Cooking-Induced Oxidation and Structural Changes in Chicken Protein: Their Impact on In Vitro Gastrointestinal Digestion and Intestinal Flora Fermentation Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sample Pretreatment and Cooking Procedure
2.3. In Vitro Oral-Gastrointestinal Digestion and Colonic Fermentation
2.3.1. In Vitro Oral-Gastrointestinal Digestion
2.3.2. In Vitro Colonic Fermentation
2.4. Determination of Protein Oxidation
2.4.1. Quantification of Free Sulfhydryl Content
2.4.2. Quantification of Total Carbonyl Content
2.4.3. Determination of Schiff Bases (SB)
2.5. Analysis of Protein Structure
2.5.1. Determination of Protein Surface Hydrophobicity
2.5.2. Detection of the Secondary Structure of Protein
2.6. Determination of In Vitro Protein Digestion Characteristics
2.6.1. Determination of Protein Digestibility
2.6.2. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.6.3. Microstructure
2.7. Intestinal Flora Analysis of 16S rRNA Gene Sequencing
2.8. Quantification of Short-Chain Fatty Acids (SCFAs)
2.9. Statistical Analyses
3. Results and Discussion
3.1. Impact of Various Cooking Methods on the Degree of Chicken Protein Oxidation
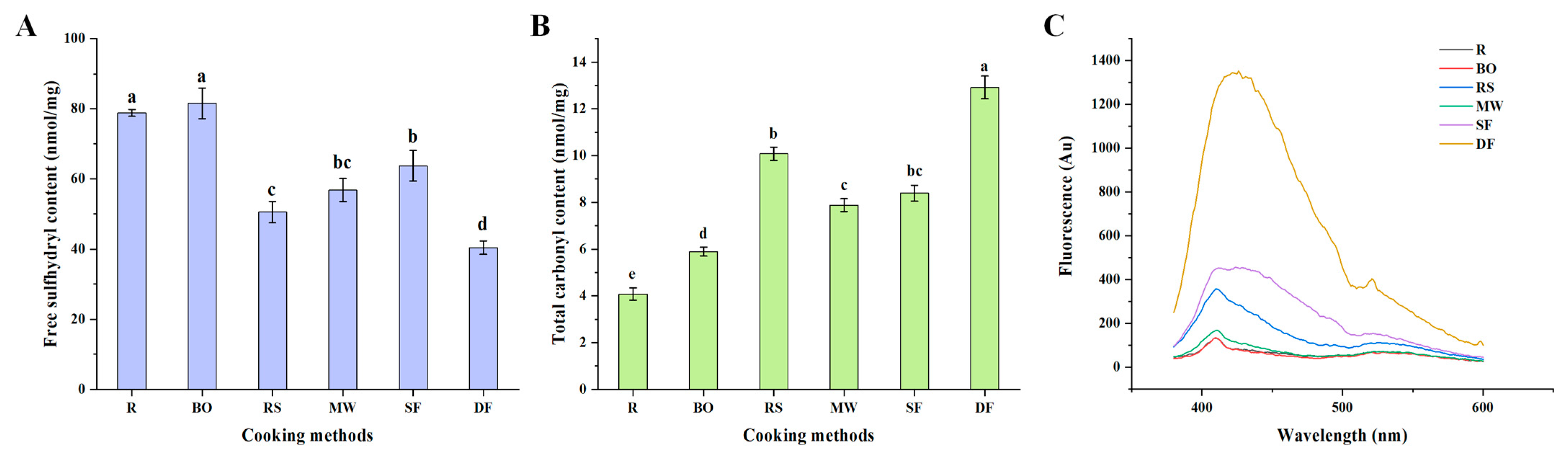
3.1.1. Free Sulfhydryl Content
3.1.2. Total Carbonyl Content
3.1.3. Schiff Base (SB) Content
3.2. Impact of Various Cooking Methods on the Protein Structure of Chicken
3.2.1. Protein Surface Hydrophobicity
3.2.2. Protein Secondary Structure
3.3. Impact of Various Cooking Methods on Digestive Characteristics of Chicken Protein
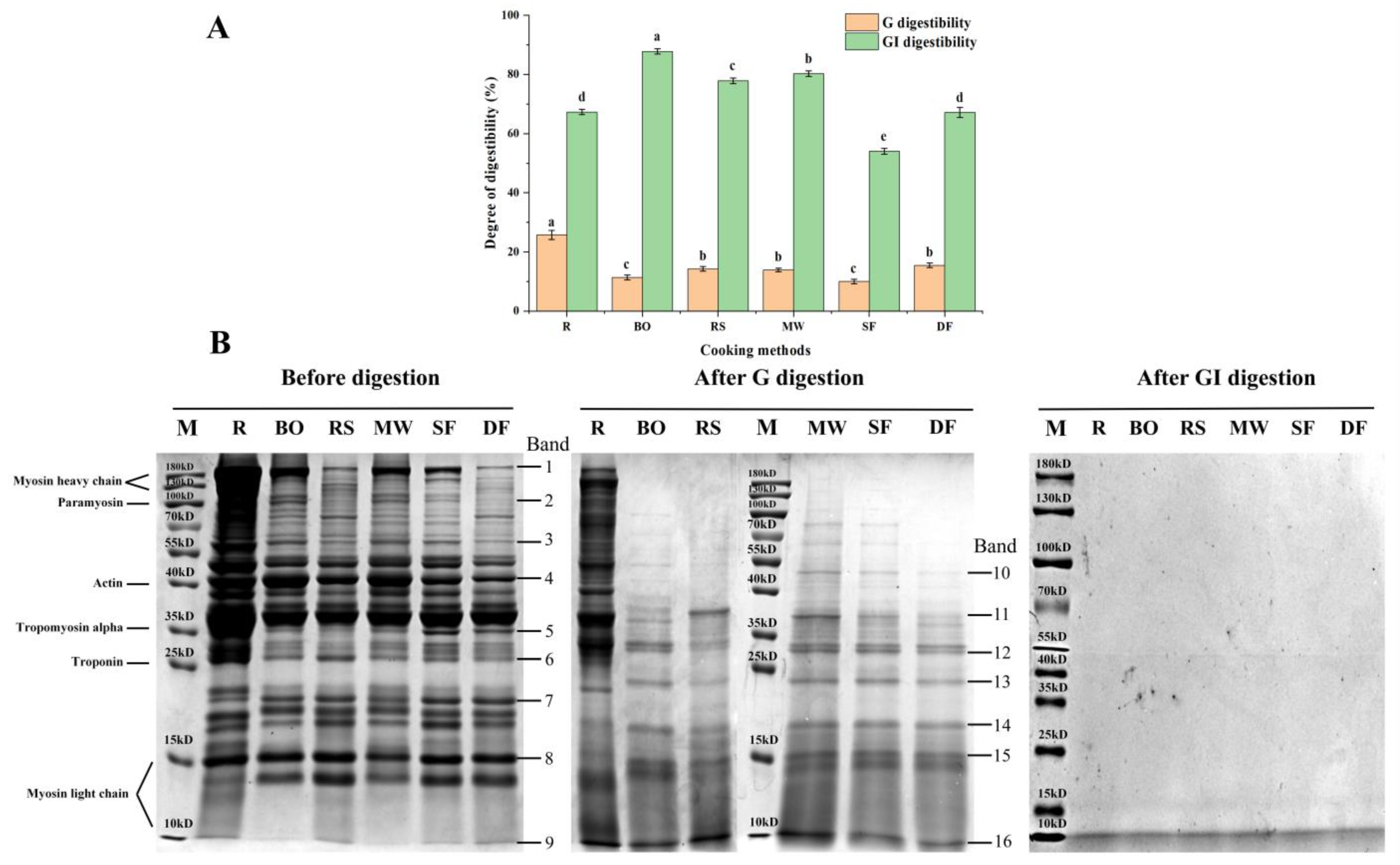
3.3.1. Digestibility
3.3.2. SDS-PAGE Patterns
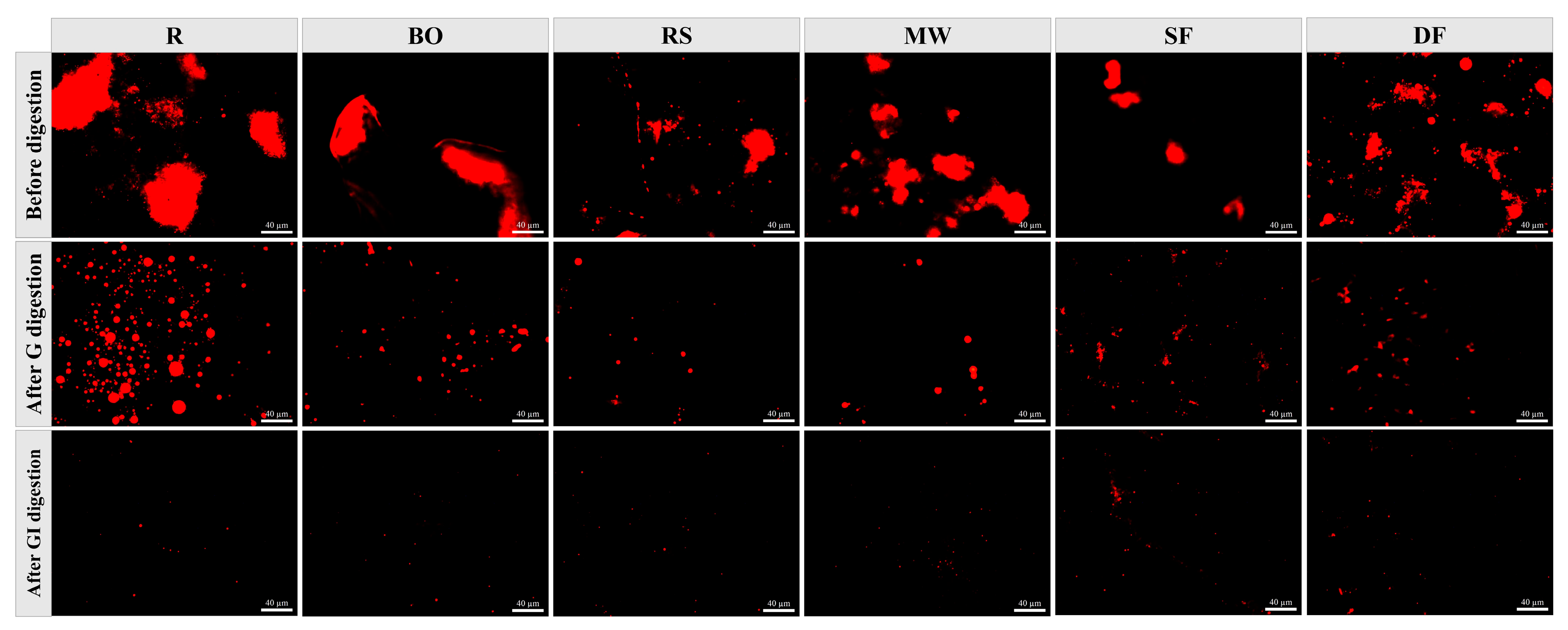
3.3.3. Microstructure
3.4. Impact of Various Cooking Methods on the Colonic Fermentation Characteristics of Chicken
3.4.1. Microbial Community
3.4.2. SCFA Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rinwi, T.G.; Ma, J.; Sun, D.-W. Effects of isochoric freezing on myofibrillar protein conformational structures of chicken breasts. LWT 2023, 181, 114768. [Google Scholar] [CrossRef]
- Sobral, M.M.C.; Casal, S.; Faria, M.A.; Cunha, S.C.; Ferreira, I.M.P.L.V.O. Influence of culinary practices on protein and lipid oxidation of chicken meat burgers during cooking and in vitro gastrointestinal digestion. Food Chem. Toxicol. 2020, 141, 111401. [Google Scholar] [CrossRef]
