Effects of Peanuts and Pistachios on Gut Microbiota and Metabolic Syndrome: A Review
Abstract
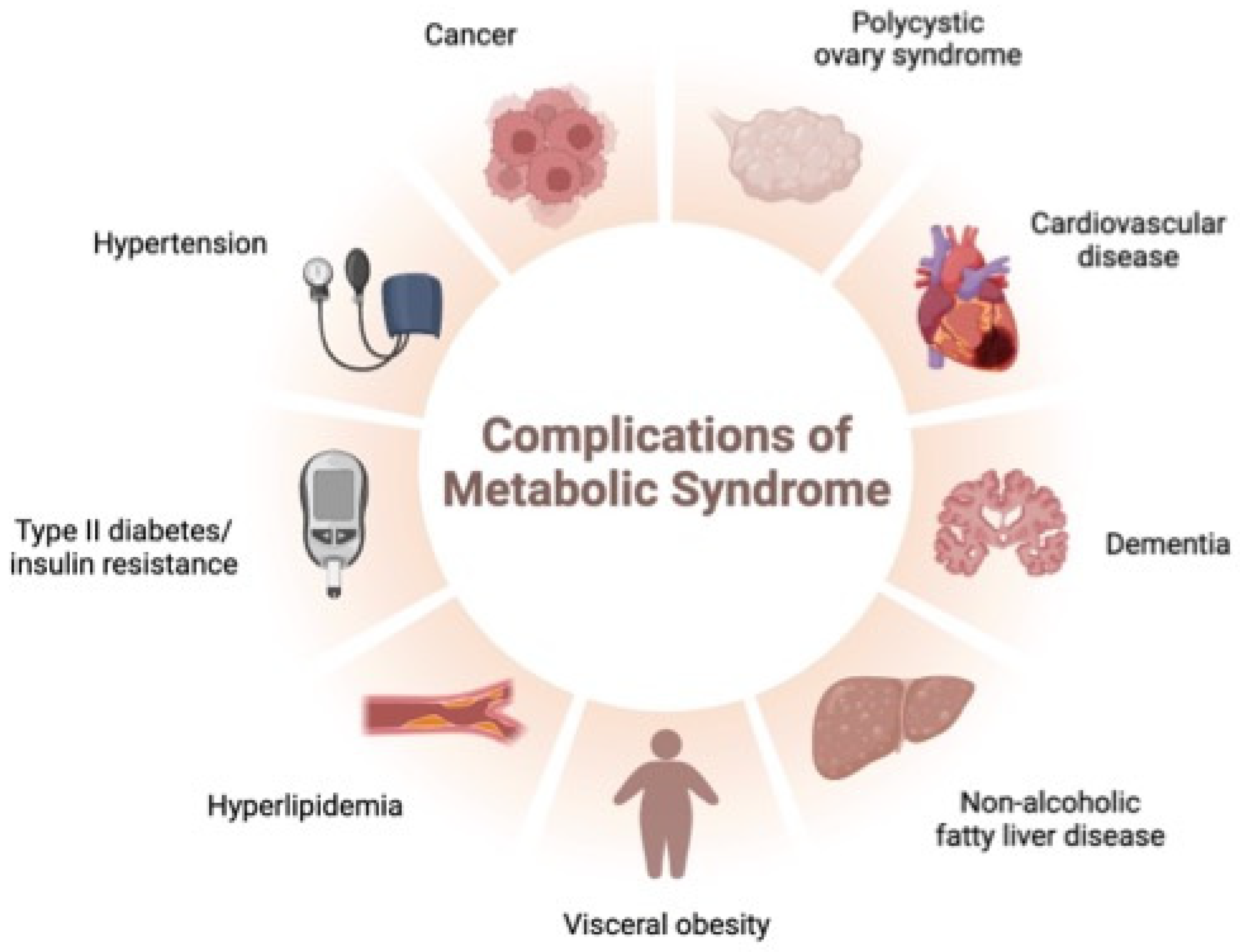
:1. Introduction
2. Peanuts
2.1. General Characteristics of Peanuts
2.2. Global Production
2.3. Nutritional Profile and Potential Health Benefits
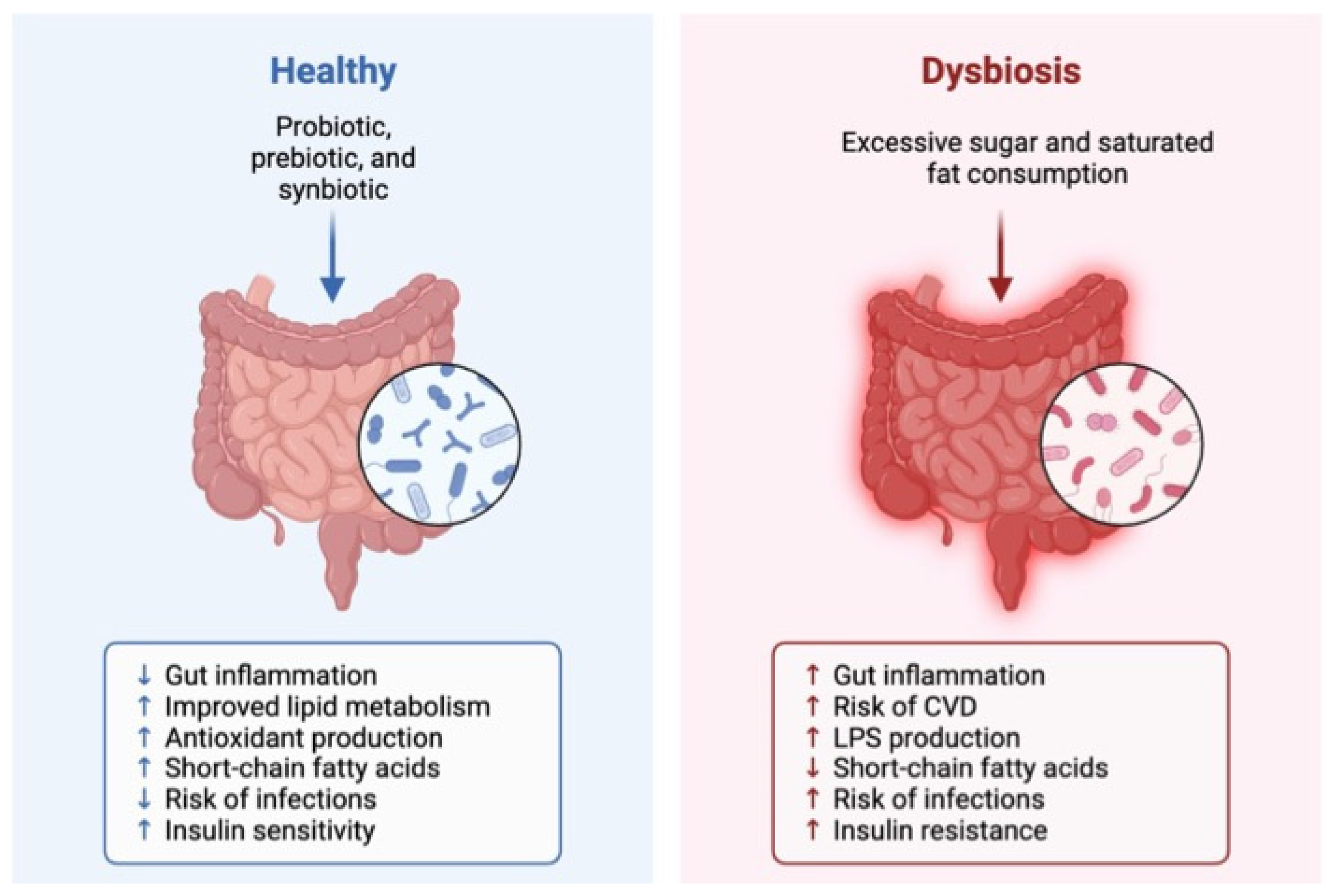
2.4. The Impact of Peanuts on the Gut Microbiota and Its Relationship with the Occurrence of MetS Risk Factors
2.4.1. The Microbiota Improvement
2.4.2. The Role of the Metabolome
2.4.3. The Impact of Lipopolysaccharides
2.4.4. The Global Impact of Peanut Consumption
3. Pistachios
3.1. General Characteristics of Pistachios
3.2. Global Production
3.3. Nutritional Profile and Potential Health Benefits
3.4. The Impact of Pistachio on the Gut Microbiota
3.4.1. The Microbiota Improvement
3.4.2. The Global Impact of Pistachio Consumption
4. Findings, Limitations, and Areas for Future Research
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Jia, H.; Cai, X.; Zhong, H.; Feng, Q.; Sunagawa, S.; Arumugam, M.; Kultima, J.R.; Prifti, E.; Nielsen, T. An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol. 2014, 32, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food components and dietary habits: Keys for a healthy gut microbiota composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tapia, M.; Tovar, A.R.; Torres, N. Diet as regulator of gut microbiota and its role in health and disease. Arch. Med. Res. 2019, 50, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Engevik, M.A.; Spinler, J.K.; Versalovic, J. Healthy human gastrointestinal microbiome: Composition and function after a decade of exploration. Dig. Dis. Sci. 2020, 65, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Van Treuren, W.; Dodd, D. Microbial contribution to the human metabolome: Implications for health and disease. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 345–369. [Google Scholar] [CrossRef]
- Allam-Ndoul, B.; Castonguay-Paradis, S.; Veilleux, A. Gut microbiota and intestinal trans-epithelial permeability. Int. J. Mol. Sci. 2020, 21, 6402. [Google Scholar] [CrossRef]
- Wu, J.; Lin, Z.; Wang, X.; Zhao, Y.; Zhao, J.; Liu, H.; Johnston, L.J.; Lu, L.; Ma, X. Limosilactobacillus reuteri SLZX19-12 protects the colon from infection by enhancing stability of the gut microbiota and barrier integrity and reducing inflammation. Microbiol. Spectr. 2022, 10, e02124-21. [Google Scholar] [CrossRef]
- Soderholm, A.T.; Pedicord, V.A. Intestinal epithelial cells: At the interface of the microbiota and mucosal immunity. Immunology 2019, 158, 267–280. [Google Scholar] [CrossRef]
