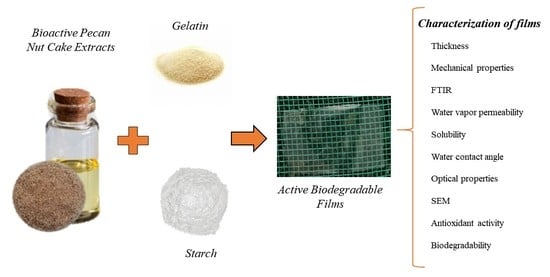
Design of Biodegradable Films Using Pecan Nut Cake Extracts for Food Packing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Extraction of Samples
Phenolic Compounds Evaluation
2.3. Film Preparation
2.4. Characterization of Films
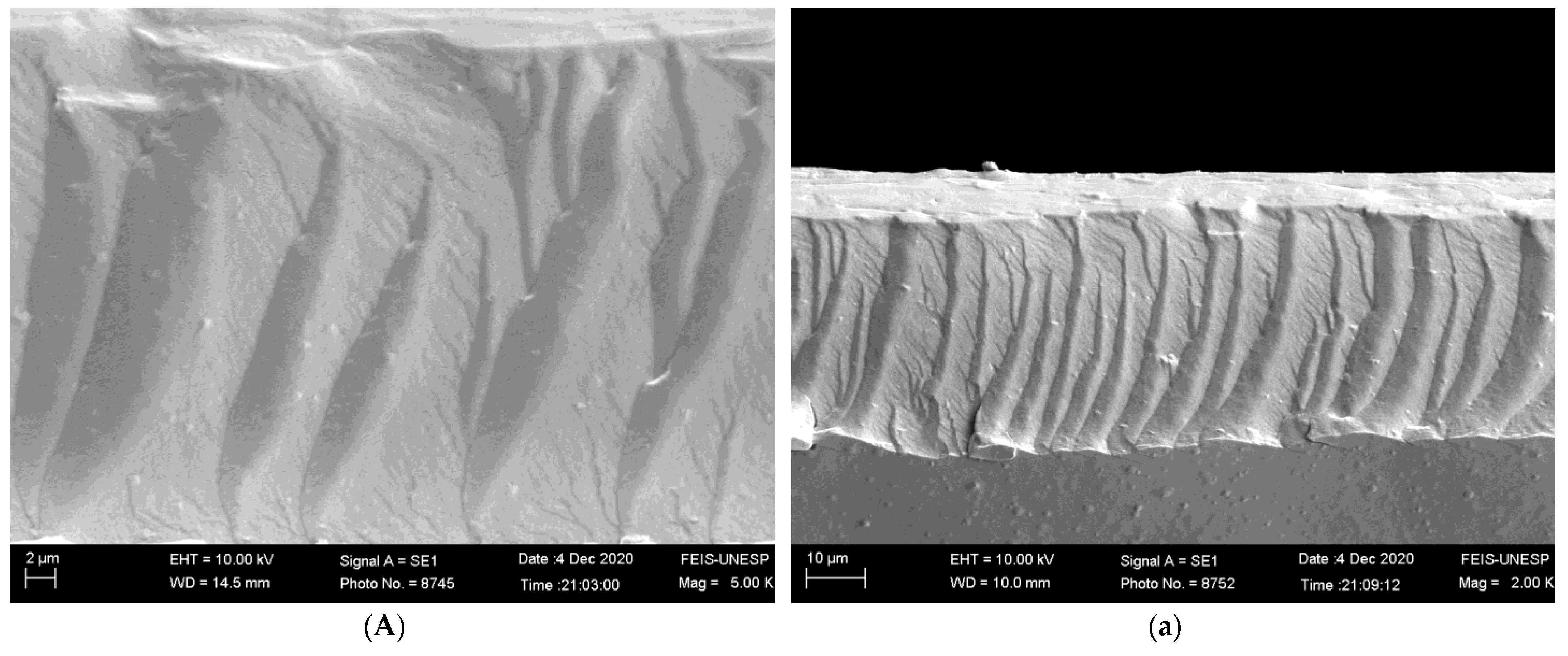
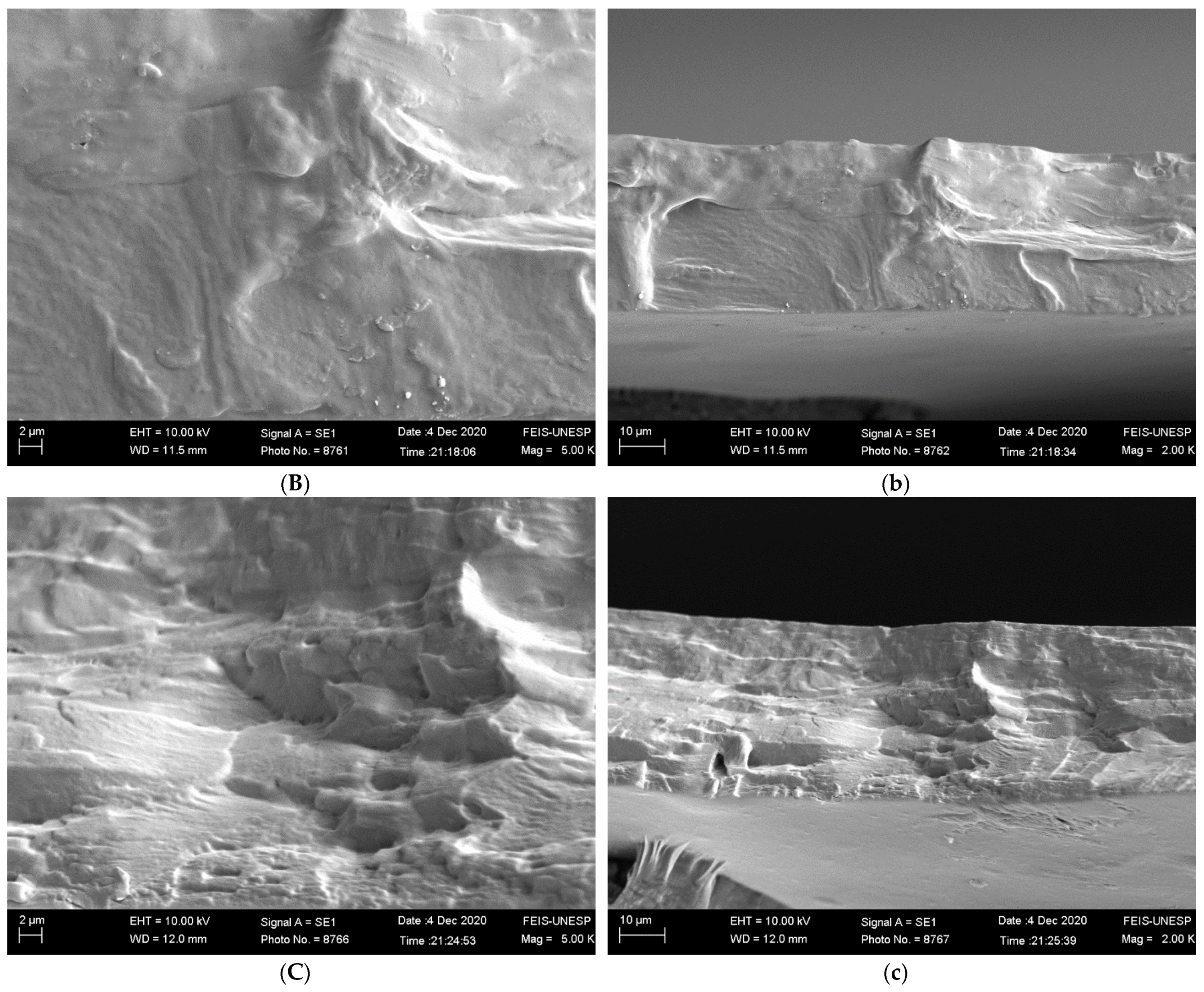
2.4.1. Scanning Electron Microscopy (SEM)
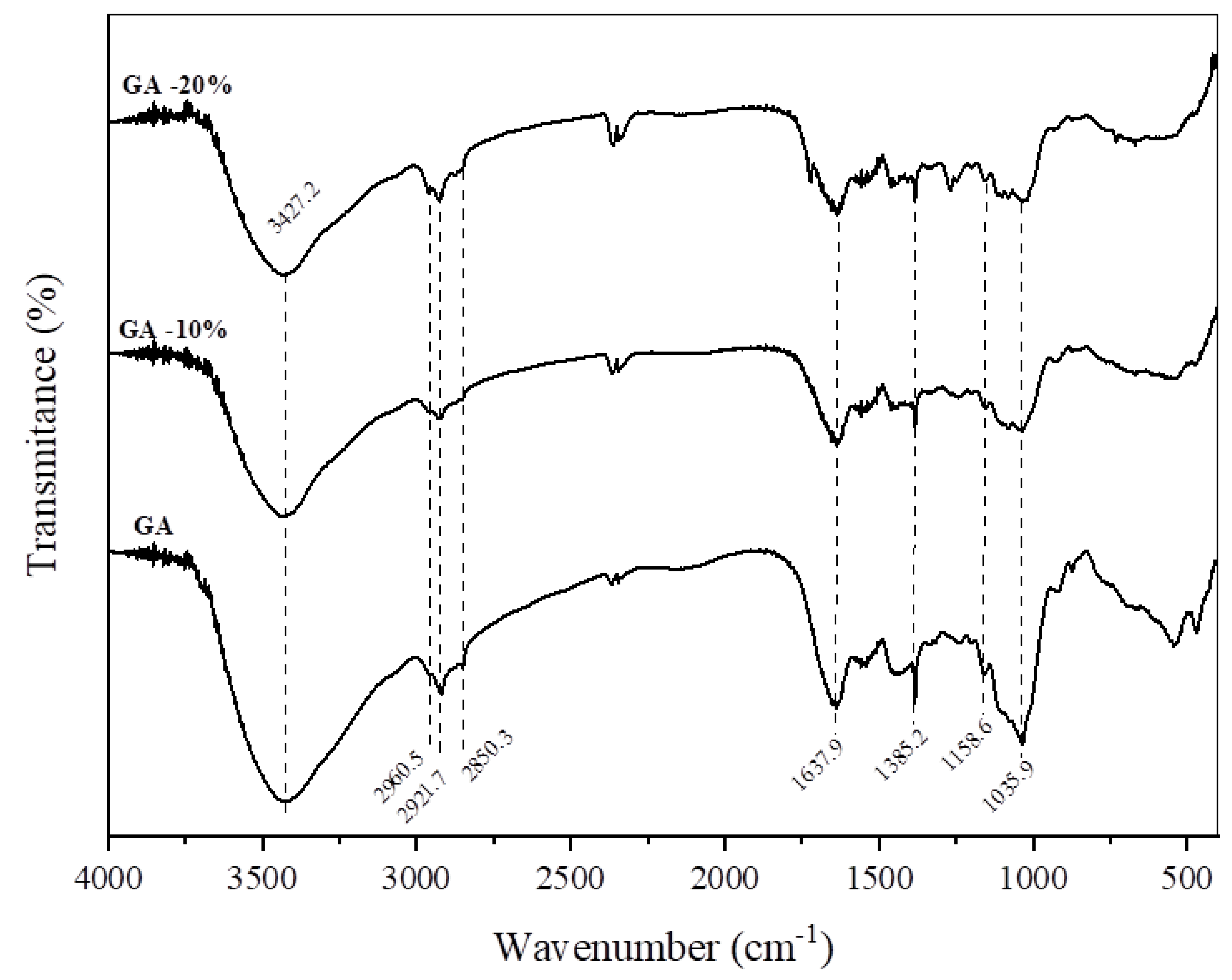
2.4.2. FTIR
2.4.3. Thickness
2.4.4. Mechanical Properties
2.4.5. Water Vapor Permeability (WVP)
2.4.6. Solubility
2.4.7. Water Contact Angle
2.4.8. Optical Properties
2.4.9. In Vitro Bioactive Activity of Films
2.4.10. Biodegradability
2.5. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic Compounds of PNC Extracts
3.2. Films Characterisation
3.2.1. Scanning Electron Microscopy (SEM)
3.2.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.2.3. Thickness
3.2.4. Mechanical Properties
3.2.5. Water Vapor Permeability (WVP)
3.2.6. Solubility
3.2.7. Water Contact Angle
3.2.8. Optical Properties