- Abete, I.; Romaguera, D.; Vieira, A.R.; Lopez de Munain, A.; Norat, T. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: A meta-analysis of cohort studies. Br. J. Nutr. 2014, 112, 762–775. [Google Scholar] [CrossRef]
- Wang, X.; Yao, Y.; Yu, J.; Cui, H.; Hayat, K.; Zhang, X.; Ho, C.-T. Evolution of lean meat tenderness stimulated by coordinated variation of water status, protein structure and tissue histology during cooking of braised pork. Food Res. Int. 2023, 171, 113081. [Google Scholar] [CrossRef]
- Hu, L.; Ren, S.; Shen, Q.; Chen, J.; Ye, X.; Ling, J. Proteomic study of the effect of different cooking methods on protein oxidation in fish fillets. RSC Adv. 2017, 7, 27496–27505. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, X.; Liu, X.; Zhang, Y.; Zhao, K.; Zhang, K.; Wang, W. Effects of different cooking methods on physicochemical, textural properties of yak meat and its changes with intramuscular connective tissue during in vitro digestion. Food Chem. 2023, 422, 136188. [Google Scholar] [CrossRef]
- De La Pomélie, D.; Santé-Lhoutellier, V.; Sayd, T.; Gatellier, P. Oxidation and nitrosation of meat proteins under gastro-intestinal conditions: Consequences in terms of nutritional and health values of meat. Food Chem. 2018, 243, 295–304. [Google Scholar] [CrossRef]
- He, J.; Zhou, G.; Bai, Y.; Wang, C.; Zhu, S.; Xu, X.; Li, C. The effect of meat processing methods on changes in disulfide bonding and alteration of protein structures: Impact on protein digestion products. RSC Adv. 2018, 8, 17595–17605. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, D.; Zhu, S.; Nian, Y.; Xu, X.; Zhou, G.; Li, C. Overheating induced structural changes of type I collagen and impaired the protein digestibility. Food Res. Int. 2020, 134, 109225. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, H.; Dong, S.; Jin, W.; Han, K.; Ren, Y.; Zeng, M. Glycation of fish protein impacts its fermentation metabolites and gut microbiota during in vitro human colonic fermentation. Food Res. Int. 2018, 113, 189–196. [Google Scholar] [CrossRef]
- Windey, K.; De Preter, V.; Verbeke, K. Relevance of protein fermentation to gut health. Mol. Nutr. Food Res. 2012, 56, 184–196. [Google Scholar] [CrossRef]
- Jantchou, P.; Morois, S.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C.; Carbonnel, F. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Am. J. Gastroenterol. 2010, 105, 2195–2201. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, M.; Yang, Y.; Mu, C.; Su, Y.; Zhu, W. Differential effect of early antibiotic intervention on bacterial fermentation patterns and mucosal gene expression in the colon of pigs under diets with different protein levels. Appl. Microbiol. Biotechnol. 2017, 101, 2493–2505. [Google Scholar] [CrossRef]
