A Comparative Study of Skimmed Milk and Cassava Flour on the Viability of Freeze-Dried Lactic Acid Bacteria as Starter Cultures for Yogurt Fermentation
Abstract
:1. Introduction
2. Materials and Methods
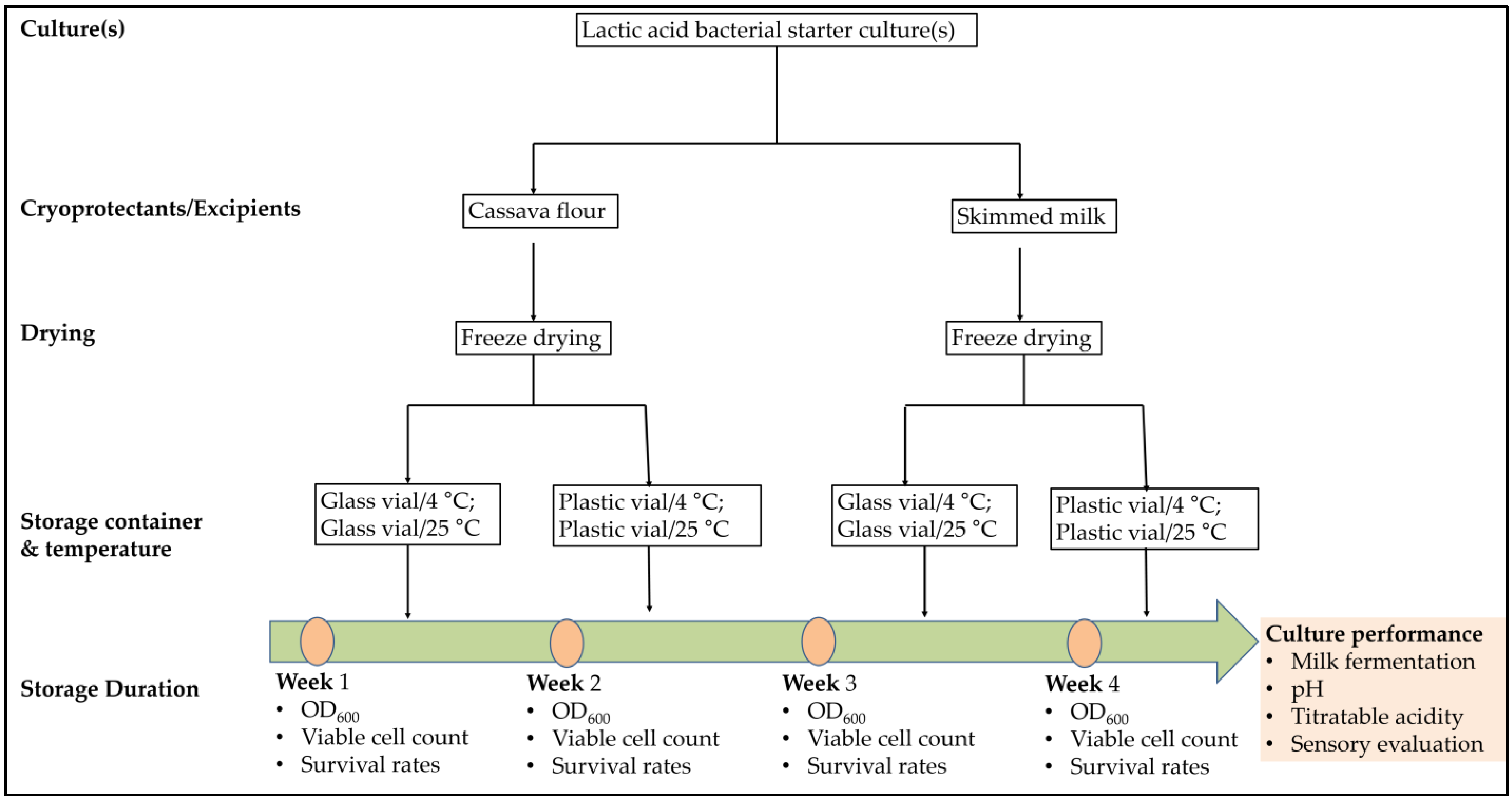
2.1. Study Design
2.2. Lactic Acid Bacterial Strains
2.3. Preparation of Cultures for Freeze Drying
2.4. Preparation of Freeze-Drying Career Materials
2.5. Freeze-Drying Procedure
2.6. Storage Conditions for Freeze-Dried Cultures
2.7. Viability Assays
2.7.1. Preparation of Resuscitation Media
2.7.2. Measurement of Optical Density (OD)/Viable Cell Counting
2.7.3. Determination of Survival Rates
2.8. Performance Evaluation of Freeze-Dried Culture in Milk Fermentation
Inoculation of Cow Milk Samples and Fermentation
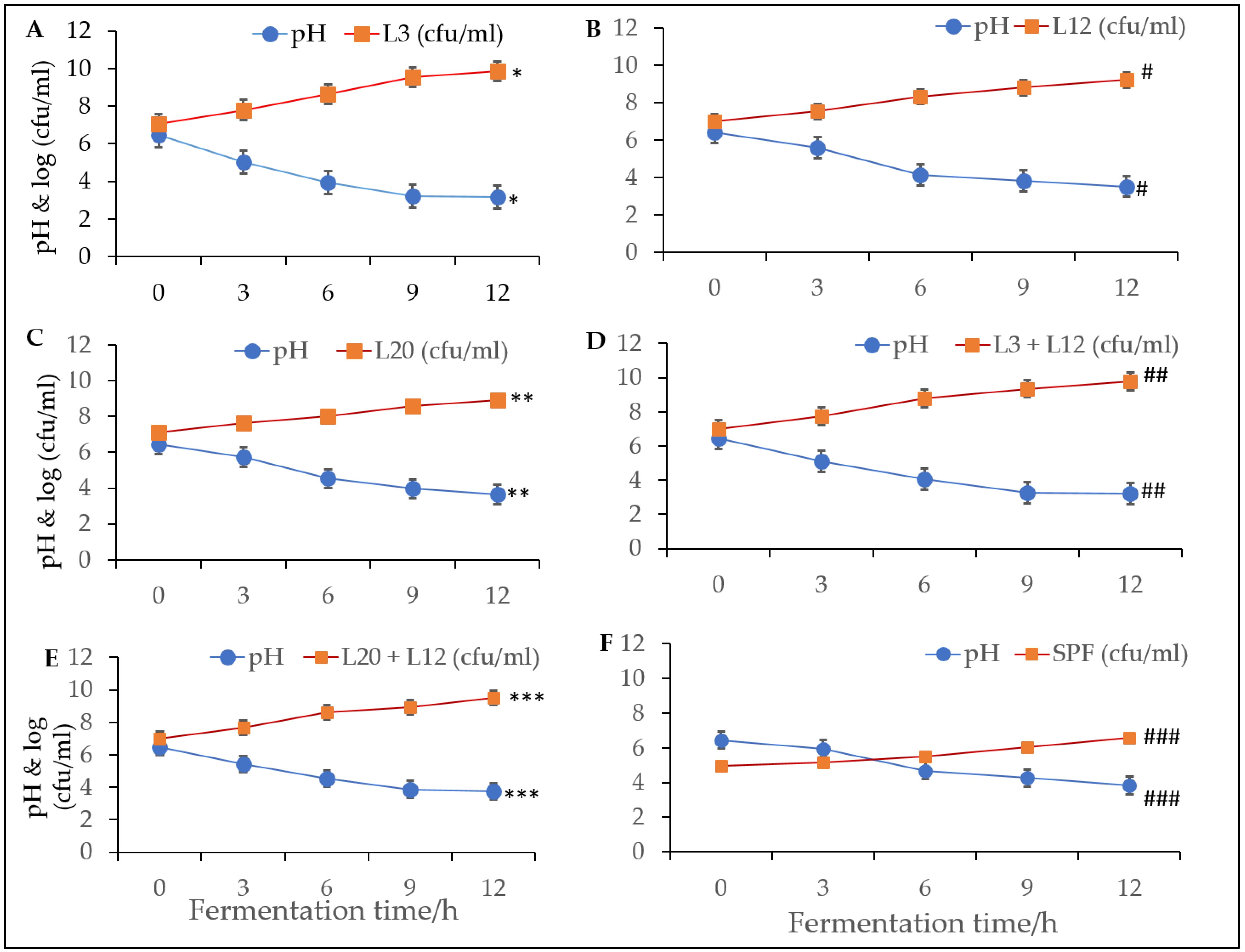
2.9. Bacterial Growth and Acidification of Milk
2.10. Consumer Sensory Evaluation of Fermented Milk
2.11. Statistical Analysis
3. Results
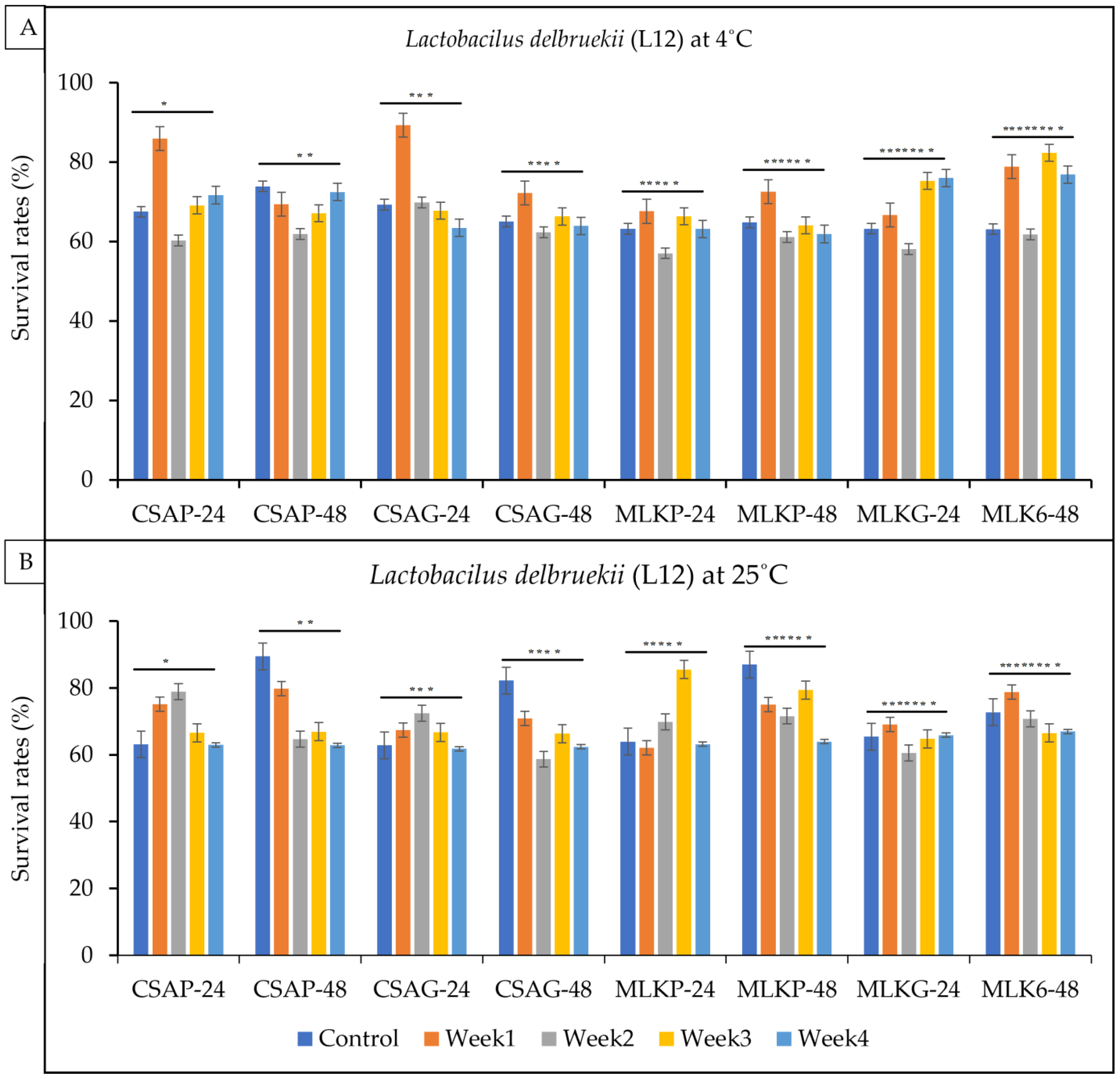
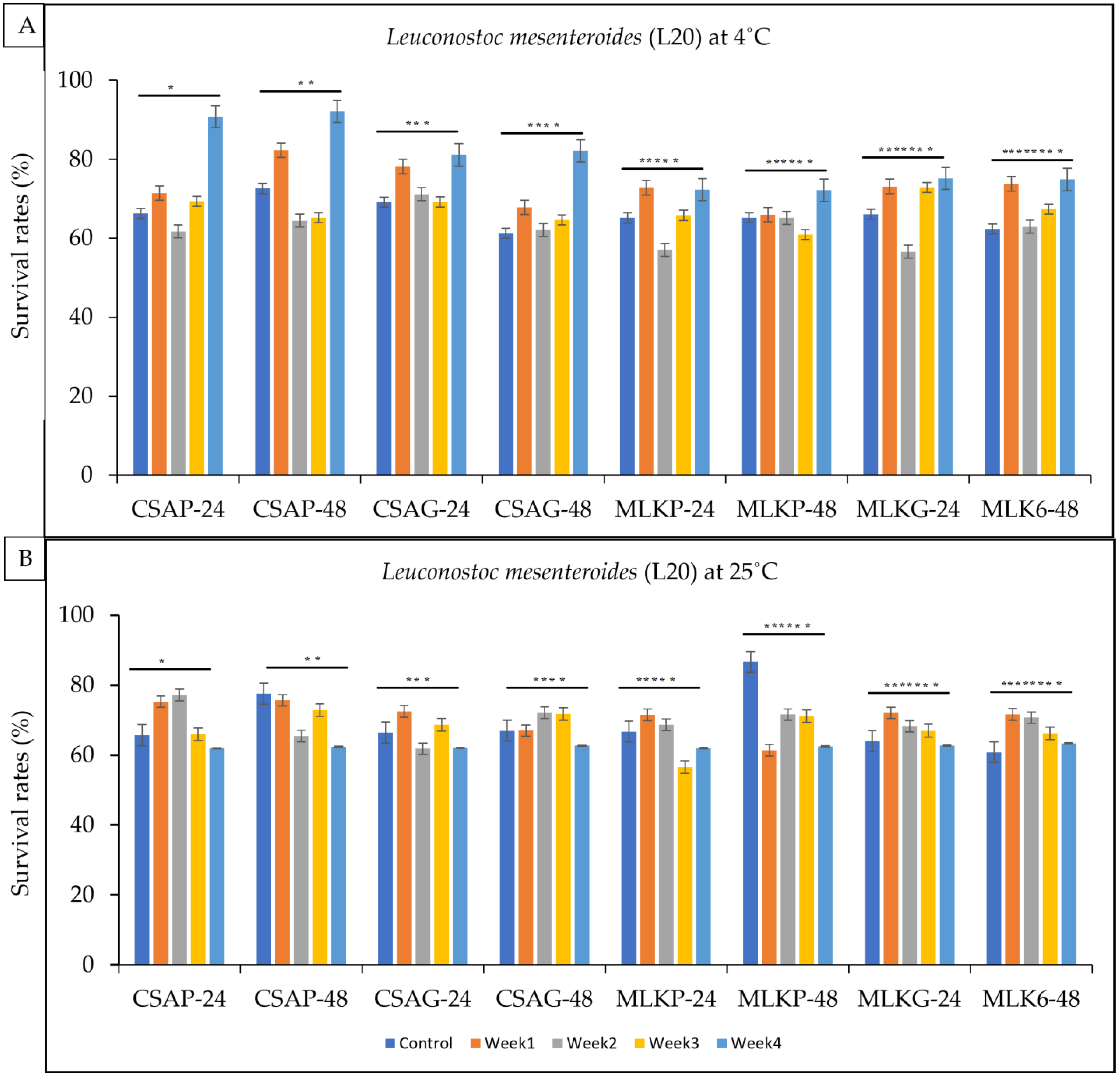
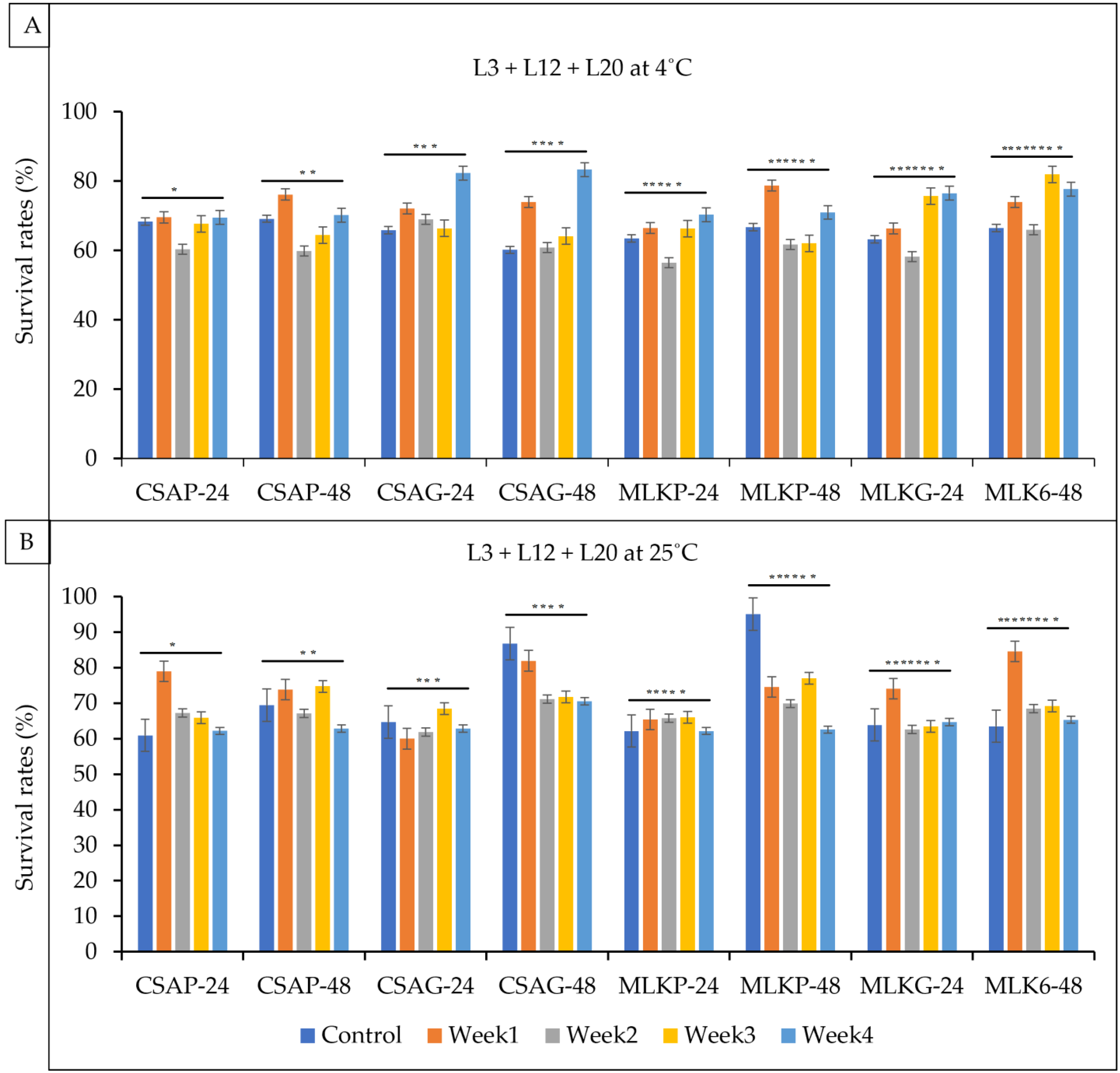
3.1. Viability of Single Strains Lactic Acid Bacteria after Freeze Drying
3.2. Viability of Single Strains Lactic Acid Bacteria following Freeze-Drying after Weeks of Storage
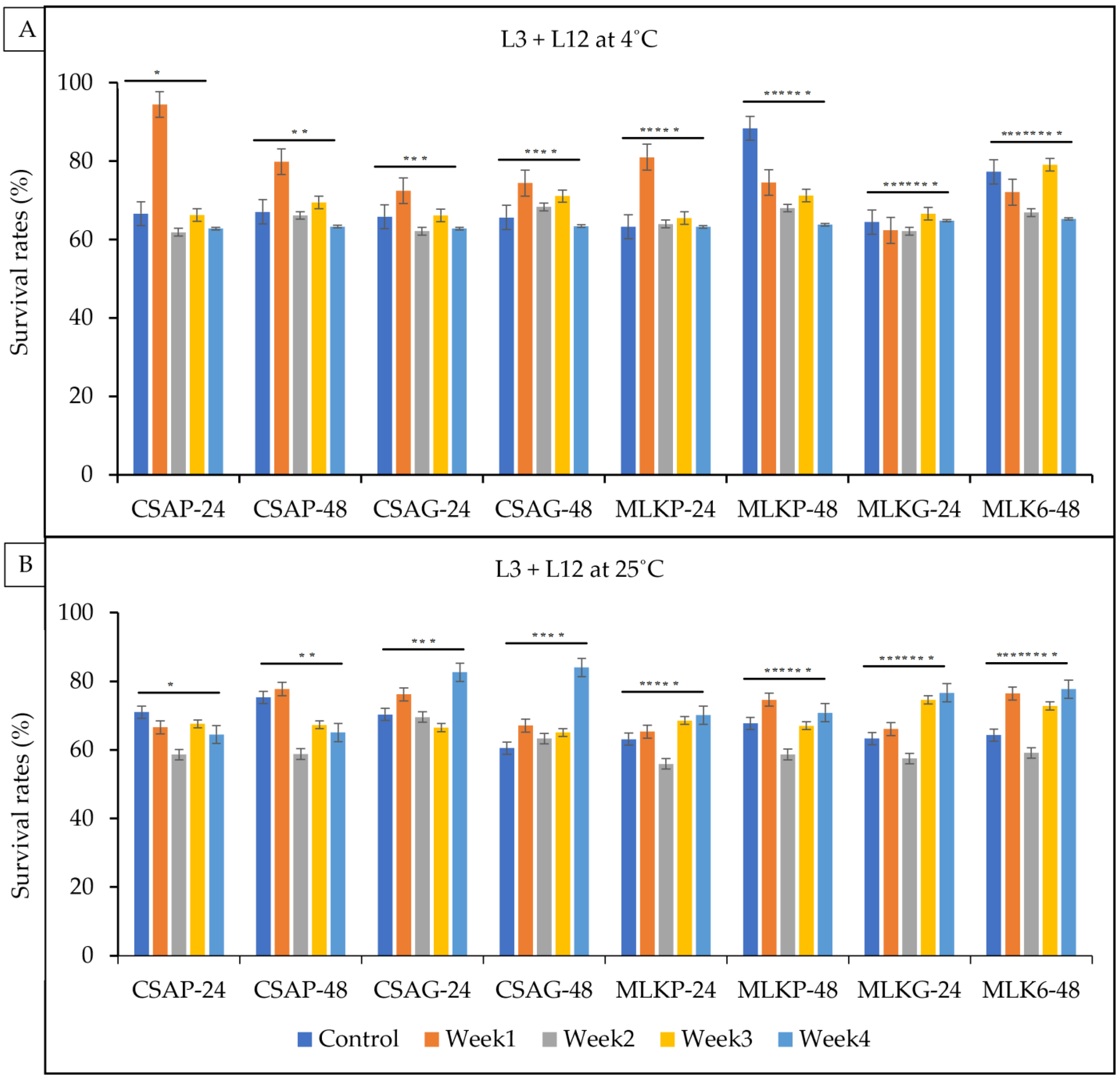
3.3. Viability of Combined Strains Lactic Acid Bacteria after Freeze-Drying After
3.4. Viability of Combined Strains Lactic Acid Bacteria following Freeze Drying after Weeks of Storage
3.5. Performance of Freeze-Dried LAB Starter Cultures during Milk Fermentation
3.6. Consumer Sensory Evaluation of Yogurts Fermented with Freeze-Dried Starter Culture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zang, J.; Xu, Y.; Xia, W.; Regenstein, J.M. Quality, functionality, and microbiology of fermented fish: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1228–1242. [Google Scholar] [CrossRef]
- Maicas, S. The role of yeasts in fermentation processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef]
- Adebo, O.A. African sorghum-based fermented foods: Past, current and future prospects. Nutrition 2020, 12, 1111. [Google Scholar] [CrossRef] [PubMed]
- Voidarou, C.; Antoniadou, M.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Bezirtzoglou, E. Fermentative foods: Microbiology, biochemistry, potential human health benefits and public health issues. Foods 2020, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Wirawati, C.U.; Sudarwanto, M.B.; Lukman, D.W.; Wientarsih, I.; Srihanto, E.A. Diversity of lactic acid bacteria in dadih produced by either backslopping or spontaneous fermentation from two different regions of West Sumatra, Indonesia. Vet. World 2019, 12, 823. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Jeong, D.; Song, K.Y.; Seo, K.H. Comparison of traditional and backslopping methods for kefir fermentation based on physicochemical and microbiological characteristics. LWT 2018, 97, 503–507. [Google Scholar] [CrossRef]
