Development and Optimization of Black Rice (Oryza sativa L.) Sourdough Fermented by Levilactobacillus brevis LUC 247 for Physicochemical Characteristics and Antioxidant Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Microorganism, and Cultivation
2.2. Methods
2.2.1. Effect of Black Rice on Sourdough Characteristics
2.2.2. Determination of Lactic Acid Bacterial Cell Count
2.2.3. Determination of pH Value and Titratable Acid Content
2.2.4. Determination of Organic Acid Content and Fermentation Quotient
2.2.5. Antioxidant Capacity
2.2.6. Determination of Total Polyphenol Content and Total Anthocyanin Content
2.2.7. Volatile Compound Analysis
2.2.8. Color Analysis
2.2.9. Determination of Water Activity and Moisture
2.2.10. Production of Black Rice Sourdough Bread
2.2.11. Analysis of Physical Properties of Black Rice Sourdough Bread
2.2.12. Test for Storage Property of Black Rice Sourdough Bread
2.2.13. Statistical Analysis
3. Results and Discussion
3.1. Effect of Black Rice Powder Content on the Growth of L. brevis
3.2. pH and Titratable Acid Content of Black Rice Sourdough
3.3. Organic Acid Content and Fermentation Quotient of Black Rice Sourdough
3.4. Anthocyanin and Total Phenol Contents and Antioxidant Capacity of Black Rice Sourdough
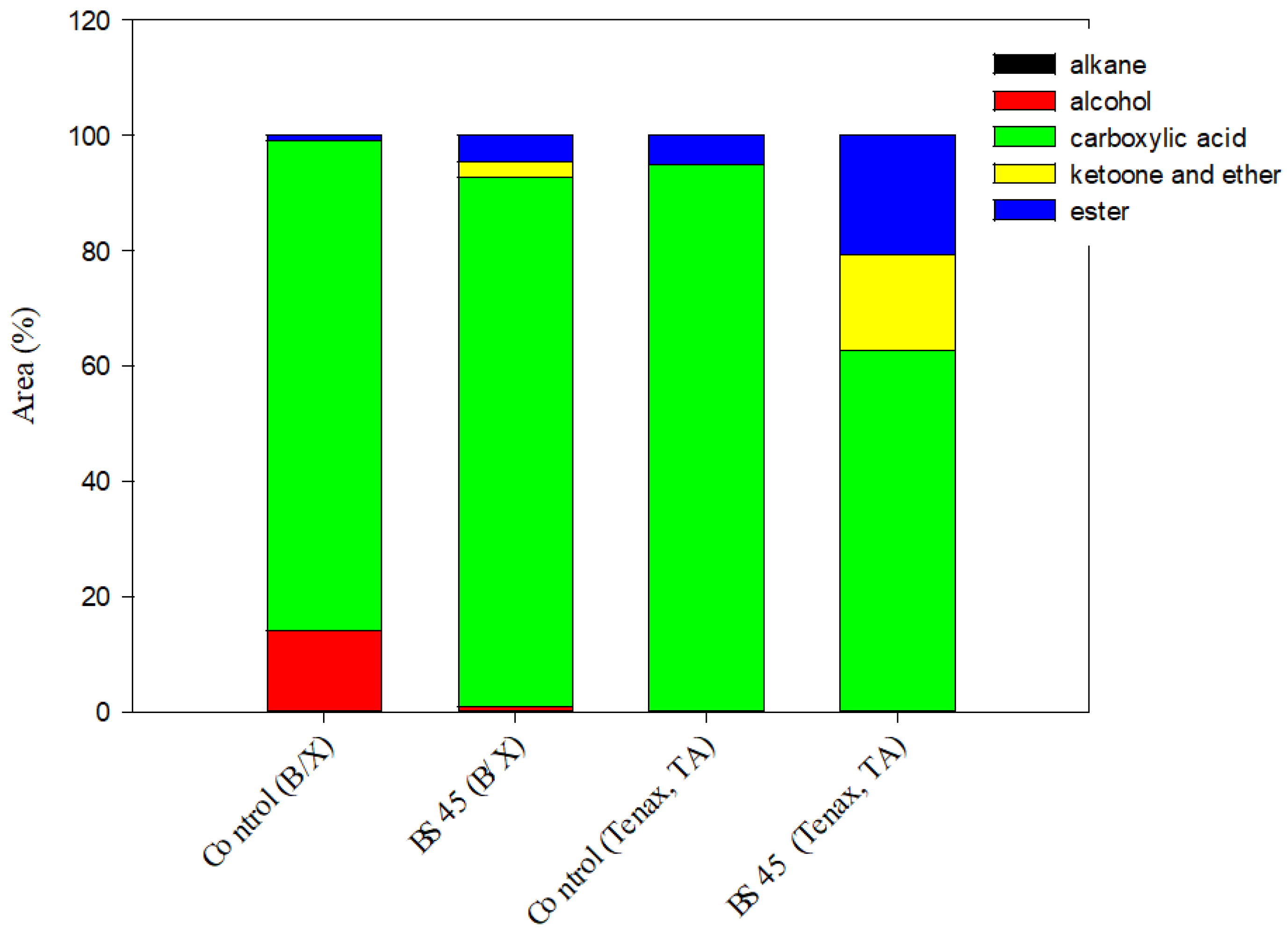
3.5. Volatile Compounds of Black Rice Sourdough
3.6. Variation of Lactic Acid Bacteria Content in Type III Black Rice Sourdough Powder at Different Storage Temperatures
3.7. Variation of Moisture Content and Water Activity of Type III Black Rice Sourdough Powder at Different Storage Temperatures
3.8. Variation of Total Phenol Content and Anthocyanin Contents in Type III Black Rice Sourdough Powder at Different Storage Temperatures
3.9. Color Change of Type III Black Rice Sourdough Powder at Different Storage Temperatures
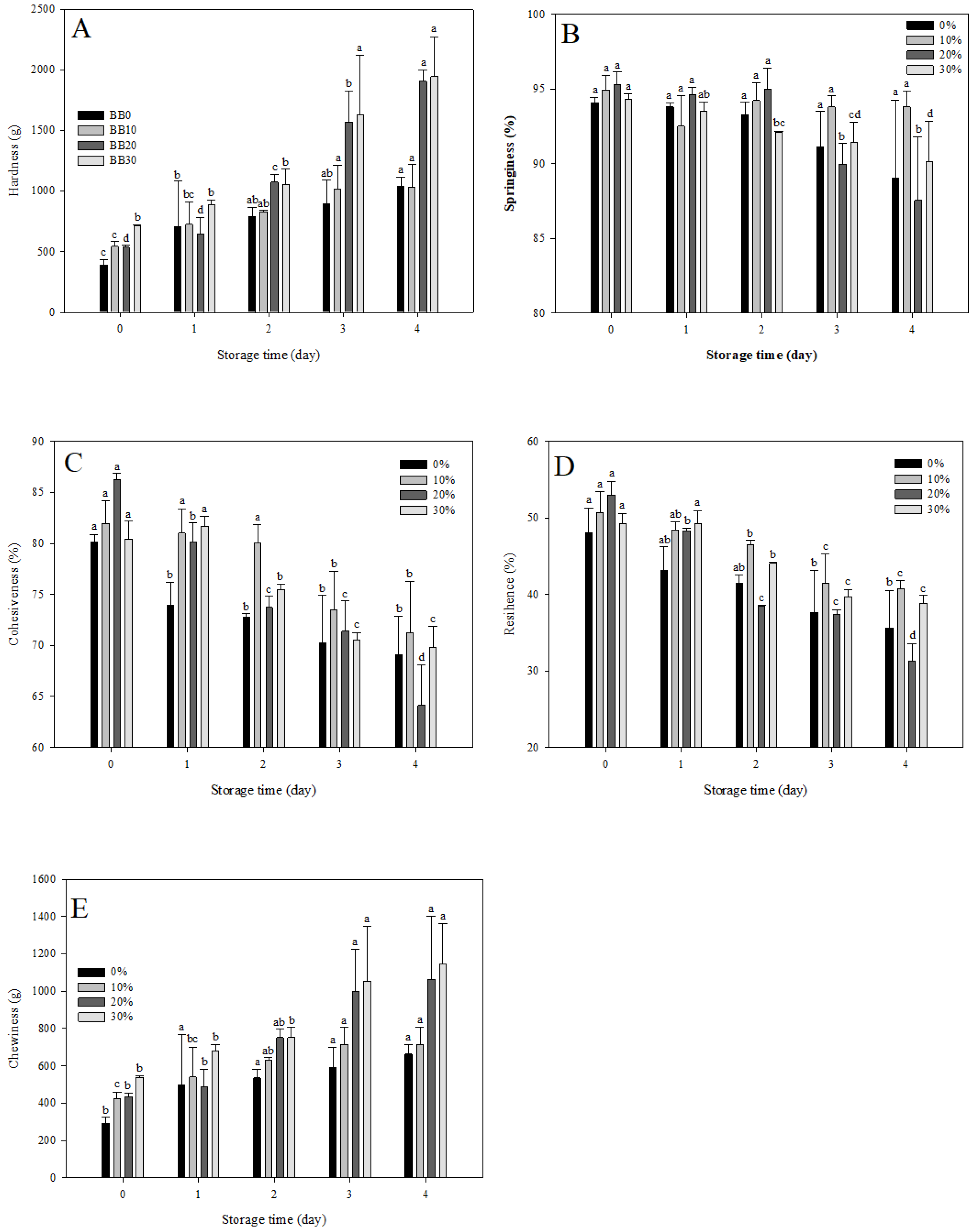
3.10. Texture Analysis of Black Rice Sourdough Bread during Storage
3.11. Analysis of Fungal Growth of Black Rice Sourdough Bread during Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savedboworn, W.; Niyomart, S.; Naknovn, J.; Phattayakorn, K. Impact of inulin on viability and storage stability of probiotic Lactobacillus plantarum TISTR 2075 in fermented rice extract. Agric. Nat. Resour. 2017, 51, 463–469. [Google Scholar] [CrossRef]
- Sumczynski, D.; Kotásková, E.; Družbíková, H.; Mlček, J. Determination of contents and antioxidant activity of free and bound phenolics compounds and in vitro digestibility of commercial black and red rice (Oryza sativa L.) varieties. Food Chem. 2016, 211, 339–346. [Google Scholar] [CrossRef]