- Laukens, D.; Brinkman, B.M.; Raes, J.; De Vos, M.; Vandenabeele, P. Heterogeneity of the gut microbiome in mice: Guidelines for optimizing experimental design. FEMS Microbiol. Rev. 2016, 40, 117–132. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Bäuerl, C.; Cortés-Macías, E.; Calvo-Lerma, J.; Carmen Collado, M.; Barba, F.J. The impact of liquid-pressurized extracts of Spirulina, Chlorella and Phaedactylum tricornutum on in vitro antioxidant, antiinflammatory and bacterial growth effects and gut microbiota modulation. Food Chem. 2023, 401, 134083. [Google Scholar] [CrossRef]
- Neves Casarotti, S.; Fernanda Borgonovi, T.; de Mello Tieghi, T.; Sivieri, K.; Lúcia Barretto Penna, A. Probiotic low-fat fermented goat milk with passion fruit by-product: In vitro effect on obese individuals’ microbiota and on metabolites production. Food Res. Int. 2020, 136, 109453. [Google Scholar] [CrossRef]
- Zou, X.-L.; Kang, Z.-L.; Li, Y.-P.; Ma, H.-J. Effect of sodium bicarbonate on solubility, conformation and emulsion properties of pale, soft and exudative meat myofibrillar proteins. LWT 2022, 157, 113097. [Google Scholar] [CrossRef]
- Duque-Estrada, P.; Berton-Carabin, C.C.; Nieuwkoop, M.; Dekkers, B.L.; Janssen, A.E.M.; van der Goot, A.J. Protein oxidation and in vitro gastric digestion of processed soy-based matrices. J. Agric. Food Chem. 2019, 67, 9591–9600. [Google Scholar] [CrossRef]
- Traore, S.; Aubry, L.; Gatellier, P.; Przybylski, W.; Jaworska, D.; Kajak-Siemaszko, K.; Santé-Lhoutellier, V. Effect of heat treatment on protein oxidation in pig meat. Meat Sci. 2012, 91, 14–21. [Google Scholar] [CrossRef]
- Chelh, I.; Gatellier, P.; Santé-Lhoutellier, V. Technical note: A simplified procedure for myofibril hydrophobicity determination. Meat Sci. 2006, 74, 681–683. [Google Scholar] [CrossRef]
- Li, K.; Fu, L.; Zhao, Y.-Y.; Xue, S.-W.; Wang, P.; Xu, X.-L.; Bai, Y.-H. Use of high-intensity ultrasound to improve emulsifying properties of chicken myofibrillar protein and enhance the rheological properties and stability of the emulsion. Food Hydrocoll. 2020, 98, 105275. [Google Scholar] [CrossRef]
