- Waters, B.W.; Hung, Y.-C. Evaluation of different methods for determination of properties of chlorine-based sanitizers. Food Control 2013, 30, 41–47. [Google Scholar] [CrossRef]
- Pangloli, P.; Hung, Y.-C. Efficacy of slightly acidic electrolyzed water in killing or reducing Escherichia coli o157:h7 on iceberg lettuce and tomatoes under simulated food service operation conditions. J. Food Sci. 2011, 76, M361–M366. [Google Scholar] [CrossRef]
- Afari, G.K.; Hung, Y.-C. A meta-analysis on the effectiveness of electrolyzed water treatments in reducing foodborne pathogens on different foods. Food Control 2018, 93, 150–164. [Google Scholar] [CrossRef]
- He, Y.; Zhao, X.; Chen, L.; Zhao, L.; Yang, H. Effect of electrolysed water generated by sodium chloride combined with sodium bicarbonate solution against Listeria innocua in broth and on shrimp. Food Control 2021, 127, 108134. [Google Scholar] [CrossRef]
- Ngnitcho, P.-F.K.; Khan, I.; Tango, C.N.; Hussain, M.S.; Oh, D.H. Inactivation of bacterial pathogens on lettuce, sprouts, and spinach using hurdle technology. Innov. Food Sci. Emerg. Technol. 2017, 43, 68–76. [Google Scholar] [CrossRef]
- Len, S.V.; Hung, Y.C.; Erickson, M.; Kim, C.U. Ultraviolet spectrophotometric characterization and bactericidal prop-erties of electrolyzed oxidizing water as influenced by amperage and pH. J. Food Prot. 2000, 63, 1534–1537. [Google Scholar] [CrossRef]
- Hao, J.; Qiu, S.; Li, H.; Chen, T.; Liu, H.; Li, L. Roles of hydroxyl radicals in electrolyzed oxidizing water (EOW) for the inactivation of Escherichia coli. Int. J. Food Microbiol. 2012, 155, 99–104. [Google Scholar] [CrossRef]
- Ding, T.; Xuan, X.-T.; Li, J.; Chen, S.-G.; Liu, D.-H.; Ye, X.-Q.; Shi, J.; Xue, S.J. Disinfection efficacy and mechanism of slightly acidic electrolyzed water on Staphylococcus aureus in pure culture. Food Control 2016, 60, 505–510. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Holley, R.A. Factors influencing the microbial safety of fresh produce: A review. Food Microbiol. 2012, 32, 1–19. [Google Scholar] [CrossRef]