- Özel, B.; Şimşek, Ö.; Settanni, L.; Erten, H. The influence of backslopping on lactic acid bacteria diversity in tarhana fermentation. Int. J. Food Microbiol. 2020, 335, 108886. [Google Scholar] [CrossRef]
- Venema, K.; Surono, I.S. Microbiota composition of dadih—A traditional fermented buffalo milk of West Sumatra. Lett. Appl. Microbiol. 2019, 68, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Agyei, D.; Owusu-Kwarteng, J.; Akabanda, F.; Akomea-Frempong, S. Indigenous African fermented dairy products: Processing technology, microbiology and health benefits. Crit. Rev. Food Sci. Nutr. 2020, 60, 991–1006. [Google Scholar] [CrossRef]
- Motey, G.A.; Owusu-Kwarteng, J.; Obiri-Danso, K.; Ofori, L.A.; Ellis, W.O.; Jespersen, L. In vitro properties of potential probiotic lactic acid bacteria originating from Ghanaian indigenous fermented milk products. World J. Microbiol. Biotechnol. 2021, 37, 1–13. [Google Scholar] [CrossRef]
- Sessou, P.; Keisam, S.; Tuikhar, N.; Gagara, M.; Farougou, S.; Jeyaram, K. High-Throughput Illumina MiSeq amplicon sequencing of yeast communities associated with indigenous dairy products from republics of benin and niger. Front. Microbiol. 2019, 10, 594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayivi, R.D.; Gyawali, R.; Krastanov, A.; Aljaloud, S.O.; Worku, M.; Tahergorabi, R.; Ibrahim, S.A. Lactic acid bacteria: Food safety and human health applications. Dairy 2020, 1, 202–232. [Google Scholar] [CrossRef]
- Owusu-Kwarteng, J.; Akabanda, F.; Agyei, D.; Jespersen, L. Microbial safety of milk production and fermented dairy products in Africa. Microorganisms 2020, 8, 752. [Google Scholar] [CrossRef]
- Abi Khalil, R.; Yvon, S.; Couderc, C.; Belahcen, L.; Jard, G.; Sicard, D.; Ayoub, M.J. Microbial communities and main features of labneh Ambaris, a traditional Lebanese fermented goat milk product. Int. J. Dairy Sci. 2022, 106, 868–883. [Google Scholar] [CrossRef] [PubMed]
- Galli, V.; Venturi, M.; Mari, E.; Guerrini, S.; Granchi, L. Selection of yeast and lactic acid bacteria strains, isolated from spontaneous raw milk fermentation, for the production of a potential probiotic fermented milk. Fermentation 2022, 8, 407. [Google Scholar] [CrossRef]
- Vinderola, G.; Champagne, C.; Desfossés-Foucault, É. The production of lactic acid bacteria starters and probiotic cultures: An industrial perspective. In Lactic Acid Bacteria, 5th ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 317–336. [Google Scholar]