- Koh, A.; Bäckhed, F. From association to causality: The role of the gut microbiota and its functional products on host metabolism. Mol. Cell 2020, 78, 584–596. [Google Scholar] [CrossRef]
- Bastiaanssen, T.F.; Cowan, C.S.; Claesson, M.J.; Dinan, T.G.; Cryan, J.F. Making sense of… the microbiome in psychiatry. Int. J. Neuropsychopharmacol. 2019, 22, 37–52. [Google Scholar] [CrossRef]
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306–314. [Google Scholar] [CrossRef]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.; Sandhu, K.V.; Bastiaanssen, T.F.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Ye, L.; Liddle, R.A. Gastrointestinal hormones and the gut connectome. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.-J.; Zhang, W. Role of dietary nutrients in the modulation of gut microbiota: A narrative review. Nutrients 2020, 12, 381. [Google Scholar] [CrossRef] [PubMed]
- Simões, C.D. Effect of diet on the human large intestinal microbiota. Rev. Nutrícias 2014, 23, 20–23. [Google Scholar]
- Wu, J.; Wang, K.; Wang, X.; Pang, Y.; Jiang, C. The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell 2021, 12, 360–373. [Google Scholar] [CrossRef]
- Earnest, C.P.; Mikus, C.R.; Lemieux, I.; Arsenault, B.J.; Church, T.S. Examination of encapsulated phytosterol ester supplementation on lipid indices associated with cardiovascular disease. Nutrition 2007, 23, 625–633. [Google Scholar] [CrossRef]
- Yang, F.; Chen, G.; Ma, M.; Qiu, N.; Zhu, L.; Li, J. Fatty acids modulate the expression levels of key proteins for cholesterol absorption in Caco-2 monolayer. Lipids Health Dis. 2018, 17, 32. [Google Scholar] [CrossRef]
- Taheri, S.E.H.; Bazargan, M.; Vosough, P.R.; Sadeghian, A. A Comprehensive Insight into Peanut: Chemical Structure of Compositions, Oxidation Process, and Storage Conditions. J. Food Compos. Anal. 2023, 125, 105770. [Google Scholar] [CrossRef]
- Ghanavati, M.; Rahmani, J.; Clark, C.C.; Hosseinabadi, S.M.; Rahimlou, M. Pistachios and cardiometabolic risk factors: A systematic review and meta-analysis of randomized controlled clinical trials. Complement. Ther. Med. 2020, 52, 102513. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D.; Taylor, A.M.; Swanson, K.S.; Novotny, J.A.; Baer, D.J. Almond consumption and processing affects the composition of the gastrointestinal microbiota of healthy adult men and women: A randomized controlled trial. Nutrients 2018, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, J.; Li, Z.; Ortiz, R.M. Almond snacking for 8 wk increases alpha-diversity of the gastrointestinal microbiome and decreases Bacteroides fragilis abundance compared with an isocaloric snack in college freshmen. Curr. Dev. Nutr. 2019, 3, nzz079. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 20 September 2020).