- Iraki, M.; Mygdalia, A.; Chatzopoulou, P.; Katsantonis, D. Impact of the combination of sourdough fermentation and hop extract addition on baking properties, antioxidant capacity and phenolics bioaccessibility of rice bran-enhanced bread. Food Chem. 2019, 285, 231–239. [Google Scholar] [CrossRef]
- Lang, G.H.; Lindemann, I.S.; Ferreira, C.D.; Hoffmann, J.F.; Vanier, N.L.; Oliveira, M. Effects of drying temperature and long-term storage conditions on black rice phenolic compounds. Food Chem. 2019, 287, 197–204. [Google Scholar] [CrossRef]
- Marlett, J.A.; McBurney, M.I.; Slavin, J.L. Position of the American Dietetic Association: Health Implications of Dietary Fiber. J. Acad. Nutr. Die. 2002, 102, 993–1000. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, Q.; Shen, L.; Luo, J.; Zhu, R.; Mao, J.; Zhao, M.; Cai, C. Phenolic profile and antioxidant activity of Chinese rice wine fermented with different rice materials and starters. Lebensm. Wiss. Technol. 2019, 111, 226–234. [Google Scholar] [CrossRef]
- Premakumara, G.A.S.; Abeysekera, W.K.S.M.; Ratnasooriya, W.D.; Chandrasekharan, N.V.; Bentota, A.P. Antioxidant, anti-amylase and anti-glycation potential of brans of some Sri Lankan traditional and improved rice (Oryza sativa L.) varieties. J. Cereal Sci. 2013, 58, 451–456. [Google Scholar] [CrossRef]
- Kavitake, D.; Kandasamy, S.; Devi, P.B.; Shetty, P.H. Recent developments on encapsulation of lactic acid bacteria as potential starter culture in fermented foods—A review. Food Biosci. 2018, 21, 34–44. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, W.J.; Kang, S.S. Inhibitory effect of bacteriocin-producing Lactobacillus brevis DF01 and Pediococcus acidilactici K10 isolated from kimchi on enteropathogenic bacterial adhesion. Food Biosci. 2019, 30, 100425. [Google Scholar] [CrossRef]
- Wu, Q.; Shah, N.P. Restoration of GABA production machinery in Lactobacillus brevis by accessible carbohydrates, anaerobiosis and early acidification. Food Microbiol. 2018, 69, 151–158. [Google Scholar] [CrossRef]
- Iannitti, T.; Palmieri, B. Therapeutical use of probiotic formulations in clinical practice. Clin. Nutr. 2010, 29, 701–725. [Google Scholar] [CrossRef]
- Aarti, C.; Khusro, A.; Varghese, R.; Arasu, M.V.; Agastian, P.; Al-Dhabi, N.A.; Soundharrajan, I.; Choi, K.C. In vitro studies on probiotic and antioxidant properties of Lactobacillus brevis strain LAP2 isolated from Hentak, a fermented fish product of North-East India. Lebensm. Wiss. Technol. 2017, 86, 438–446. [Google Scholar] [CrossRef]