- Xuan, X.; Wang, M.; Ahn, J.; Ma, Y.; Chen, S.; Ye, X.; Liu, D.; Ding, T. Storage stability of slightly acidic electrolyzed water and circulating electrolyzed water and their property changes after application. J. Food Sci. 2016, 81, E610–E617. [Google Scholar] [CrossRef]
- Liao, X.; Xiang, Q.; Cullen, P.J.; Su, Y.; Chen, S.; Ye, X.; Liu, D.; Ding, T. Plasma-activated water (PAW) and slightly acidic electrolyzed water (SAEW) as beef thawing media for enhancing microbiological safety. LWT 2020, 117, 108649. [Google Scholar] [CrossRef]
- Song, H.; Lee, J.Y.; Lee, H.-W.; Ha, J.-H. Inactivation of bacteria causing soft rot disease in fresh cut cabbage using slightly acidic electrolyzed water. Food Control 2021, 128, 108217. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, Y.; Yang, R.; Zhao, W. Combined effect of slightly acidic electrolyzed water and ascorbic acid to im-prove quality of whole chilled freshwater prawn (Macrobrachium rosenbergii). Food Control 2020, 108, 106820. [Google Scholar] [CrossRef]
- Tantratian, S.; Kaephen, K. Shelf-life of shucked oyster in epigallocatechin-3-gallate with slightly acidic electrolyzed water washing under refrigeration temperature. LWT-Food Sci. Technol. 2020, 118, 108733. [Google Scholar] [CrossRef]
- Jo, H.-Y.; Tango, C.N.; Oh, D.-H. Influence of different organic materials on chlorine concentration and sanitization of slightly acidic electrolyzed water. Lebensm. Wiss. Technol. 2018, 92, 187–194. [Google Scholar] [CrossRef]
- Sheng, X.; Shu, D.; Tang, X.; Zang, Y. Effects of slightly acidic electrolyzed water on the microbial quality and shelf life extension of beef during refrigeration. Food Sci. Nutr. 2018, 6, 1975–1981. [Google Scholar] [CrossRef]