- Xue, S.; Wang, C.; Kim, Y.H.B.; Bian, G.; Han, M.; Xu, X.; Zhou, G. Application of high-pressure treatment improves the in vitro protein digestibility of gel-based meat product. Food Chem. 2020, 306, 125602. [Google Scholar] [CrossRef]
- Wang, H.; Gao, S.; Zhang, D.; Wang, Y.; Zhang, Y.; Jiang, S.; Li, B.; Wu, D.; Lv, G.; Zou, X.; et al. Encapsulation of catechin or curcumin in co-crystallized sucrose: Fabrication, characterization and application in beef meatballs. LWT 2022, 168, 113911. [Google Scholar] [CrossRef]
- Liu, W.; Lanier, T.C. Combined use of variable pressure scanning electron microscopy and confocal laser scanning microscopy best reveal microstructure of comminuted meat gels. LWT Food Sci. Technol. 2015, 62, 1027–1033. [Google Scholar] [CrossRef]
- Xie, J.; Sun, N.; Huang, H.; Xie, J.; Chen, Y.; Hu, X.; Hu, X.; Dong, R.; Yu, Q. Catabolism of polyphenols released from mung bean coat and its effects on gut microbiota during in vitro simulated digestion and colonic fermentation. Food Chem. 2022, 396, 133719. [Google Scholar] [CrossRef]
- Ivane, N.M.A.; Elysé, F.K.R.; Haruna, S.A.; Pride, N.; Richard, E.; Foncha, A.C.; Dandago, M.A. The anti-oxidative potential of ginger extract and its constituent on meat protein isolate under induced Fenton oxidation. J. Proteom. 2022, 269, 104723. [Google Scholar] [CrossRef]
- Jiang, S.; Feng, X.; Zhang, F.; Wang, R.; Zeng, M. Effects of cooking methods on the Maillard reaction products, digestibility, and mineral bioaccessibility of Pacific oysters (Crassostrea gigas). LWT 2021, 141, 110943. [Google Scholar] [CrossRef]
- Weber, D.; Davies, M.J.; Grune, T. Determination of protein carbonyls in plasma, cell extracts, tissue homogenates, isolated proteins: Focus on sample preparation and derivatization conditions. Redox Biol. 2015, 5, 367–380. [Google Scholar] [CrossRef]
- Santé-Lhoutellier, V.; Astruc, T.; Marinova, P.; Greve, E.; Gatellier, P. Effect of meat cooking on physicochemical state and in vitro digestibility of myofibrillar proteins. J. Agric. Food Chem. 2008, 56, 1488–1494. [Google Scholar] [CrossRef]
- Gatellier, P.; Kondjoyan, A.; Portanguen, S.; Santé-Lhoutellier, V. Effect of cooking on protein oxidation in n-3 polyunsaturated fatty acids enriched beef. Implication on nutritional quality. Meat Sci. 2010, 85, 645–650. [Google Scholar] [CrossRef]
- Yongsawatdigul, J.; Park, J.W. Thermal denaturation and aggregation of threadfin bream actomyosin. Food Chem. 2003, 83, 409–416. [Google Scholar] [CrossRef]