- Lim, S.M.; Jeong, J.J.; Jang, S.E.; Han, M.J.; Kim, D.H. A mixture of the probiotic strains Bifidobacterium longum CH57 and Lactobacillus brevis CH23 ameliorates colitis in mice by inhibiting macrophage activation and restoring the Th17/Treg balance. J. Funct. Foods. 2016, 27, 295–309. [Google Scholar] [CrossRef]
- Maloy, K.J.; Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011, 474, 298–306. [Google Scholar] [CrossRef]
- Harada, G.; Pattarawat, P.; Ito, K.; Matsumoto, T.; Hasegawa, T.; Katakura, Y. Lactobacillus brevis T2102 suppresses the growth of colorectal cancer cells by activating SIRT1. J. Funct. Foods 2016, 23, 444–452. [Google Scholar] [CrossRef]
- Lin, Q. Submerged fermentation of Lactobacillus rhamnosus YS9 for γ-aminobutyric acid (GABA) production. Braz. J. Microbiol. 2013, 44, 183–187. [Google Scholar] [CrossRef]
- Decock, P.; Cappelle, S. Bread technology and sourdough technology. Trends Food Sci. Technol. 2005, 16, 113–120. [Google Scholar] [CrossRef]
- Siepmann, F.B.; De Almeida, B.S.; Waszczynskyj, N.; Spier, M.R. Influence of temperature and of starter culture on biochemical characteristics and the aromatic compounds evolution on type II sourdough and wheat bread. Lebensm. Wiss. Technol. 2019, 108, 199–206. [Google Scholar] [CrossRef]
- Ripari, V.; Teresa, C.; Enrico, B. Microbiological characterisation and volatiles profile of model, ex-novo, and traditional italian white wheat sourdoughs. Food Chem. 2016, 205, 297–307. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; Lavermicocca, P.; De Vincenzi, M.; Giovannini, C.; Faccia, M.; Gobbetti, M. Proteolysis by Sourdough Lactic Acid Bacteria: Effects on Wheat Flour Protein Fractions and Gliadin Peptides Involved in Human Cereal Intolerance. Appl. Environ. Microbiol. 2002, 68, 623–633. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Jinshui, Z. Lifestyles of sourdough Lactobacilli—Do they matter for microbial ecology and bread quality? Int. J. Food Microbi. 2019, 302, 15–23. [Google Scholar] [CrossRef]
- Clément, H.; Prost, C.; Chiron, H.; Ducasse, M.B.; Della Valle, G.; Courcoux, P.; Onno, B. The effect of organic wheat flour by-products on sourdough performances assessed by a multi-criteria approach. Food Res. Int. 2018, 106, 974–981. [Google Scholar] [CrossRef]
- Fraberger, V.; Ammer, C.; Domig, K.J. Functional Properties and Sustainability Improvement of Sourdough Bread by Lactic Acid Bacteria. Microorganisms 2020, 8, 1895. [Google Scholar] [CrossRef]