- WHO. Raised Cholesterol. 2019. Available online: https://www.who.int/gho/ncd/risk_factors/cholesterol_text/en/ (accessed on 3 October 2020).
- WHO. Noncommunicable Diseases. 2021. Available online: https://www.who.int/health-topics/noncommunicable-diseases#tab=tab_1 (accessed on 28 October 2023).
- Finicelli, M.; Squillaro, T.; Di Cristo, F.; Di Salle, A.; Melone, M.A.B.; Galderisi, U.; Peluso, G. Metabolic syndrome, Mediterranean diet, and polyphenols: Evidence and perspectives. J. Cell. Physiol. 2019, 234, 5807–5826. [Google Scholar] [CrossRef] [PubMed]
- Dabke, K.; Hendrick, G.; Devkota, S. The gut microbiome and metabolic syndrome. J. Clin. Investig. 2019, 129, 4050–4057. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.M.; Agostini-Costa, T.d.S.; Gimenes, M.A.; Silveira, D. Chemical composition and biological activities of Arachis species. J. Agric. Food Chem. 2011, 59, 4321–4330. [Google Scholar] [CrossRef]
- Suchoszek-Łukaniuk, K.; Jaromin, A.; Korycińska, M.; Kozubek, A. Health benefits of peanut (Arachis hypogaea L.) seeds and peanut oil consumption. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2011; pp. 873–880. [Google Scholar]
- U.S.D.A. (United States Department of Agriculture). Agriculture Research Service. National Nutrient Database for Standard Reference Release Full Repots (All Nutrients). 2016. Available online: http://ndb.nal.usda.gov/ndb/food?fgcd=&manu=&lfacet=&count=&max=35&sort=&qlookup=peanut&offset=&format=Full&new=&measureby= (accessed on 20 January 2022).
- F.A.O. (Food and Agriculture Organization of the United Nations). Statistical Databases. Available online: https://www.fao.org/food-agriculture-statistics/en/ (accessed on 22 January 2023).
- FAS-USDA. Peanut 2023 World Production: 50,411 (1000 MT). Available online: https://ipad.fas.usda.gov/cropexplorer/cropview/commodityView.aspx?cropid=2221000 (accessed on 5 December 2023).
- Higgs, J. The beneficial role of peanuts in the diet–an update and rethink! Peanuts and their role in CHD. Nutr. Food Sci. 2002, 32, 214–218. [Google Scholar] [CrossRef]
- Guimón, J.; Guimón, P. How ready-to-use therapeutic food shapes a new technological regime to treat child malnutrition. Technol. Forecast. Soc. Chang. 2012, 79, 1319–1327. [Google Scholar] [CrossRef]
- Prasad, P.; Kochhar, A. Nutritional intervention to combat malnutrition among children under the age of five: A review. Int. J. Health Sci. Res. 2015, 5, 374–380. [Google Scholar]
- U.S. Department of Agriculture (U.S.D.A.). Agricultural Research Service. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170147/nutrients (accessed on 4 April 2021).
- Ejigui, J.; Savoie, L.; Marin, J.; Desrosiers, T. Influence of traditional processing methods on the nutritional composition and antinutritional factors of red peanuts (Arachis hypogea) and small red kidney beans (Phaseolus vulgaris). J. Biol. Sci. 2005, 5, 597–605. [Google Scholar]
- Jonnala, R.S.; Dunford, N.T.; Chenault, K. Nutritional composition of genetically modified peanut varieties. J. Food Sci. 2005, 70, S254–S256. [Google Scholar] [CrossRef]
- Campos-Mondragón, M.; De La Barca, A.C.; Durán-Prado, A.; Campos-Reyes, L.; Oliart-Ros, R.; Ortega-García, J.; Medina-Juárez, L.; Angulo, O. Nutritional composition of new peanut (Arachis hypogaea L.) cultivars. Grasas Aceites 2009, 60, 161–167. [Google Scholar]
- Lee, G.Y.; Han, S.N. The role of vitamin E in immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 26. Nutrient Data Laboratory Home Page. 2013. Available online: http://www.ars.usda.gov/ba/bhnrc/ndl (accessed on 2 October 2023).