- Yan, S.; Xu, J.; Zhang, S.; Li, Y. Effects of flexibility and surface hydrophobicity on emulsifying properties: Ultrasound-treated soybean protein isolate. LWT 2021, 142, 110881. [Google Scholar] [CrossRef]
- Yu, M.-M.; Fan, Y.-C.; Liu, Y.-X.; Yin, F.-W.; Li, D.-Y.; Liu, X.-Y.; Zhou, D.-Y.; Zhu, B.-W. Effects of antioxidants of bamboo leaves on protein digestion and transport of cooked abalone muscles. Food Funct. 2022, 13, 1785–1796. [Google Scholar] [CrossRef]
- Mitra, B.; Rinnan, Å.; Ruiz-Carrascal, J. Tracking hydrophobicity state, aggregation behaviour and structural modifications of pork proteins under the influence of assorted heat treatments. Food Res. Int. 2017, 101, 266–273. [Google Scholar] [CrossRef]
- Wang, T.; Huang, Y.; Tang, X.; Wang, H.; Li, B.; Meng, X.; Jiang, S. Effects of different cooking methods on peanut allergenicity. Food Biosci. 2022, 47, 101757. [Google Scholar] [CrossRef]
- Calabrò, E.; Magazù, S. Comparison between conventional convective heating and microwave heating: An FTIR spectroscopy study of the effects of microwave oven cooking of bovine breast meat. J. Electromagn. Anal. Appl. 2012, 4, 433–439. [Google Scholar] [CrossRef]
- Yu, P. Protein secondary structures (α-helix and β-sheet) at a cellular level and protein fractions in relation to rumen degradation behaviours of protein: A new approach. Br. J. Nutr. 2005, 94, 655–665. [Google Scholar] [CrossRef]
- Mancini, S.; Mattioli, S.; Paolucci, S.; Fratini, F.; Dal Bosco, A.; Tuccinardi, T.; Paci, G. Effect of cooking techniques on the in vitro protein digestibility, fatty acid profile, and oxidative status of mealworms (Tenebrio molitor). Front. Vet. Sci. 2021, 8, 675572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Wang, W.; Zhang, J. Effect of boiling and frying on nutritional value and in vitro digestibility of rabbit meat. Afr. J. Food Sci. 2014, 8, 92–103. [Google Scholar] [CrossRef]
- Yu, T.-Y.; Morton, J.D.; Clerens, S.; Dyer, J.M. Cooking-induced protein modifications in meat. Compr. Rev. Food Sci. F. 2017, 16, 141–159. [Google Scholar] [CrossRef]
- Kajak-Siemaszko, K.; Aubry, L.; Peyrin, F.; Bax, M.L.; Gatellier, P.; Astruc, T.; Przybylski, W.; Jaworska, D.; Gaillard-Martinie, B.; Santé-Lhoutellier, V. Characterization of protein aggregates following a heating and freezing process. Food Res. Int. 2011, 44, 3160–3166. [Google Scholar] [CrossRef]