- De Angelis, M.; Gallo, G.; Corbo, M.R.; McSweeney, P.L.H.; Faccia, M.; Giovine, M.; Gobbetti, M. Phytase activity in sourdough lactic acid bacteria: Purification and characterization of a phytase from Lactobacillus sanfranciscensis CB1. Int. J. Food Microbi. 2003, 87, 259–270. [Google Scholar] [CrossRef]
- Svanberg, U.; Lorri, W.; Sandbeag, S.A. Lactic fermentation of non-tannin and high-tannin cereals: Effects on in vitro estimation of iron availability and phytate hydrolysis. J. Food Sci. 2003, 58, 408–412. [Google Scholar] [CrossRef]
- Ua-Arak, T.; Jakob, F.; Vogel, R.F. Characterization of growth and exopolysaccharide production of selected acetic acid bacteria in buckwheat sourdoughs. Int. J. Food Microbi. 2016, 239, 103–112. [Google Scholar] [CrossRef]
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and functional paths of lactic acid bacteria in plant foods: Get out of the labyrinth. Curr. Opin. Biotechnol. 2018, 49, 64–72. [Google Scholar] [CrossRef]
- Lattanzi, A.; Minervini, F.; Gobbetti, M. Assessment of comparative methods for storing type-I wheat sourdough. Lebensm. Wiss. Technol. 2014, 59, 948–955. [Google Scholar] [CrossRef]
- Duodu, K.G.; Taylor, J.R.N. The quality of breads made with non-wheat flours. In Breadmaking; Woodhead Publishing: Sawston, UK, 2012; pp. 754–782. [Google Scholar] [CrossRef]
- Reale, A.; Di Renzo, T.; Preziuso, M.; Panfili, G.; Cipriano, L.; Messia, M.C. Stabilization of sourdough starter by spray drying technique: New breadmaking perspective. Lebensm. Wiss. Technol. 2019, 99, 468–475. [Google Scholar] [CrossRef]
- Coda, R.; Rizzello, C.G.; Gobbetti, M. Use of sourdough fermentation and pseudo-cereals and leguminous flours for the making of a functional bread enriched of γ-aminobutyric acid (GABA). Int. J. Food Microbiol. 2010, 137, 236–245. [Google Scholar] [CrossRef]
- Rühmkorf, C.; Jungkunz, S.; Wagner, M.; Vogel, R.F. Optimization of homoexopolysaccharide formation by lactobacilli in gluten-free sourdoughs. Food Microbio. 2012, 32, 286–294. [Google Scholar] [CrossRef]
- Chiang, S.H.; Yang, K.M.; Wang, S.Y.; Chen, C.W. Enzymatic treatment in black tea manufacturing processing: Impact on bioactive compounds, quality, and bioactivities of black tea. Lebensm. Wiss. Technol. 2022, 163, 113560. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.; Wrolstad, R. Total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines. In Official Methods of Analysis of AOAC International; Oxford University Press: Oxfordshire, UK, 2005; Chapter 37. [Google Scholar]