- Harmankaya, M.; Özcan, M.M.; Al Juhaimi, F. Mineral contents and proximate composition of Pistacia vera kernels. Environ. Monit. Assess. 2014, 186, 4217–4221. [Google Scholar] [CrossRef] [PubMed]
- Tsantili, E.; Konstantinidis, K.; Christopoulos, M.; Roussos, P. Total phenolics and flavonoids and total antioxidant capacity in pistachio (Pistachia vera L.) nuts in relation to cultivars and storage conditions. Sci. Hortic. 2011, 129, 694–701. [Google Scholar] [CrossRef]
- Shakerardekani, A.; Karim, R.; Vaseli, N. The effect of processing variables on the quality and acceptability of pistachio milk. J. Food Process. Preserv. 2013, 37, 541–545. [Google Scholar] [CrossRef]
- F.D.A. (Food and Drug Administration). Food Labeling. Revision of the Nutrition and Supplement Facts Labels; Food and Drug Administration: Silver Spring, MD, USA, 2016.
- E.U.; EuroCommerce; Food and Drink Europe. Guidance on the Provision of Food Information to Consumers; Regulation (E.U.) No. 1169/2011; Food and Drink Europe: Brussels, Belgium, 2013. [Google Scholar]
- U.S.D.A. (U.S. Department of Agriculture). Agricultural Research Service. FoodData Central. 2022. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170184/nutrients (accessed on 7 February 2022).
- Tomaino, A.; Martorana, M.; Arcoraci, T.; Monteleone, D.; Giovinazzo, C.; Saija, A. Antioxidant activity and phenolic profile of pistachio (Pistacia vera L., variety Bronte) seeds and skins. Biochimie 2010, 92, 1115–1122. [Google Scholar] [CrossRef]
- NDT; Nutrient Data Laboratory; Beltsville Human Nutrition Research Center Agricultural Research Service. U.S.D.A. Database for the Flavonoid Content of Selected Foods Release 3.3; U.S. Department of Agriculture: Washington, DC, USA, 2018.
- Barbour, J.A.; Howe, P.R.; Buckley, J.D.; Wright, G.C.; Bryan, J.; Coates, A.M. Lower energy intake following consumption of Hi-oleic and regular peanuts compared with iso-energetic consumption of potato crisps. Appetite 2014, 82, 124–130. [Google Scholar] [CrossRef]
- Wang, M.L.; Khera, P.; Pandey, M.K.; Wang, H.; Qiao, L.; Feng, S.; Tonnis, B.; Barkley, N.A.; Pinnow, D.; Holbrook, C.C. Genetic mapping of QTLs controlling fatty acids provided insights into the genetic control of fatty acid synthesis pathway in peanut (Arachis hypogaea L.). PLoS ONE 2015, 10, e0119454. [Google Scholar] [CrossRef]
- Pandey, M.K.; Wang, M.L.; Qiao, L.; Feng, S.; Khera, P.; Wang, H.; Tonnis, B.; Barkley, N.A.; Wang, J.; Holbrook, C.C. Identification of QTLs associated with oil content and mapping FAD2 genes and their relative contribution to oil quality in peanut (Arachis hypogaea L.). BMC Genet. 2014, 15, 133. [Google Scholar] [CrossRef]
- Yamaki, T.; Nagamine, I.; Fukumoto, K.; Yano, T.; Miyahara, M.; Sakurai, H. High oleic peanut oil modulates promotion stage in lung tumorigenesis of mice treated with methyl nitrosourea. Food Sci. Technol. Res. 2005, 11, 231–235. [Google Scholar] [CrossRef]
- Waitzberg, D.L. Nutrição Oral, Enteral e Parenteral na Prática Clínica, 5th ed.; Editora Atheneu: Rio de Janeiro, Brazil, 2017. [Google Scholar]
- Batal, A.; Dale, N.; Café, M. Nutrient composition of peanut meal. J. Appl. Poult. Res. 2005, 14, 254–257. [Google Scholar] [CrossRef]
- King, J.C.; Blumberg, J.; Ingwersen, L.; Jenab, M.; Tucker, K.L. Tree nuts and peanuts as components of a healthy diet. J. Nutr. 2008, 138, 1736S–1740S. [Google Scholar] [CrossRef] [PubMed]
- Abdualrahman, M.A.Y. Chemical, In-vitro Protein Digestibility, Minerals and Amino Acids Composition of Edible Peanut Seeds (Arachis hypogaea L.). Sci. Int. 2013, 1, 199–202. [Google Scholar]
- Mukhopadhyay, S.; Panda, P.K.; Behera, B.; Das, C.K.; Hassan, M.K.; Das, D.N.; Sinha, N.; Bissoyi, A.; Pramanik, K.; Maiti, T.K. In vitro and in vivo antitumor effects of Peanut agglutinin through induction of apoptotic and autophagic cell death. Food Chem. Toxicol. 2014, 64, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Inoue, N.; Shimizu-Ibuka, A.; Tadaishi, M.; Takita, T.; Arai, S.; Mura, K. Serum cholesterol reduction by feeding a high-cholesterol diet containing a lower-molecular-weight polyphenol fraction from peanut skin. Biosci. Biotechnol. Biochem. 2012, 76, 834–837. [Google Scholar] [CrossRef]
- Parilli-Moser, I.; Hurtado-Barroso, S.; Guasch-Ferré, M.; Lamuela-Raventós, R.M. Effect of Peanut Consumption on Cardiovascular Risk Factors: A Randomized Clinical Trial and Meta-Analysis. Front. Nutr. 2022, 9, 853378. [Google Scholar] [CrossRef]
- Mingrou, L.; Guo, S.; Ho, C.T.; Bai, N. Review on chemical compositions and biological activities of peanut (Arachis hypogeae L.). J. Food Biochem. 2022, 46, e14119. [Google Scholar] [CrossRef]