- Wen, S.; Zhou, G.; Li, L.; Xu, X.; Yu, X.; Bai, Y.; Li, C. Effect of cooking on in vitro digestion of pork proteins: A peptidomic perspective. J. Agric. Food Chem. 2015, 63, 250–261. [Google Scholar] [CrossRef]
- Xie, Y.; Cai, L.; Zhao, D.; Liu, H.; Xu, X.; Zhou, G.; Li, C. Real meat and plant-based meat analogues have different in vitro protein digestibility properties. Food Chem. 2022, 387, 132917. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Zou, X.; He, J.; Xu, X.; Zhou, G.; Li, C. In vitro protein digestibility of pork products is affected by the method of processing. Food Res. Int. 2017, 92, 88–94. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, Y.; Tan, Y.; Zhang, Z.; McClements, D.J. Digestibility and gastrointestinal fate of meat versus plant-based meat analogs: An in vitro comparison. Food Chem. 2021, 364, 130439. [Google Scholar] [CrossRef]
- Dou, Z.; Chen, C.; Huang, Q.; Fu, X. In vitro digestion of the whole blackberry fruit: Bioaccessibility, bioactive variation of active ingredients and impacts on human gut microbiota. Food Chem. 2022, 370, 131001. [Google Scholar] [CrossRef]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Program, N.C.S.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; et al. Topographical and temporal diversity of the human skin microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.; Zhao, K.; Li, H.; Liu, J.; Da, S.; Ciren, D.; Tang, H. In vitro digestion and fermentation combined with microbiomics and metabolomics reveal the mechanism of superfine yak bone powder regulating lipid metabolism by altering human gut microbiota. Food Chem. 2023, 410, 135441. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, S.; Saulnier, D.M.; Pot, B.; Versalovic, J. How can probiotics and prebiotics impact mucosal immunity? Gut Microbes 2010, 1, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Yang, Y.; Luo, Z.; Guan, L.; Zhu, W. The colonic microbiome and epithelial transcriptome are altered in rats fed a high-protein diet compared with a normal-protein diet. J. Nutr. 2016, 146, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, M.; Huang, Y.; Xie, J.; Shen, M.; Xie, M. A comprehensive review of advanced glycosylation end products and N- Nitrosamines in thermally processed meat products. Food Control 2022, 131, 108449. [Google Scholar] [CrossRef]