- Chen, W.; Hang, F. Lactic acid bacteria starter. In Lactic Acid Bacteria; Chen, W., Ed.; Science Press Beijing: Beijing, China, 2019; pp. 93–143. [Google Scholar]
- Brizuela, N.S.; Semorile, L.C.; Bravo-Ferrada, B.M.; Tymczyszyn, E.E. Encapsulation of Lactic Acid Bacteria in Sugar Matrices To Be Used as Starters in the Food Industry. In Basic Protocols in Encapsulation of Food Ingredients; Humana: New York, NY, USA, 2021; pp. 55–61. [Google Scholar]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrition 2019, 11, 1591. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, F.; Pénicaud, C.; Tymczyszyn, E.E.; Gómez-Zavaglia, A.; Passot, S. Factors influencing the membrane fluidity and the impact on production of lactic acid bacteria starters. Appl. Microbiol. Biotechnol. 2019, 103, 6867–6883. [Google Scholar] [CrossRef] [Green Version]
- El-Dein, A.N.; Daba, G.; Mostafa, F.A.; Soliman, T.N.; Awad, G.A.; Farid, M.A. Utilization of autochthonous lactic acid bacteria attaining safety attributes, probiotic properties, and hypocholesterolemic potential in the production of a functional set yogurt. Biocatal. Agric. Biotechnol. 2022, 43, 102448. [Google Scholar] [CrossRef]
- Sandhya, M.; Disha, K. Expension in the field of Freeze-drying: An Advanced Review. Res. J. Pharm. Technol. 2020, 13, 2468–2474. [Google Scholar] [CrossRef]
- Guowei, S.; Yang, X.; Li, C.; Huang, D.; Lei, Z.; He, C. Comprehensive optimization of composite cryoprotectant for Saccharomyces boulardii during freeze-drying and evaluation of its storage stability. Prep. Biochem. Biotechnol. 2019, 49, 846–857. [Google Scholar] [CrossRef]
- de Melo Carvalho, T. Consistent Scale-Up of The Freeze-Drying Process. Ph.D. Thesis, Technical University of Denmark, Lyngby, Denmark, October 2018. [Google Scholar]
- Estilarte, M.L.; Tymczyszyn, E.E.; de los Ángeles Serradell, M.; Carasi, P. Freeze-drying of Enterococcus durans: Effect on their probiotics and biopreservative properties. LWT 2021, 137, 110496. [Google Scholar] [CrossRef]
- Jeantet, R.; Jan, G. Improving the drying of Propionibacterium freudenreichii starter cultures. Appl. Microbiol. Biotechnol. 2021, 105, 3485–3494. [Google Scholar] [CrossRef]
- Wang, J.; He, L.; An, W.; Yu, D.; Liu, S.; Shi, K. Lyoprotective effect of soluble extracellular polymeric substances from Oenococcus oeni during its freeze-drying process. Process Biochem. 2019, 84, 205–212. [Google Scholar] [CrossRef]