- Liang, D.; Wang, Q.; Zhao, D.; Han, X.; Hao, J. Systematic application of slightly acidic electrolyzed water (SAEW) for natural microbial reduction of buckwheat sprouts. Lebensm. Wiss. Technol. 2019, 108, 14–20. [Google Scholar] [CrossRef]
- Hussain, M.S.; Kwon, M.; Park, E.-J.; Seheli, K.; Huque, R.; Oh, D.-H. Disinfection of Bacillus cereus biofilms on leafy green vegetables with slightly acidic electrolyzed water, ultrasound and mild heat. Lebensm. Wiss. Technol. 2019, 116, 108582. [Google Scholar] [CrossRef]
- Veasey, S.; Muriana, P.M. Evaluation of Electrolytically-Generated Hypochlorous Acid (‘Electrolyzed Water’) for Sanitation of Meat and Meat-Contact Surfaces. Foods 2016, 5, 42. [Google Scholar] [CrossRef]
- Cheng, X.; Tian, Y.; Zhao, C.; Qu, T.; Ma, C.; Liu, X.; Yu, Q. Bactericidal effect of strong acid electrolyzed water against flow enterococcus faecalis biofilms. J. Endod. 2016, 42, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Wang, S.; Chen, T.; Gao, W.; Zhu, S.; He, J.; Han, Z. Inactivation mechanism of Escherichia coli induced by slightly acidic electrolyzed water. Sci. Rep. 2017, 7, 6279. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.X.; Cao, M.J.; Liu, G.M.; Chen, Q.C.; Sun, L.C.; Chen, H.X. Antibacterial activity and mechanisms of de-polymerized fucoidans isolated from Laminaria japonica. Carbohydr. Polym. 2017, 172, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Xuan, X.; Li, J.; Suo, Y.; Liu, D.; Ye, X.; Chen, S.; Ding, T. Bactericidal action of slightly acidic electrolyzed water against Escherichia coli and Staphylococcus aureus via multiple cell targets. Food Control 2017, 79, 380–385. [Google Scholar] [CrossRef]
- Zendehdel, R.; Shirazi, F.H. Discrimination of human cell lines by infrared spectroscopy and mathematical modeling. Iran. J. Pharm. Res. IJPR 2015, 14, 803–810. [Google Scholar] [PubMed]
- Tango, C.N.; Khan, I.; Kounkeu, P.-F.N.; Momna, R.; Hussain, M.S.; Oh, D.-H. Slightly acidic electrolyzed water combined with chemical and physical treatments to decontaminate bacteria on fresh fruits. Food Microbiol. 2017, 67, 97–105. [Google Scholar] [CrossRef]