- Li, Y.; Xue, C.; Quan, W.; Qin, F.; Wang, Z.; He, Z.; Zeng, M.; Chen, J. Assessment the influence of salt and polyphosphate on protein oxidation and Nε-(carboxymethyl)lysine and Nε-(carboxyethyl)lysine formation in roasted beef patties. Meat Sci. 2021, 177, 108489. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Jin, W.; Mao, Z.; Dong, S.; Zhang, Q.; Yang, Y.; Chen, B.; Wu, H.; Zeng, M. Microbiome and butyrate production are altered in the gut of rats fed a glycated fish protein diet. J. Funct. Foods 2018, 47, 423–433. [Google Scholar] [CrossRef]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci. Technol. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial short-chain fatty acids modulate CD8(+) T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Gibson, G.R.; Beatty, E.; Cummings, J.H. Estimation of short-chain fatty acid production from protein by human intestinal bacteria based on branched-chain fatty acid measurements. FEMS Microbiol. Lett. 1992, 101, 81–88. [Google Scholar] [CrossRef]




| Cooking Methods | Secondary Structure Proportions (%) | |||
|---|---|---|---|---|
| α-Helix | β-Sheet | β-Turn | Random Coil | |
| R | 40.41 ± 0.24 a | 12.14 ± 0.17 e | 7.25 ± 0.18 a | 40.20 ± 0.26 a |
| BO | 35.41 ± 0.83 b | 43.57 ± 0.38 c | 3.39 ± 0.38 c | 17.63 ± 0.08 c |
| RS | 34.50 ± 0.85 b | 41.25 ± 0.17 d | 3.77 ± 0.50 c | 20.48 ± 1.41 c |
| MW | 35.30 ± 1.38 b | 42.27 ± 0.52 cd | 3.41 ± 0.33 c | 19.03 ± 1.19 c |
| SF | 14.11 ± 0.74 c | 50.75 ± 0.95 b | 5.17 ± 0.37 b | 29.97 ± 1.90 b |
| DF | 14.99 ± 0.19 c | 52.61 ± 0.33 a | 6.03 ± 0.06 b | 26.38 ± 0.09 b |
| Bands Number | Relative Intensities | |||||
|---|---|---|---|---|---|---|
| R | BO | RS | MW | SF | DF | |
| Before digestion | ||||||
| 1 | 1.527 ± 0.079 a | 1.269 ± 0.059 b | 0.645 ± 0.027 d | 1.170 ± 0.054 b | 0.910 ± 0.027 c | 0.430 ± 0.019 e |
| 2 | 1.244 ± 0.179 a | 0.826 ± 0.105 b | 0.598 ± 0.083 bcd | 0.691 ± 0.092 bc | 0.429 ± 0.059 cd | 0.241 ± 0.022 d |
| 3 | 0.905 ± 0.054 a | 0.612 ± 0.038 b | 0.454 ± 0.030 c | 0.588 ± 0.038 b | 0.452 ± 0.025 c | 0.354 ± 0.020 c |
| 4 | 1.870 ± 0.045 a | 1.830 ± 0.071 a | 1.544 ± 0.076 bc | 1.746 ± 0.063 ab | 1.432 ± 0.057 cd | 1.264 ± 0.032 d |
| 5 | 0.952 ± 0.020 a | 0.543 ± 0.036 cd | 0.473 ± 0.034 d | 0.540 ± 0.035 cd | 0.699 ± 0.045 b | 0.634 ± 0.032 bc |
| 6 | 0.972 ± 0.080 a | 0.537 ± 0.030 b | 0.631 ± 0.032 b | 0.500 ± 0.030 b | 0.520 ± 0.022 b | 0.487 ± 0.023 b |
| 7 | 0.610 ± 0.032 b | 0.718 ± 0.029 ab | 0.717 ± 0.035 ab | 0.662 ± 0.034 ab | 0.730 ± 0.037 ab | 0.760 ± 0.030 a |
| 8 | 1.526 ± 0.028 a | 1.485 ± 0.021 ab | 1.471 ± 0.024 ab | 1.327 ± 0.024 c | 1.417 ± 0.026 bc | 1.418 ± 0.031 bc |