- Noguerol, A.T.; Igual, M.; Pagán, M.J. Comparison of biopreservatives obtained from a starter culture of Pediococcus acidilactici by different techniques. Food Biosci. 2021, 42, 101114. [Google Scholar] [CrossRef]
- Yao, A.A.; Dortu, C.; Egounlety, M.; Pinto, C.; Edward, V.A.; Huch, M.; Franz, C.M.A.; Holzapfel, W.; Mbugua, S.; Mengu, M.; et al. Production of Freeze-Dried Lactic Acid Bacteria Starter Culture for Cassava Fermentation into Gari. Afr. J. Biotechnol. 2009, 8, 4996–5004. [Google Scholar]
- Abrahamsen, R.K.; Narvhus, J.A. Can ultrasound treatment replace conventional high temperature short time pasteurization of milk? A critical review. Int. Dairy J. 2022, 131, 105375. [Google Scholar] [CrossRef]
- Fortune, A.; Owusu-Kwarteng, J.; Tano-Debrah, K.; Parkouda, C.; Jespersen, L. The Use of Lactic Acid Bacteria Starter Culture in the Production of Nunu, a Spontaneously Fermented Milk Product in Ghana. Int. J. Food Sci. 2014, 2014, 721067. [Google Scholar]
- Owusu-Kwarteng, J.; Wuni, A.; Akabanda, F.; Jespersen, L. Prevalence and characteristics of Listeria monocytogenes isolates in raw milk, heated milk and nunu, a spontaneously fermented milk beverage in Ghana. Beverages 2018, 4, 40. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Banat, F.; Taher, H. A review on the lactic acid fermentation from low-cost renewable materials: Recent developments and challenges. Environ. Technol. Innov. 2020, 20, 101138. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, A.; Tomer, V. Process optimization for the development of a synbiotic beverage based on lactic acid fermentation of nutricereals and milk-based beverage. LWT 2020, 131, 109774. [Google Scholar] [CrossRef]
- Hwang, C.E.; Cho, K.M.; Kim, S.C.; Joo, O.S. Change in physicochemical properties, phytoestrogen content, and antioxidant activity during lactic acid fermentation of soy powder milk obtained from colored small soybean. Korean J. Food Preserv. 2018, 25, 696–705. [Google Scholar] [CrossRef]
- Abesinghe, A.M.N.L.; Islam, N.; Vidanarachchi, J.K.; Prakash, S.; Silva, K.F.S.T.; Karim, M.A. Effects of ultrasound on the fermentation profile of fermented milk products incorporated with lactic acid bacteria. Int. Dairy J. 2019, 90, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Li, X.; Chen, H.; Guan, K.; Qi, X.; Yang, L.; Ma, Y. Evaluation of FAAs and FFAs in yogurts fermented with different starter cultures during storage. J. Food Compost. Anal. 2021, 96, 103666. [Google Scholar] [CrossRef]
- Celik, O.F.; Temiz, H. Lactobacilli isolates as potential aroma producer starter cultures: Effects on the chemical, physical, microbial, and sensory properties of yogurt. Food Biosci. 2022, 48, 101802. [Google Scholar] [CrossRef]