- Fenner, D.C.; Burge, B.; Kayser, H.P.; Wittenbrink, M.M. The anti-microbial activity of electrolysed oxidizing water against microorganisms relevant in veterinary medicine. J. Veter. Med. Ser. B 2006, 53, 133–137. [Google Scholar] [CrossRef]
- Hao, J.; Wu, T.; Li, H.; Liu, H. Differences of bactericidal efficacy on Escherichia coli, Staphylococcus aureus, and Bacillus subtilis of slightly and strongly acidic electrolyzed water. Food Bioprocess Technol. 2017, 10, 155–164. [Google Scholar] [CrossRef]
- Churklam, W.; Chaturongakul, S.; Ngamwongsatit, B.; Aunpad, R. The mechanisms of action of carvacrol and its syn-ergism with nisin against Listeria monocytogenes on sliced bologna sausage. Food Control 2020, 108, 106864. [Google Scholar] [CrossRef]
- Li, H.; Liang, D.; Huang, J.; Cui, C.; Rao, H.; Zhao, D.; Hao, J. The bactericidal efficacy and the mechanism of action of slightly acidic electrolyzed water on Listeria monocytogenes’ survival. Foods 2021, 10, 2671. [Google Scholar] [CrossRef]
- Alvarez-Ordóñez, A.; Halisch, J.; Prieto, M. Changes in fourier transform infrared spectra of Salmonella enterica serovars Typhimurium and Enteritidis after adaption to stressful growth conditions. Int. J. Food Microbiol. 2010, 142, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Al-Qadiri, H.M.; Al-Alami, N.I.; Al-Holy, M.A.; Rasco, B.A. Using Fourier transform infrared (FT-IR) absorbance spec-troscopy and multivariate analysis to study the effect of chlorine-induced bacterial injury in water. J. Agric. Food Chem. 2008, 56, 8992–8997. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Q.; Wu, D.; Al-Qadiri, H.M.; Al-Alami, N.I.; Kang, D.H.; Shin, J.H.; Tang, J.; Jabal, J.M.F.; Aston, E.D.; et al. Using of infrared spectroscopy to study the survival and injury of Escherichia coli O157: H7, Campylobacter jejuni and Pseudomonas aeruginosa under cold stress in low nutrient media. Food Microbiol. 