- Tafti, A.G.; Peighambardoust, S.H.; Hesari, J.; Bahrami, A.; Bonab, E.S. Physico-chemical and functional properties of spray-dried sourdough in breadmaking. Food Sci. Technol. Int. 2013, 19, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Kiumarsi, M.; Shahbazi, M.; Yeganehzad, S.; Majchrzak, D.; Lieleg, O.; Winkeljann, B. Relation between structural, mechanical and sensory properties of gluten-free bread as affected by modified dietary fibers. Food Chem. 2019, 277, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, L.; Qian, H.; Zhang, H.; Li, Y.; Wu, G.; Qi, X.; Xu, M.; Rao, Z. Effect of selected strains on physical and organoleptic properties of breads. Food Chem. 2019, 276, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Axel, C.; Brosnan, B.; Zannini, E.; Furey, A.; Coffey, A.; Arendt, E.K. Antifungal sourdough lactic acid bacteria as biopreservation tool in quinoa and rice bread. Int. J. Food Microbiol. 2016, 239, 86–94. [Google Scholar] [CrossRef]
- Demirci, T.; Aktaş, K.; Sözeri, D.; Öztürk, H.İ.; Oğul, A. Rice bran improve probiotic viability in yoghurt and provide added antioxidative benefits. J. Funct. Foods 2017, 36, 396–403. [Google Scholar] [CrossRef]
- Pereira, A.L.F.; Maciel, T.C.; Rodrigues, S. Probiotic beverage from cashew apple juice fermented with Lactobacillus casei. Food Res Int. 2017, 44, 1276–1283. [Google Scholar] [CrossRef]
- Rehman, S.-U.; Paterson, A.; Piggott, J.R. Flavour in sourdough breads: A review. Trends Food Sci. Technol. 2006, 17, 557–566. [Google Scholar] [CrossRef]
- Ravyts, F.; De Vuyst, L. Prevalence and impact of single-strain starter cultures of lactic acid bacteria on metabolite formation in sourdough. Food Micr. 2011, 28, 1129–1139. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chou, C.C. Effect of heat treatment on total phenolic and anthocyanin contents as well as antioxidant activity of the extract from Aspergillus awamori-fermented black soybeans, a healthy food ingredient. Int. J. Food. Sci. Nutr. 2009, 60, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Geng, X.; Egashira, Y.; Sanada, H. Purification and Characterization of a Feruloyl Esterase from the Intestinal Bacterium Lactobacillus acidophilus. Appl. Environ. Microbiol. 2004, 70, 2367–2372. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, R.; Deng, Y.; Zhang, Y.; Xiao, J.; Huang, F.; Wen, W.; Zhang, M. Fermentation and complex enzyme hydrolysis enhance total phenolics and antioxidant activity of aqueous solution from rice bran pretreated by steaming with α-amylase. Food Chem. 2017, 221, 636–643. [Google Scholar] [CrossRef]
- Hansen, A.; Schieberle, P. Generation of aroma compounds during sourdough fermentation: Applied and fundamental aspects. Trends Food Sci. Technol. 2005, 16, 85–94. [Google Scholar] [CrossRef]