- Musa-Veloso, K.; Paulionis, L.; Poon, T.; Lee, H.Y. The effects of almond consumption on fasting blood lipid levels: A systematic review and meta-analysis of randomised controlled trials. J. Nutr. Sci. 2016, 5, e34. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, A.; Wang, Q.; Zhou, J. High oleic acid peanut oil and extra virgin olive oil supplementation attenuate metabolic syndrome in rats by modulating the gut microbiota. Nutrients 2019, 11, 3005. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Wu, Q.; Osada, H.; Yoshida, M.; Pan, W.; Qi, J. Peanut skin extract ameliorates the symptoms of type 2 diabetes mellitus in mice by alleviating inflammation and maintaining gut microbiota homeostasis. Aging 2020, 12, 13991. [Google Scholar] [CrossRef]
- Bimro, E.T.; Hovav, R.; Nyska, A.; Glazer, T.A.; Madar, Z. High oleic peanuts improve parameters leading to fatty liver development and change the microbiota in mice intestine. Food Nutr. Res. 2020, 64, 4278. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ding, H.; Liu, Q.; Wei, Y.; Zhang, Y.; Wang, Y.; Lu, Y.; Ma, A.; Li, Z.; Hu, Y. Effects of peanut meal extracts fermented by Bacillus natto on the growth performance, learning and memory skills and gut microbiota modulation in mice. Br. J. Nutr. 2020, 123, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Lv, C.; Wang, H.; Lu, Q.; Ye, M.; Zhu, X.; Liu, R. Peanut skin extract ameliorates high-fat diet-induced atherosclerosis by regulating lipid metabolism, inflammation reaction and gut microbiota in ApoE−/− mice. Food Res. Int. 2022, 154, 111014. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, L.; Wang, D.; Huang, M.; Zhao, J.; Malik, V.; Liu, X.; Sun, L.; Lin, X.; Chen, Y. Gut microbiota composition is associated with responses to peanut intervention in multiple parameters among adults with metabolic syndrome risk. Mol. Nutr. Food Res. 2021, 65, 2001051. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, L.; Liu, H.; Shen, J.; Zhang, Y.; Lu, L.; Zhang, X.; Ma, X. Bacillus subtilis M6 improves intestinal barrier, antioxidant capacity and gut microbial composition in A.A. broiler. Front. Nutr. 2022, 9, 965310. [Google Scholar] [CrossRef]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 2019, 26, 222–235.e225. [Google Scholar] [CrossRef]
- Kverka, M.; Zakostelska, Z.; Klimesova, K.; Sokol, D.; Hudcovic, T.; Hrncir, T.; Rossmann, P.; Mrazek, J.; Kopecny, J.; Verdu, E. Oral administration of Parabacteroides distasonis antigens attenuates experimental murine colitis through modulation of immunity and microbiota composition. Clin. Exp. Immunol. 2011, 163, 250–259. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Santos-Marcos, J.A.; Perez-Jimenez, F.; Camargo, A. The role of diet and intestinal microbiota in the development of metabolic syndrome. J. Nutr. Biochem. 2019, 70, 1–27. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.-S. The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients 2016, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The firmicutes/bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Wang, Y.; Zhang, Q.; Zou, R.; Guo, M.; Zheng, H. Characteristics of gut microbiota in people with obesity. PLoS ONE 2021, 16, e0255446. [Google Scholar] [CrossRef] [PubMed]
- Salguero, M.V.; Al-Obaide, M.A.; Singh, R.; Siepmann, T.; Vasylyeva, T.L. Dysbiosis of Gram-negative gut microbiota and the associated serum lipopolysaccharide exacerbates inflammation in type 2 diabetic patients with chronic kidney disease. Exp. Ther. Med. 2019, 18, 3461–3469. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, Q.; Dong, Z.; Yan, Y.; Fu, Y.; Liu, X.; Zhao, B.; Duan, X. Phosphatidylcholine ameliorates lps-induced systemic inflammation and cognitive impairments via mediating the gut–brain axis balance. J. Agric. Food Chem. 2020, 68, 14884–14895. [Google Scholar] [CrossRef]
- Getachew, B.; Aubee, J.I.; Schottenfeld, R.S.; Csoka, A.B.; Thompson, K.M.; Tizabi, Y. Ketamine interactions with gut-microbiota in rats: Relevance to its antidepressant and anti-inflammatory properties. BMC Microbiol. 2018, 18, 222. [Google Scholar] [CrossRef]
- Płóciennikowska, A.; Hromada-Judycka, A.; Borzęcka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Casas-Agustench, P.; Salas-Huetos, A. Cultural and historical aspects of Mediterranean nuts with emphasis on their attributed healthy and nutritional properties. Nutr. Metab. Cardiovasc. Dis. 2011, 21, S1–S6. [Google Scholar] [CrossRef]
- Dreher, M.L. Pistachio nuts: Composition and potential health benefits. Nutr. Rev. 2012, 70, 234–240. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Database of the Food and Agriculture Organization of the United Nations. 2016. Available online: http://faostat.fao.org/ (accessed on 5 July 2022).