2011, 28, 537–546. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, J.; Lim, Z.Y.; Aggarwal, A.; Yang, H.; Wang, S. Evaluation of the metabolic response of Escherichia coli to electrolysed water by 1H NMR spectroscopy. LWT 2017, 79, 428–436. [Google Scholar] [CrossRef]
- Maquelin, K.; Kirschner, C.; Choo-Smith, L.-P.; Braak, N.v.D.; Endtz, H.; Naumann, D.; Puppels, G. Identification of medically relevant microorganisms by vibrational spectroscopy. J. Microbiol. Methods 2002, 51, 255–271. [Google Scholar] [CrossRef]
- Ng, L.K.; Sherburne, R.; Taylor, D.E.; Stiles, M.E. Morphological forms and viability of Campylobacter species studied by electron microscopy. J. Bacteriol. 1985, 164, 338–343. [Google Scholar] [CrossRef]
- Fröhling, A.; Baier, M.; Ehlbeck, J.; Knorr, D.; Schlüter, O. Atmospheric pressure plasma treatment of Listeria innocua and Escherichia coli at polysaccharide surfaces: Inactivation kinetics and flow cytometric characterization. Innov. Food Sci. Emerg. Technol. 2012, 13, 142–150. [Google Scholar] [CrossRef]
- Rodino, S.; Butu, M.; Negoescu, C.; Caunii, A.; Cristina, R.; Butnariu, M. Spectrophotometric method for quantitative determination of nystatin antifungal agent in pharmaceutical formulations. Dig. J. Nanomater. 2014, 9, 1215–1222. [Google Scholar]
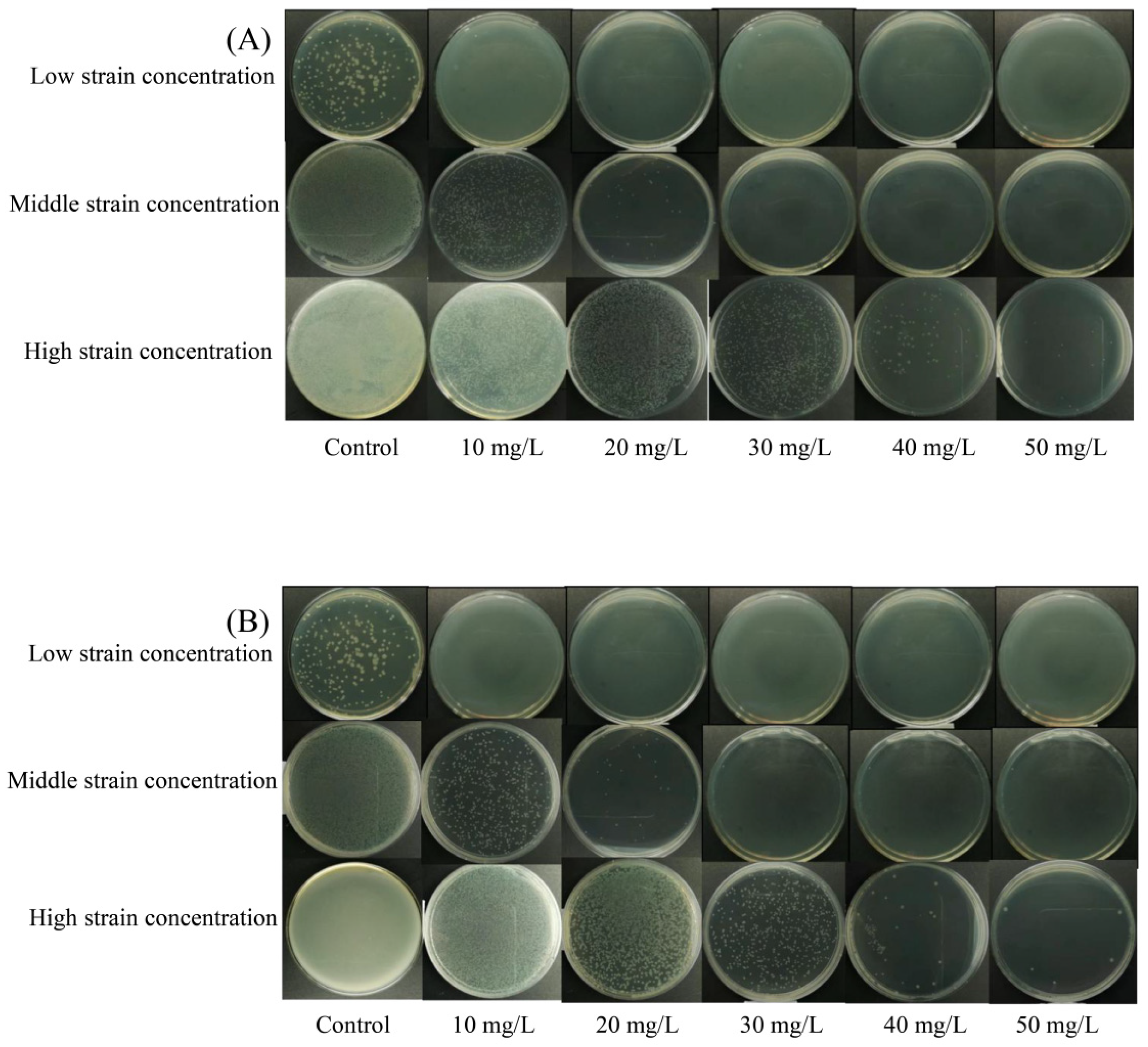
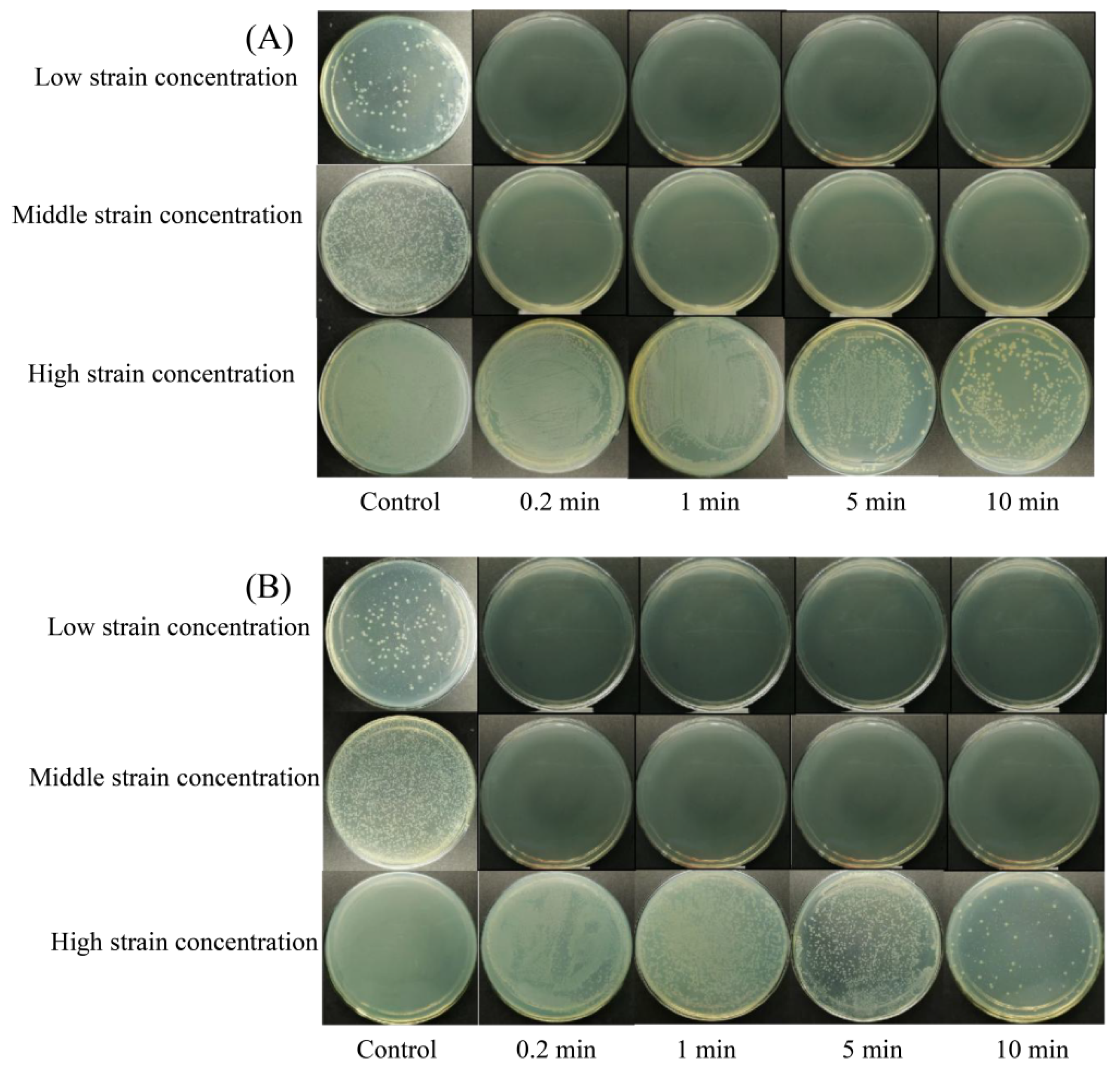

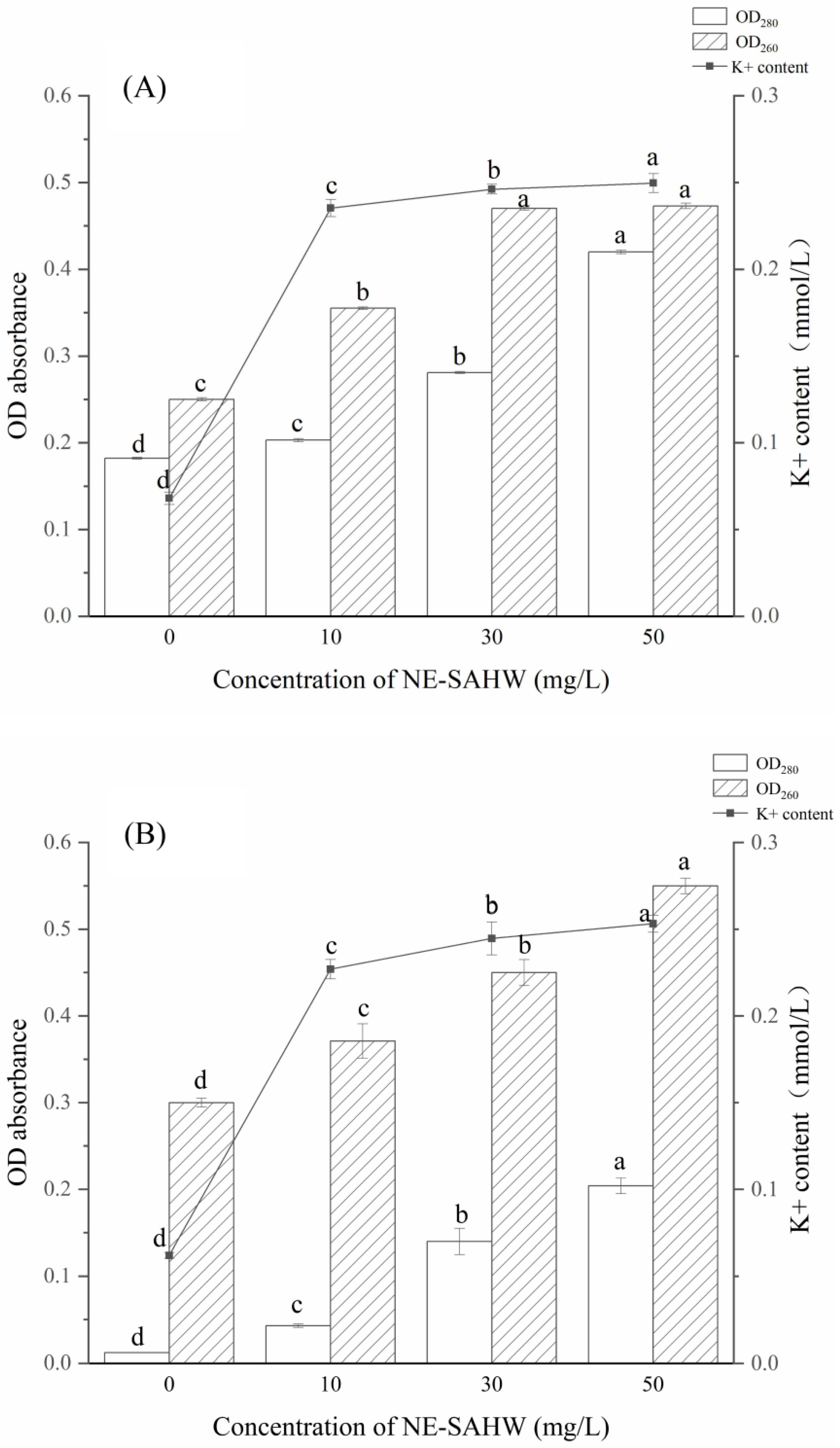
| Initial Populations of the Strains | Concentration of NE-SAHW (mg/L) | Log Reduction (log10 CFU/mL) | ||
|---|---|---|---|---|
| P. fragi | P. fluorescens | |||
| Low population levels | P. fragi 2.27 log10 CFU/mL P. fluorescens 2.08 log10 CFU/mL | 10 | >1.97 | >1.81 |
| 20 | ||||
| 30 | ||||
| 40 | ||||
| 50 | ||||
| Medium population levels | P. fragi 5.20 log10 CFU/mL P. fluorescens 5.06 log10 CFU/mL | 10 | 1.99 | 1.96 |
| 20 | 3.93 | 3.98 | ||
| 30 | >4.9 | >4.76 | ||
| 40 | ||||
| 50 | ||||