- Choi, I.S.; Ko, S.H.; Kim, H.M.; Chun, H.H.; Lee, K.H.; Yang, J.E.; Jeong, S.; Park, H.W. Shelf-life extension of freeze-dried Lactobacillus brevis WiKim0069 using supercooling pretreatment. Lebensm. Wiss. Technol. 2019, 112, 108230. [Google Scholar] [CrossRef]
- Chávez, B.E.; Ledebore, A.M. Drying of Prebiotics: Optimization of. Formulation and Process to Enhance Storage Survival. Dry. Technol. 2007, 25, 1193–1201. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Fan, Z. Influential Factor of Anthocyanin Stability and the Potential Pathways Improving Their Stability. Hans J. Food Nutri. Sci. 2017, 6, 137–142. [Google Scholar] [CrossRef]
- Hou, Z.; Qin, P.; Zhang, Y.; Cui, S.; Ren, G. Identification anthocyanins isolated from black rice (Oryza sativa L.) and their degradation kinetics. Food Res. Int. 2013, 50, 691–697. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, W.; Zhou, X.; Wu, Z.; Hui, A.; He, Y.; Gao, H.; Chen, P. Effect of high hydrostatic pressure on the bioactive compounds, antioxidant activity and in vitro digestibility of cooked black rice during refrigerated storage. J. Cereal Sci. 2019, 86, 54–59. [Google Scholar] [CrossRef]
- Phimolsiripol, Y.; Mukprasirt, A.; Schoenlechner, R. Quality improvement of rice-based gluten-free bread using different dietary fibre fractions of rice bran. J. Cereal Sci. 2012, 56, 389–395. [Google Scholar] [CrossRef]



| Materials | Black Rice Sourdough | ||||
|---|---|---|---|---|---|
| BS0 * | BS15 | BS30 | BS45 | BS60 | |
| Black rice powder (g) | 0 | 30 | 60 | 90 | 120 |
| Bread flour (g) | 200 | 170 | 140 | 110 | 80 |
| Sterilized distilled water (mL) | 200 | 200 | 200 | 200 | 200 |
| Bacterial suspension (mL) ** | 4 | 4 | 4 | 4 | 4 |
| Sample | Fermentation Time (h) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | 24 | 36 | 48 | ||
| Anthocyanin (μg/g sourdough) | BS0 | N.D. * | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| BS15 | 4.23 ± 0.19 dF | 2.23 ± 0.39 dG | 8.13 ± 0.39 dE | 16.70 ± 2.03 dD | 28.72 ± 3.22 dC | 31.73 ± 0.33 dB | 42.40 ± 0.26 dA | |
| BS30 | 15.92 ± 0.13 cC | 8.91 ± 0.20 cD | 16.36 ± 0.33 cC | 37.85 ± 0.70 cB | 54.44 ± 0.88 cA | 56.44 ± 0.58 cA | 56.22 ± 0.39 cA | |
| BS45 | 36.63 ± 0.15 bC | 25.27 ± 0.21 bD | 23.60 ± 0.51 bD | 52.66 ± 0.91 bB | 75.41 ± 1.20 bA | 78.15 ± 1.46 bA | 71.58 ± 0.51 bA | |
| BS60 | 63.46 ± 1.77 aB | 48.76 ± 1.20 aC | 52.99 ± 0.70 aC | 62.34 ± 0.77 aB | 86.83 ± 0.0 aA | 86.28 ± 0.29 aA | 81.94 ± 1.17 aA | |