- Arab, M.; Yousefi, M.; Khanniri, E.; Azari, M.; Ghasemzadeh-Mohammadi, V.; Mollakhalili-Meybodi, N. A comprehensive review on yogurt syneresis: Effect of processing conditions and added additives. J. Food Sci. Technol. 2022, 10, 1–10. [Google Scholar] [CrossRef]
- Ghosh, D. Comparative study on nutritional profile analysis of herbal yogurt. Int. J. Eng. Res. Technol. 2019, 8, 174–177. [Google Scholar]
- Wirjantoro, T.I.; Phianmongkhol, A. The viability of lactic acid bacteria and Bifidobacterium bifidum in yoghurt powder during storage. J. Nat. Sci. 2009, 8, 95–104. [Google Scholar]
- Soro-Yao, A.A.; Béra, F.; Franz, C.M.A.; Holzapfel, W. Survival Rate Analysis of Freeze-Dried Lactic Acid Bacteria Using the Arrhenius and z -Value Models. J. Food Prot. 2008, 71, 431–434. [Google Scholar]
- Berner, D.; Viernstein, H. Effect of protective agents on the viability of Lactococcus lactis subjected to freeze-thawing and freeze-drying. Sci. Pharm. 2006, 74, 137–149. [Google Scholar] [CrossRef] [Green Version]
- Ward, K.R.; Matejtschuk, P. The principles of freeze-drying and application of analytical technologies. In Cryopreservation and Freeze-Drying Protocols, 4th ed.; Wolkers, W.F., Oldenhof, H., Eds.; Humana Press: New York, NY, USA, 2021; pp. 99–127. [Google Scholar]
- Jofré, A.; Aymerich, T.; Garriga, M. Impact of different cryoprotectants on the survival of freeze-dried Lactobacillus rhamnosus and Lactobacillus casei/paracasei during long-term storage. Benef. Microbes 2015, 6, 381–386. [Google Scholar] [CrossRef] [Green Version]
- Ambros, S.; Mayer, R.; Schumann, B.; Kulozik, U. Microwave-freeze drying of lactic acid bacteria: Influence of process parameters on drying behavior and viability. Innov. Food Sci. Emerg. Technol. 2018, 48, 90–98. [Google Scholar] [CrossRef]
- Miyamoto-Shinohara, Y.; Imaizumi, T.; Sukenobe, J.; Murakami, Y.; Kawamura, S.; Komatsu, Y. Survival rate of microbes after freeze-drying and long-term storage. Cryobiology 2000, 41, 251–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, F.; Girardeau, A.; Passot, S. Freeze-drying of lactic acid bacteria: A stepwise approach for developing a freeze-drying protocol based on physical properties. In Cryopreservation and Freeze-Drying Protocols, 4th ed.; Wolkers, W.F., Oldenhof, H., Eds.; Humana Press: New York, NY, SUA, 2021; pp. 703–719. [Google Scholar]
- Jouki, M.; Khazaei, N.; Rezaei, F.; Taghavian-Saeid, R. Production of synbiotic freeze-dried yoghurt powder using microencapsulation and cryopreservation of L. plantarum in alginate-skim milk microcapsules. Int. Dairy J. 2021, 122, 105133. [Google Scholar] [CrossRef]