- Shakerardekani, A.; Karim, R.; Ghazali, H.M.; Chin, N.L. Development of pistachio (Pistacia vera L.) spread. J. Food Sci. 2013, 78, S484–S489. [Google Scholar] [CrossRef]
- Bailey, H.M.; Stein, H.H. Raw and roasted pistachio nuts (Pistacia vera L.) are ‘good’sources of protein based on their digestible indispensable amino acid score as determined in pigs. J. Sci. Food Agric. 2020, 100, 3878–3885. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, M.; Fallico, B. Anthocyanins, chlorophylls and xanthophylls in pistachio nuts (Pistacia vera) of different geographic origin. J. Food Compos. Anal. 2007, 20, 352–359. [Google Scholar] [CrossRef]
- Rabadán, A.; Gallardo-Guerrero, L.; Gandul-Rojas, B.; Álvarez-Ortí, M.; Pardo, J.E. Effect of roasting conditions on pigment composition and some quality parameters of pistachio oil. Food Chem. 2018, 264, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Pumilia, G.; Cichon, M.J.; Cooperstone, J.L.; Giuffrida, D.; Dugo, G.; Schwartz, S.J. Changes in chlorophylls, chlorophyll degradation products and lutein in pistachio kernels (Pistacia vera L.) during roasting. Food Res. Int. 2014, 65, 193–198. [Google Scholar] [CrossRef]
- Mateos, R.; Salvador, M.D.; Fregapane, G.; Goya, L. Why Should Pistachio Be a Regular Food in Our Diet? Nutrients 2022, 14, 3207. [Google Scholar] [CrossRef]
- Hernández-Alonso, P.; Cañueto, D.; Giardina, S.; Salas-Salvadó, J.; Cañellas, N.; Correig, X.; Bulló, M. Effect of pistachio consumption on the modulation of urinary gut microbiota-related metabolites in prediabetic subjects. J. Nutr. Biochem. 2017, 45, 48–53. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Carlsen, M.H.; Phillips, K.M.; Bøhn, S.K.; Holte, K.; Jacobs, D.R., Jr.; Blomhoff, R. Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States. Am. J. Clin. Nutr. 2006, 84, 95–135. [Google Scholar] [CrossRef]
- Yanni, A.E.; Mitropoulou, G.; Prapa, I.; Agrogiannis, G.; Kostomitsopoulos, N.; Bezirtzoglou, E.; Kourkoutas, Y.; Karathanos, V.T. Functional modulation of gut microbiota in diabetic rats following dietary intervention with pistachio nuts (Pistacia vera L.). Metab. Open 2020, 7, 100040. [Google Scholar] [CrossRef]
- Terzo, S.; Mulè, F.; Caldara, G.F.; Baldassano, S.; Puleio, R.; Vitale, M.; Cassata, G.; Ferrantelli, V.; Amato, A. Pistachio consumption alleviates inflammation and improves gut microbiota composition in mice fed a high-fat diet. Int. J. Mol. Sci. 2020, 21, 365. [Google Scholar] [CrossRef]
- Ukhanova, M.; Wang, X.; Baer, D.J.; Novotny, J.A.; Fredborg, M.; Mai, V. Effects of almond and pistachio consumption on gut microbiota composition in a randomised cross-over human feeding study. Br. J. Nutr. 2014, 111, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Bisignano, C.; Filocamo, A.; Chessa, S.; Sarò, M.; Torre, G.; Faulks, R.M.; Dugo, P. Bioaccessibility of pistachio polyphenols, xanthophylls, and tocopherols during simulated human digestion. Nutrition 2013, 29, 338–344. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Y.; Wang, X.; Kong, L.; Johnston, L.J.; Lu, L.; Ma, X. Dietary nutrients shape gut microbes and intestinal mucosa via epigenetic modifications. Crit. Rev. Food Sci. Nutr. 2022, 62, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Valdés, L.; Cuervo, A.; Salazar, N.; Ruas-Madiedo, P.; Gueimonde, M.; González, S. The relationship between phenolic compounds from diet and microbiota: Impact on human health. Food Funct. 2015, 6, 2424–2439. [Google Scholar] [CrossRef] [PubMed]
- Lamuel-Raventos, R.M.; Onge, M.-P.S. Prebiotic nut compounds and human microbiota. Crit. Rev. Food Sci. Nutr. 2017, 57, 3154–3163. [Google Scholar] [CrossRef]
- Kay, C.D.; Gebauer, S.K.; West, S.G.; Kris-Etherton, P.M. Pistachios increase serum antioxidants and lower serum oxidized-LDL in hypercholesterolemic adults. J. Nutr. 2010, 140, 1093–1098. [Google Scholar] [CrossRef]
- Kocyigit, A.; Koylu, A.; Keles, H. Effects of pistachio nuts consumption on plasma lipid profile and oxidative status in healthy volunteers. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 202–209. [Google Scholar] [CrossRef]
- Sari, I.; Baltaci, Y.; Bagci, C.; Davutoglu, V.; Erel, O.; Celik, H.; Ozer, O.; Aksoy, N.; Aksoy, M. Effect of pistachio diet on lipid parameters, endothelial function, inflammation, and oxidative status: A prospective study. Nutrition 2010, 26, 399–404. [Google Scholar] [CrossRef]
- Gulati, S.; Misra, A.; Pandey, R.M.; Bhatt, S.P.; Saluja, S. Effects of pistachio nuts on body composition, metabolic, inflammatory and oxidative stress parameters in Asian Indians with metabolic syndrome: A 24-wk, randomized control trial. Nutrition 2014, 30, 192–197. [Google Scholar] [CrossRef]
- Parham, M.; Heidari, S.; Khorramirad, A.; Hozoori, M.; Hosseinzadeh, F.; Bakhtyari, L.; Vafaeimanesh, J. Effects of pistachio nut supplementation on blood glucose in patients with type 2 diabetes: A randomized cross-over trial. Rev. Diabet. Stud. RDS 2014, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.; West, S.; Augustin, L.; Esfahani, A.; Vidgen, E.; Bashyam, B.; Sauder, K.; Campbell, J.; Chiavaroli, L.; Jenkins, A. Acute effects of pistachio consumption on glucose and insulin, satiety hormones and endothelial function in the metabolic syndrome. Eur. J. Clin. Nutr. 2014, 68, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Mayo. Nuts and Your Heart: Eating Nuts for Heart Health. Available online: https://www.mayoclinic.org/diseases-conditions/heart-disease/in-depth/nuts/art-20046635 (accessed on 1 November 2023).