| Total phenolic (gallic acid equivalent;mg/g sourdough) | BS0 | 0.55 ± 0.01 bD | 0.65 ± 0.01 bD | 1.14 ± 0.00 cC | 1.29 ± 0.01 cC | 1.40 ± 0.01 dB | 1.70 ± 0.06 bA | 1.78 ± 0.07 bA |
| BS15 | 0.54 ± 0.02 bD | 0.55 ± 0.01 cD | 1.53 ± 0.01 bC | 1.55 ± 0.05 bC | 1.59 ± 0.03 cC | 1.79 ± 0.02 bB | 2.01 ± 0.02 aA | |
| BS30 | 0.60 ± 0.02 aD | 0.58 ± 0.01 cD | 1.29 ± 0.02 cC | 1.74 ± 0.03 aB | 1.87 ± 0.08 bB | 2.11 ± 0.03 aA | 2.17 ± 0.02 aA | |
| BS45 | 0.68 ± 0.02 aD | 0.71 ± 0.02 aD | 0.88 ± 0.01 dC | 1.63 ± 0.03 bB | 2.00 ± 0.01 aA | 2.29 ± 0.05 aA | 2.17 ± 0.01 aa | |
| BS60 | 0.68 ± 0.00 aC | 0.78 ± 0.01 aC | 1.64 ± 0.02 aB | 1.69 ± 0.03 aB | 2.00 ± 0.01 aA | 2.15 ± 0.05 aA | 2.06 ± 0.03 aA | |
| DPPH RSA (Vit. C equivalent; μg/g sourdough) | BS0 | 48.25 ± 3.29 dD | 53.04 ± 6.31 eD | 75.42 ± 0.99 eC | 75.55 ± 2.67 dC | 93.45 ± 3.68 dB | 104.27 ± 2.88 dA | 73.32 ± 1.72 dC |
| BS15 | 123.73 ± 1.45 cD | 137.68 ± 5.09 dD | 178.76 ± 3.97 dC | 334.39 ± 2.06 cB | 494.17 ± 5.02 cA | 521.94 ± 7.77 cA | 504.58 ± 3.02 cA | |
| BS30 | 182.86 ± 2.02 bC | 217.41 ± 4.90 cB | 237.68 ± 2.74 cB | 657.64 ± 5.97 bA | 652.53 ± 8.47 bA | 675.91 ± 3.59 bA | 671.16 ± 7.40 bA | |
| BS45 | 193.53 ± 3.63 bE | 313.35 ± 3.02 bD | 378.01 ± 4.66 bD | 726.50 ± 9.21 aC | 954.37 ± 22.28 aB | 998.57 ± 7.30 aA | 923.32 ± 6.23 aB | |
| BS60 | 380.05 ± 5.27 aE | 468.05 ± 8.07 aD | 573.81 ± 3.84 aC | 734.55 ± 8.63 aB | 965.15 ± 18.12 aA | 995.65 ± 19.24 aA | 905.42 ± 12.40 aA | |
| Reducing power (Vit. C equivalent; μg/g sourdough) | BS0 | 59.65 ± 1.26 eC | 60.83 ± 1.28 eC | 58.03 ± 1.28 dC | 63.05 ± 0.75 dC | 78.03 ± 0.57 eB | 76.88 ± 1.36 dB | 90.63 ± 2.47 dA |
| BS15 | 120.28 ± 0.51 dC | 115.18 ± 2.04 dC | 143.97 ± 0.51 cB | 308.90 ± 1.89 cA | 345.04 ± 6.70 dA | 339.93 ± 1.67 cA | 331.87 ± 6.25 cA | |
| BS30 | 189.32 ± 3.9 cD | 176.08 ± 4.29 cD | 192.17 ± 4.20 bD | 397.88 ± 4.22 bC | 479.03 ± 9.39 cB | 506.02 ± 2.10 bA | 477.05 ± 13.87 bB | |
| BS45 | 254.72 ± 3.21 bC | 248.09 ± 3.62 bC | 232.27 ± 3.21 bC | 547.26 ± 4.54 aB | 589.83 ± 3.49 bB | 688.10 ± 5.59 aA | 616.03 ± 14.21 aB | |
| BS60 | 330.65 ± 5.78 aD | 304.82 ± 4.34 aD | 437.38 ± 8.87 aC | 569.73 ± 4.19 aB | 653.53 ± 21.08 aA | 665.38 ± 1.42 aA | 629.17 ± 7.59 aA | |
| Storage Temperature (°C) | Storage Time (Week) | Lactic Acid Bacteria (Log CFU/g Sourdough Powder) | Water Activity (Aw) | Moisture (%) | Total Phenolic (mg/g Sourdough Powder) | Anthocyanin (μg/g Sourdough Powder) | Color | |||
|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ΔE* | |||||||