- Enache, I.M.; Vasile, A.M.; Enachi, E.; Barbu, V.; Stănciuc, N.; Vizireanu, C. Co-microencapsulation of anthocyanins from cornelian cherry fruits and lactic acid bacteria in biopolymeric matrices by freeze-drying: Evidence on functional properties and applications in food. Polymers 2020, 12, 906. [Google Scholar] [CrossRef] [Green Version]
- Cescutti, P. Cescutti, Bacterial capsular polysaccharides and exopolysaccharides. In Microbial Glycobiology; Holst, O., Brennan, J., Eds.; Academic Press: Cambridge, MA, USA, 2010; pp. 93–108. [Google Scholar]
- Bibi, A.; Xiong, Y.; Rajoka, M.S.R.; Mehwish, H.M.; Radicetti, E.; Umair, M.; Aadil, R.M. Recent advances in the production of exopolysaccharide (EPS) from Lactobacillus sp and its application in the food industry: A review. Sustainability 2021, 13, 12429. [Google Scholar] [CrossRef]
- Brabet, C.; Raimbault, M.; Figueroa, C.; Dufour, D.; Chuzel, G. Study of natural fermentation of cassava starch in Colombia. In III: Establishment of an Exopolysaccharide (EPS). Producing Lactic Acid Bacteria Strain Bank; CIRAD: Paris, France, 1994. [Google Scholar]
- Carvalho, A.; Teixeira, G.; Silva, J.; Malcat, F.X. Relevant Factors for the Preparation of Freeze-Dried Lactic Acid Bacteria. Relevant Factors for the Preparation of Freeze-Dried Lactic Acid Bacteria. Int. Dairy J. 2004, 14, 835–847. [Google Scholar] [CrossRef]
- OPS Diagnostics, Bacteria Freeze-Drying Protocol. Available online: https://opsdiagnostics.com/notes/ranpri/rpbacteriafdprotocol.htm (accessed on 14 November 2022).
- Suo, X.; Huang, S.; Wang, J.; Fu, N.; Jeantet, R.; Chen, X.D. Effect of culturing lactic acid bacteria with varying skim milk concentration on bacteria survival during heat treatment. J. Food Eng. 2021, 294, 110396. [Google Scholar] [CrossRef]
- Degeest, B.; Vaningelgem, F.; De Vuyst, L. Microbial Physiology, Fermentation Kinetics, and Process Engineering of Heteropolysaccharide Production by Lactic Acid Bacteria. Int. Dairy J. 2001, 11, 747–757. [Google Scholar] [CrossRef]
- Portner, D.C.; Leuschner, R.G.; Murray, B.S. Optimising the viability during storage of freeze-dried cell preparations of Campylobacter jejuni. Cryobiology 2007, 54, 265–270. [Google Scholar] [CrossRef]
- Higl, B.; Kurtmann, L.; Carlsen, C.U.; Ratjen, J.; Först, F.; Skibsted, L.H.; Risbo, J. Impact of water activity, temperature, and physical state on the storage stability of Lactobacillus paracasei ss paracasei freeze-dried in a lactose matrix. Biotechnol. Prog. 2007, 23, 794–800. [Google Scholar] [CrossRef]