- Zabor, E.C.; Kaizer, A.M.; Hobbs, B.P. Randomized controlled trials. Chest 2020, 158, S79–S87. [Google Scholar] [CrossRef] [PubMed]
- Alamout, M.M.; Rahmanian, M.; Aghamohammadi, V.; Mohammadi, E.; Nasiri, K. Effectiveness of mindfulness based cognitive therapy on weight loss, improvement of hypertension and attentional bias to eating cues in overweight people. Int. J. Nurs. Sci. 2020, 7, 35–40. [Google Scholar] [CrossRef]
- Evans, M.; Lewis, E.D.; Antony, J.M.; Crowley, D.C.; Guthrie, N.; Blumberg, J.B. Breaking new frontiers: Assessment and re-evaluation of clinical trial design for nutraceuticals. Front. Nutr. 2022, 9, 958753. [Google Scholar] [CrossRef]
- Uzbay, T. Germ-free animal experiments in the gut microbiota studies. Curr. Opin. Pharmacol. 2019, 49, 6–10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of M.D.P.I. and/or the editor(s). M.D.P.I. and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |



| Components | Peanut [32,38,42,43] | Pistachio [44,45,46,47,48,49,50,51] |
|---|---|---|
| Moisture (g/100 g) | 6 | 4.5 |
| Ash (g/100 g) | 2 | 2.8 |
| Protein (g/100 g) | 25 | 20.4 |
| Lipid (g/100 g) | 50 | 47.4 |
| Dietary fiber (g/100 g) | 8 | 10 |
| Carbohydrate (g/100 g) | 20 | 14 |
| Energy (kcal g/100g) | 674 | 620 |
| Potassium (mg/100 g) | 690 | 1020 |
| Phosphorus (mg/100 g) | 380 | 490 |
| Magnesium (mg/100 g) | 171 | 121 |
| Calcium (mg/100 g) | 89 | 23 |
| Sodium (mg/100 g) | 10 | |
| Zinc (mg/100 g) | 4.33 | 2.20 |
| Iron (mg/100 g) | 2.55 | 3.92 |
| Manganese (mg/100 g) | 1.69 | - |
| Saturated fatty acids (g/100 g) | 6 | 6 |
| Monounsaturated fatty acids (g/100 g) | 25 | 23 |
| Polyunsaturated fatty acids (g/100 g) | 15 | 14 |
| Tocopherols (mg/100 g) | 8.33 | 20.6 |
| Zeaxanthin (mg/100 g) | - | 2.9 |
| Beta-carotene (mg/100 g) | - | 0.305 |
| Total phenolic compounds (μmol TE/g DW) | 16.2 | 1677 |
| Food Intervention | Type of Study | Model | Main Results | References |
|---|---|---|---|---|
| High Oleic Acid Peanut Oil (HOPO) | Animal | Male Sprague Dawley rats fed for 12 weeks with 10% HOPO plus a high-fat diet and water containing 10% fructose | Overall results: ↑ insulin sensitivity. ↓ liver TG, fat accumulation; plasma fasting insulin, HOMA-IR, TC, TG, and LDL levels. Gut microbiota results: ↑ Family level: Clostridiaceae_1, Anaeroplasmataceae, Bifidobacteriaceae, Erysipelotrichaceae, and Planococcaceae; genus level: Olsenella, Peptoclostridium, Ruminococcaceae_UCG-009, Weissella, Bifidobacterium, [Eubacterium]_fissicatena_group, [Eubacterium]_coprostanoligenes_group, Ruminococcaceae_NK4A214_group, Clostridium_sensu_stricto_1, Ruminococcaceae_UCG-014, and Faecalibaculum. ↓ Family level: Lachnospiraceae, Micrococcaceae, Streptococcaceae, and Bacteroidaceae; genus level: Bilophila, Leuconostoc, [Eubacterium]_nodatum_group, Lactococcus, uncultured_bacterium_f_Coriobacteriaceae, Streptococcus, Rothia, [Ruminococcus]_torques_group, Bacteroides, Lachnoclostridium, and Blautia. | Zhao, et al. [65] |
| Peanut skin extract (PSE) with doses of 10, 80, and 160 mg/kg per day for 6 weeks | Animal | Mice with type 2 diabetes mellitus (T2DM) induced by high-fat diet for 4 months until the mice presented >7 mmol/L blood glucose concentration, obesity, polydipsia, polyphagia, and polyuria. | Overall results: ↑ glucose tolerance and insulin sensitivity. ↓ fasting blood glucose; liver, epididymal fat, heart, pancreas, and kidney weights; plasma TG and TC; pro-inflammatory cytokines in plasma and gene expression levels in adipose tissue; and LPS in the blood. Gut microbiota results: ↑ Cyanobacteria phyla. ↓ Bacillota to Bacteroidota ratio; Bifidobacterium pseudolc and Parabacteroides distasor; and Mucispirillum at the genus level. Actinomycetota and Ruminococcaceae-6 were not detected. | Xiang, et al. [66] |
| High oleic peanut (D7) and peanut cv. Hanoch (HN) | Animal | Mice (male C57BL/6J) fed for 10 weeks with normal and high-fat diets plus peanut (4%) | Overall results: ↑ plasma fasting glucose in HN; plasma TC and HDL in peanut groups; n-6/n-3 in liver tissue in peanut groups; and Srebp1C, PPARα, TNF, and iNOS gene expression in D7-group. ↓ AUC in peanut groups; plasma fatty acid, plasma TG, lipid fatty accumulation, and TG in the liver in D7-group. Gut microbiota results: ↑ diversity of bacteria in D7-group; Prevotella in D7-group; and Bacillota phyla in D7-group. ↓ Pseudomonadota (former Proteobacteria); Deferribateres; Verrucomicrobia; Bacillota/Bacteroidota ratio in peanut groups; and Bacteriodetes phyla in D7-group. | Bimro, et al. [67] |