| High population levels | P. fragi 7.23 log10 CFU/mL P. fluorescens 7.05 log10 CFU/mL | 10 | 1.83 | 1.92 |
| 20 | 3.5 | 3.17 | ||
| 30 | 4.38 | 4.35 | ||
| 40 | 5.56 | 5.78 | ||
| 50 | 6.16 | 6.23 | ||
| Initial Level of the Strains | Treatment Time (min) | Log Reduction (log10 CFU/mL) | ||
|---|---|---|---|---|
| P. fragi | P. fluorescens | |||
| Low population levels | P. fragi 2.36 log10 CFU/mL P. fluorescens 2.38 log10 CFU/mL | 0.2 | >2.16 | >2.08 |
| 1 | ||||
| 5 | ||||
| 10 | ||||
| Medium population levels | P. fragi 5.23 log10 CFU/mL P. Fluorescens 5.06 log10 CFU/mL | 0.2 | >4.93 | >3.76 |
| 1 | ||||
| 5 | ||||
| 10 | ||||
| High population levels | P. fragi 7.12 log10 CFU/mL P. fluorescens 6.96 log10 CFU/mL | 0.2 | 0.94 | 1.21 |
| 1 | 1.39 | 1.52 | ||
| 5 | 4.02 | 4.14 | ||
| 10 | 5.60 | 5.74 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Zhou, Y.; Yun, X.; Zhao, N.; Bu, H.; Dong, T. Bactericidal Efficacy and Mechanisms of Non-Electrolytic Slightly Acidic Hypochlorous Water on Pseudomonas fragi and Pseudomonas fluorescens. Foods 2023, 12, 3980. https://doi.org/10.3390/foods12213980
Chen Q, Zhou Y, Yun X, Zhao N, Bu H, Dong T. Bactericidal Efficacy and Mechanisms of Non-Electrolytic Slightly Acidic Hypochlorous Water on Pseudomonas fragi and Pseudomonas fluorescens. Foods. 2023; 12(21):3980. https://doi.org/10.3390/foods12213980
Chicago/Turabian StyleChen, Qianru, Yanfang Zhou, Xueyan Yun, Namula Zhao, Hongyu Bu, and Tungalag Dong. 2023. "Bactericidal Efficacy and Mechanisms of Non-Electrolytic Slightly Acidic Hypochlorous Water on Pseudomonas fragi and Pseudomonas fluorescens" Foods 12, no. 21: 3980. https://doi.org/10.3390/foods12213980
APA StyleChen, Q., Zhou, Y., Yun, X., Zhao, N., Bu, H., & Dong, T. (2023). Bactericidal Efficacy and Mechanisms of Non-Electrolytic Slightly Acidic Hypochlorous Water on Pseudomonas fragi and Pseudomonas fluorescens. Foods, 12(21), 3980. https://doi.org/10.3390/foods12213980