- Castro, H.P.; Teixeira, P.M.; Kirby, R. Changes in the Membrane of Lactobacillus Bulgaricus during Storage Following Freeze-Drying Changes in the Cell Membrane of Lactobacillus Bulgaricus Storage Following Freeze-Drying. Biotechnol. Lett. 1996, 18, 99–104. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Silva, J.; Ho, P.; Teixeira, P.; Malcata, F.X.; Gibbs, P. Survival of freeze-dried Lactobacillus plantarum and Lactobacillus rhamnosus during storage in the presence of protectants. Biotechnol. Lett. 2002, 24, 1587–1591. [Google Scholar] [CrossRef]
- Mofidi, A.A.; Rochelle, A.; Chou, C.I.; Mehta, H.M.; Linden, K.G.; Malley, J. Bacterial survival after ultraviolet light disinfection: Resistance, regrowth and repair. In Proceedings of the American Water Works Association Water Quality and Technology Conference (AWWA WQTC), Seattle, WA, USA, 11 January 2002; pp. 10–13. [Google Scholar]
- Hernández-Macedo, M.L.; Barancelli, G.V.; Contreras-Castillo, C.J. Microbial deterioration of vacuum-packaged chilled beef cuts and techniques for microbiota detection and characterization: A review. Braz. J. Microbiol. 2011, 42, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Parente, E.; Cogan, T.M.; Powell, I.B. Starter cultures: General aspects. In Cheese; Academic Press: Cambridge, MA, USA, 2017; pp. 201–226. [Google Scholar]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Yamauchi, R.; Maguin, E.; Horiuchi, H.; Hosokawa, M.; Sasaki, Y. The critical role of urease in yogurt fermentation with various combinations of Streptococcus thermophilus and Lactobacillus delbrueckii ss bulgaricus. Int. J. Dairy Sci. 2019, 102, 1033–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Capillas, C.; Herrero, A.M. Sensory analysis and consumer research in new product development. Foods 2021, 10, 582. [Google Scholar] [CrossRef]
- Guzel-Seydim, Z.B.; Sezgin, E.; Seydim, A.C. Influences of exopolysaccharide producing cultures on the quality of plain set type yogurt. Food Control 2005, 16, 205–209. [Google Scholar] [CrossRef]
- Sharif, M.K.; Butt, M.S.; Sharif, H.R.; Nasir, M. Sensory Evaluation and Consumer Acceptability. In Handbook of Food Science and Technology; The University of Agriculture (UAF): Faisalabad, Pakistan, 2017; pp. 362–386. [Google Scholar]
- Sung, J.M.; Kim, Y.B.; Kum, J.S.; Choi, Y.S.; Seo, D.H.; Choi, H.W.; Park, J.D. Effects of freeze-dried mulberry on antioxidant activities and fermented characteristics of yogurt during refrigerated storage. Korean J. Food Sci. Anim. Resour. 2015, 35, 807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacBean, R.D.; Hall, R.J.; Linklater, M. Analysis of pH-stat continuous cultivation and the stability of the mixed fermentation in continuous yogurt production. Biotechnol. Bioeng. 1979, 21, 1517–1541. [Google Scholar] [CrossRef]