| Peanut meal fermented by Bacillus natto with doses of 0, 0.3, 1.5, and 7.5 g/kg per day | Animal | Male Kunming mice (n = 90) fed by gavage of 0.1 mL/g body weight per day. | Overall results: ↑ better growth and development; enhancement of learning and memory capacity; preventive role in antibiotic-induced dysbacteriosis; increased richness; and uniformity of the gut microbiota. Gut microbiota results: ↑ Bacteroidota; Deferribacteres; Bacillota; Pseudomonadota; and Tenericutes. ↓ Rikenellaceae_RC9_gut_group; Peptoclostridium; Escherichia-Shigella; Lachnospiraceae_UCG-001; Parasutterella; Helicobacter; Enterobacter; Parabacteroides; Lachnospiraceae_NK4A136_group; and Bacteroides. | Jiang, et al. [68] |
| Peanut skin extract (PSE) with doses of 150 and 300 mg/kg per day for 12 weeks | Animal | ApoE−/− mice (C57BL/6J) fed 10% fat kcal per day | Overall results: ↑ HDL-c content and IL-10 anti-inflammatory cytokine. ↓ plasma TC; LDL-c content; and pro-inflammatory cytokines TNF and IL-6. Gut microbiota results: ↑ Roseburia, Rothia, Parabacteroides, and Akkermansia ↓ Bilophila and Alistipes. | Xu, et al. [69] |
| 56 g/day of peanuts divided into two portions: one packet within 1 h before lunch and one packet within 1 h before dinner | Human | Participants (n = 209) with central obesity and at least one other risk factor for MetS from a 12-week randomized clinical trial | Overall results: ↓ body weight; waist circumference, and fasting blood glucose. Gut microbiota results: ↓ Bilophila; Coprococcus_3; and Dorea. | Wang, et al. [70] |
| Food Intervention | Type of Study | Model | Main Results | References |
|---|---|---|---|---|
| 8.5 g/100 g whole and fresh pistachio diet, including skin, except the shell. Fixed amount daily in the morning. | Animal | Male Wistar rats with T1DM induced with streptozotocin solution (40 mg/kg) (diabetic) and healthy animals. Duration 4 weeks. | Overall results: Pistachio did not affect body weight or the plasma lipid profile. Gut microbiota results: ↑ bifidobacterial counts in fecal, jejunum, ileum, and caecum microbiota for healthy and diabetic rats; bifidobacterial counts in colon for healthy rats; lactobacilli count in fecal, ilium, and caecum microbiota for healthy and diabetic rats; Turicibacter and Lactobacillus genera in fecal microbiota for healthy rats; Bifidobacterium in fecal microbiota for diabetic rats; and Romboutsia levels for fecal microbiota for healthy and diabetic rats. ↓ lactobacilli count in colon microbiota for diabetic rats; enterococci counts in fecal, jejunum, cecum, and colon microbiota; E. coli population in fecal and colon microbiota of diabetic rats; E. coli population in jejunum and caecum microbiota for healthy and diabetic rats; Enterobacteriacae in ileum and cecum microbiota for healthy and diabetic rats; Enterobacteriacae and coliforms in jejunum microbiota for diabetic rats; Coliforms in ilium microbiota for healthy and diabetic rats. | Yanni, et al. [96] |
| Hyperlipidic diet with 20% of caloric intake replaced by pistachios (180 g/kg HFD) for 16 weeks | Animal | Mice (male C57BL/6J) fed for 4 weeks with a normal and high-fat diet | Overall results: ↓ TNF- α; IL-1β; number and area of adipocytes, crown-like structure density, IL-1β, TNF-α, CCL-2 mRNA expression levels; liver: IL-1β e CCL-2. Gut microbiota results: ↑ Genus level: Parabacteroides, Dorea, Allobaculum, Turicibacter, Lactobacillus, and Anaeroplasma; ↓ Ratio Bacillota/Bacteirodetes; genus level: Oscillospira, Desulfovibrio, Coprobacillus, and Bilophila | Terzo, et al. [97] |
| Three treatment groups: (1) no nuts; (2) 1.5 servings/day of almonds or pistachios; (3) 3 servings/day of almonds or pistachios. | Human | Volunteers (n = 16) were recruited to participate in two separate randomized, controlled, cross-over studies with three 18-day feeding periods separated by an elimination period of at least 2 weeks. | Gut microbiota results: ↑ Butyrate-producing bacteria. Bifidobacterial; α-diversity; proportions of the main phyla; and numbers of lactic acid bacteria and bifidobacteria were not affected. | Ukhanova, et al. [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, S.B.; Oliveira Filho, J.G.d.; Salgaço, M.K.; Jesus, M.H.D.; Egea, M.B. Effects of Peanuts and Pistachios on Gut Microbiota and Metabolic Syndrome: A Review. Foods 2023, 12, 4440. https://doi.org/10.3390/foods12244440
Campos SB, Oliveira Filho JGd, Salgaço MK, Jesus MHD, Egea MB. Effects of Peanuts and Pistachios on Gut Microbiota and Metabolic Syndrome: A Review. Foods. 2023; 12(24):4440. https://doi.org/10.3390/foods12244440
Chicago/Turabian StyleCampos, Stéphani Borges, Josemar Gonçalves de Oliveira Filho, Mateus Kawata Salgaço, Marisa Helena De Jesus, and Mariana Buranelo Egea. 2023. "Effects of Peanuts and Pistachios on Gut Microbiota and Metabolic Syndrome: A Review" Foods 12, no. 24: 4440. https://doi.org/10.3390/foods12244440
APA StyleCampos, S. B., Oliveira Filho, J. G. d., Salgaço, M. K., Jesus, M. H. D., & Egea, M. B. (2023). Effects of Peanuts and Pistachios on Gut Microbiota and Metabolic Syndrome: A Review. Foods, 12(24), 4440. https://doi.org/10.3390/foods12244440