| 9 | 0.515 ± 0.052 a | 0.234 ± 0.021 b | 0.356 ± 0.047 b | 0.236 ± 0.015 b | 0.344 ± 0.015 b | 0.484 ± 0.036 a |
| After G digestion | ||||||
| 10 | 0.711 ± 0.048 a | 0.154 ± 0.004 c | 0.158 ± 0.009 c | 0.281 ± 0.012 b | 0.176 ± 0.003 c | 0.053 ± 0.001 d |
| 11 | 0.732 ± 0.073 a | 0.299 ± 0.031 c | 0.469 ± 0.049 b | 0.535 ± 0.055 b | 0.299 ± 0.030 c | 0.184 ± 0.019 c |
| 12 | 0.733 ± 0.090 a | 0.460 ± 0.041 bc | 0.308 ± 0.013 c | 0.537 ± 0.055 b | 0.429 ± 0.037 bc | 0.341 ± 0.036 c |
| 13 | 0.401 ± 0.018 bc | 0.447 ± 0.017 ab | 0.309 ± 0.010 d | 0.475 ± 0.015 a | 0.391 ± 0.009 c | 0.318 ± 0.009 d |
| 14 | 0.495 ± 0.052 a | 0.574 ± 0.063 a | 0.486 ± 0.046 a | 0.590 ± 0.071 a | 0.487 ± 0.047 a | 0.436 ± 0.053 a |
| 15 | 0.588 ± 0.095 a | 0.697 ± 0.091 a | 0.643 ± 0.091 a | 0.867 ± 0.114 a | 0.750 ± 0.098 a | 0.703 ± 0.083 a |
| 16 | 1.131 ± 0.030 cd | 1.202 ± 0.019 bc | 1.288 ± 0.010 b | 1.520 ± 0.079 a | 1.037 ± 0.041 d | 0.875 ± 0.044 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, G.; Wang, H.; Wei, X.; Lu, M.; Yang, W.; Aalim, H.; Capanoglu, E.; Zou, X.; Battino, M.; Zhang, D. Cooking-Induced Oxidation and Structural Changes in Chicken Protein: Their Impact on In Vitro Gastrointestinal Digestion and Intestinal Flora Fermentation Characteristics. Foods 2023, 12, 4322. https://doi.org/10.3390/foods12234322
Lv G, Wang H, Wei X, Lu M, Yang W, Aalim H, Capanoglu E, Zou X, Battino M, Zhang D. Cooking-Induced Oxidation and Structural Changes in Chicken Protein: Their Impact on In Vitro Gastrointestinal Digestion and Intestinal Flora Fermentation Characteristics. Foods. 2023; 12(23):4322. https://doi.org/10.3390/foods12234322
Chicago/Turabian StyleLv, Guanhua, Hengpeng Wang, Xiaoou Wei, Minmin Lu, Wenhao Yang, Halah Aalim, Esra Capanoglu, Xiaobo Zou, Maurizio Battino, and Di Zhang. 2023. "Cooking-Induced Oxidation and Structural Changes in Chicken Protein: Their Impact on In Vitro Gastrointestinal Digestion and Intestinal Flora Fermentation Characteristics" Foods 12, no. 23: 4322. https://doi.org/10.3390/foods12234322
APA StyleLv, G., Wang, H., Wei, X., Lu, M., Yang, W., Aalim, H., Capanoglu, E., Zou, X., Battino, M., & Zhang, D. (2023). Cooking-Induced Oxidation and Structural Changes in Chicken Protein: Their Impact on In Vitro Gastrointestinal Digestion and Intestinal Flora Fermentation Characteristics. Foods, 12(23), 4322. https://doi.org/10.3390/foods12234322