- Park, D.J.; Oh, S.; Ku, K.H.; Mok, C.; Kim, S.H.; Imm, J.Y. Characteristics of Yogurt-like Products Prepared from the Combinaton of Skim Milk and Soymilk Containing Saccharified-Rice Solution. Int. J. Food Sci. Nutr. 2005, 56, 23–34. [Google Scholar] [CrossRef]



| Fermentation | Starter Culture | Codes of Starter Culture |
|---|---|---|
| Single starter culture | Lactococcus lactis | L3 |
| L. delbrueckii subsp. bulgaricus | L12 | |
| Leuconostoc mesenteroides | L20 | |
| Combined starter cultures | Lactococcus lactis + L. delbrueckii | L3 + L12 |
| Leuconostoc mesenteroides + L. delbrueckii | L20 + L12 | |
| Spontaneous fermentation (control) | No starter added | SPF |
| Starter Culture | Sensory Attribute | ||||
|---|---|---|---|---|---|
| Color | Odor | Taste | Texture | Overall Acceptability | |
| L3 | 8.04 ± 1.35 a | 7.16 ± 1.21 a | 7.23 ± 1.30 a | 7.72 ± 1.52 a | 8.36 ± 1.18 a |
| L12 | 7.95 ± 1.40 a | 7.01 ± 1.19 a | 6.65 ± 1.25 b | 7.60 ± 1.45 a | 7.22 ± 1.15 b |
| L20 | 8.00 ± 1.20 a | 8.37 ± 1.25 b | 7.14 ± 1.30 ac | 7.84 ± 1.33 ab | 7.39 ± 1.50 b |
| L3 + L12 | 8.06 ± 0.95 a | 8.83 ± 1.10 b | 7.37 ± 1.05 a | 8.08 ± 0.96 b | 8.45 ± 0.99 a |
| L20 + L12 | 7.90 ± 1.15 a | 7.20 ± 1.21 a | 6.91 ± 1.27 c | 7.75 ± 1.65 a | 7.30 ± 1.05 b |
| SPF | 7.96 ± 1.20 a | 6.03 ± 0.83 c | 6.20 ± 1.24 d | 7.02 ± 0.73 c | 6.25 ± 1.00 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, I.; Ayariga, J.A.; Xu, J.; Boakai, R.K.; Ajayi, O.S.; Owusu-Kwarteng, J. A Comparative Study of Skimmed Milk and Cassava Flour on the Viability of Freeze-Dried Lactic Acid Bacteria as Starter Cultures for Yogurt Fermentation. Foods 2023, 12, 1207. https://doi.org/10.3390/foods12061207
Ibrahim I, Ayariga JA, Xu J, Boakai RK, Ajayi OS, Owusu-Kwarteng J. A Comparative Study of Skimmed Milk and Cassava Flour on the Viability of Freeze-Dried Lactic Acid Bacteria as Starter Cultures for Yogurt Fermentation. Foods. 2023; 12(6):1207. https://doi.org/10.3390/foods12061207
Chicago/Turabian StyleIbrahim, Iddrisu, Joseph Atia Ayariga, Junhuan Xu, Robertson K. Boakai, Olufemi S. Ajayi, and James Owusu-Kwarteng. 2023. "A Comparative Study of Skimmed Milk and Cassava Flour on the Viability of Freeze-Dried Lactic Acid Bacteria as Starter Cultures for Yogurt Fermentation" Foods 12, no. 6: 1207. https://doi.org/10.3390/foods12061207
APA StyleIbrahim, I., Ayariga, J. A., Xu, J., Boakai, R. K., Ajayi, O. S., & Owusu-Kwarteng, J. (2023). A Comparative Study of Skimmed Milk and Cassava Flour on the Viability of Freeze-Dried Lactic Acid Bacteria as Starter Cultures for Yogurt Fermentation. Foods, 12(6), 1207. https://doi.org/10.3390/foods12061207