| 4 °C | 0 | 7.25 ± 0.0 a | 0.13 ± 0.01 a | 2.75 ± 0.05 a | 6.75 ± 0.26 a | 143.72 ± 4.15 a | 55.81 ± 0.14 a | 13.71 ± 0.10 ab | 2.67 ± 0.06 c | — |
| 2 | 7.13 ± 0.05 b | 0.12 ± 0.0 ab | 2.71 ± 0.04 a | 6.31 ± 0.20 a | 82.16 ± 0.88 c | 56.20 ± 0.43 a | 13.95 ± 0.24 a | 3.21 ± 0.16 a | 0.90 ± 0.03 c | |
| 4 | 7.07 ± 0.11 b | 0.10 ± 0.02 b | 2.51 ± 0.07 b | 6.19 ± 0.16 a | 89.39 ± 3.88 b | 54.44 ± 0.15 b | 13.64 ± 0.12 b | 2.76 ± 0.04 c | 1.38 ± 0.18 b | |
| 6 | 7.13 ± 0.12 b | 0.11 ± 0.01 b | 2.46 ± 0.12 b | 6.17 ± 0.19 a | 90.06 ± 2.15 b | 53.26 ± 0.35 c | 13.50 ± 0.04 c | 2.97 ± 0.07 b | 2.33 ± 0.11 a | |
| 25 °C | 0 | 7.25 ± 0.0 a | 0.13 ± 0.01 a | 2.75 ± 0.05 a | 6.75 ± 0.26 a | 143.72 ± 4.15 a | 55.81 ± 0.14 ab | 13.71 ± 0.10 ab | 2.67 ± 0.06 b | — |
| 2 | 6.98 ± 0.03 b | 0.10 ± 0.01 b | 2.44 ± 0.02 b | 6.12 ± 0.36 ab | 83.61 ± 1.26 b | 55.47 ± 0.42 b | 13.90 ± 0.32 a | 3.22 ± 0.23 a | 0.66 ± 0.10 b | |
| 4 | 6.99 ± 0.15 b | 0.09 ± 0.01 b | 2.19 ± 0.16 b | 5.86 ± 0.04 b | 87.28 ± 2.37 b | 56.22 ± 0.41 a | 13.56 ± 0.05 ab | 2.64 ± 0.05 b | 0.71 ± 0.04 b | |
| 6 | 6.95 ± 0.08 b | 0.10 ± 0.01 b | 2.33 ± 0.23 b | 5.80 ± 0.44 b | 87.06 ± 1.84 b | 54.50 ± 0.33 c | 13.44 ± 0.25 b | 2.80 ± 0.04 b | 1.19 ± 0.05 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, S.-F.; Chen, Y.-W.; Lee, S.-M.; Huang, H.-Y.; Huang, Y.-H.; Lu, Y.-C.; Chen, C.-W. Development and Optimization of Black Rice (Oryza sativa L.) Sourdough Fermented by Levilactobacillus brevis LUC 247 for Physicochemical Characteristics and Antioxidant Capacity. Foods 2023, 12, 1389. https://doi.org/10.3390/foods12071389
Lai S-F, Chen Y-W, Lee S-M, Huang H-Y, Huang Y-H, Lu Y-C, Chen C-W. Development and Optimization of Black Rice (Oryza sativa L.) Sourdough Fermented by Levilactobacillus brevis LUC 247 for Physicochemical Characteristics and Antioxidant Capacity. Foods. 2023; 12(7):1389. https://doi.org/10.3390/foods12071389
Chicago/Turabian StyleLai, Syue-Fong, Yi-Wen Chen, Shin-Mei Lee, Hsin-Yu Huang, Yu-Hsin Huang, Ying-Chen Lu, and Chih-Wei Chen. 2023. "Development and Optimization of Black Rice (Oryza sativa L.) Sourdough Fermented by Levilactobacillus brevis LUC 247 for Physicochemical Characteristics and Antioxidant Capacity" Foods 12, no. 7: 1389. https://doi.org/10.3390/foods12071389
APA StyleLai, S.-F., Chen, Y.-W., Lee, S.-M., Huang, H.-Y., Huang, Y.-H., Lu, Y.-C., & Chen, C.-W. (2023). Development and Optimization of Black Rice (Oryza sativa L.) Sourdough Fermented by Levilactobacillus brevis LUC 247 for Physicochemical Characteristics and Antioxidant Capacity. Foods, 12(7), 1389. https://doi.org/10.3390/foods12071389