3.2.9. In Vitro Bioactive Activity of Films
3.2.10. Biodegradability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvarez-Parrilla, E.; Urrea-López, R.; de La Rosa, L.A. Bioactive components and health effects of pecan nuts and their byproducts: A review. J. Food Bioact. 2018, 1, 56–92. [Google Scholar] [CrossRef]
- Oro, T.; Bolini, H.M.A.; Arellano, D.B.; Block, J.M. Physicochemical and sensory quality of crude brazilian pecan nut oil during storage. J. Am. Oil Chem. Soc. 2009, 86, 971–976. [Google Scholar] [CrossRef]
- INC, Nuts & Dried Fruits—Statistical Yearbook 2019–2020. Statistical Yearbook. Available online: https://www.nutfruit.org/industry/technical-resources?category=statistical-yearbooks (accessed on 12 February 2021).
- Polmann, G.; Badia, V.; Frena, M.; Teixeira, G.L.; Rigo, E.; Block, J.M.; Feltes, M.M.C. Enzyme-assisted aqueous extraction combined with experimental designs allows the obtaining of a high-quality and yield pecan nut oil. LWT-Food Sci. Technol. 2019, 113, 108283. [Google Scholar] [CrossRef]
- Alves, J.S.; Confortin, T.C.; Todero, I.; Rodrigues, A.S.; Ribeiro, S.R.; Sasso, S.R.; Canabarro, N.I.; Wagner, R.; Cichoski, A.J.; Mazutti, M.A.; et al. Use of compressed fluids in the recovery of pecan nut cake oil: Influence of extraction conditions on yield and extract quality. J. Supercrit. Fluids 2020, 160, 104820. [Google Scholar]
- Maciel, L.G.; Teixeira, G.L. Plant-based pecan nut cake beverage enrichment of phytochemicals and antioxidant properties using multi-stage block freeze concentration. Food Prod. Proc. Nutr. 2022, 4, 1–14. [Google Scholar] [CrossRef]
- Salvador, A.A.; Podestá, R.; Block, J.M.; Ferreira, S.R.S.S. Increasing the value of pecan nut [Carya illinoinensis (Wangenh) C. Koch] cake by means of oil extraction and antioxidant activity evaluation. J. Supercrit. Fluids 2016, 116, 215–222. [Google Scholar] [CrossRef]
- Sarkis, J.R.; Côrrea, A.P.F.; Michel, I.; Brandeli, A.; Tessaro, I.C.; Marczak, L.D.F. Evaluation of the phenolic content and antioxidant activity of different seed and nut cakes from the edible oil industry. J. Am. Oil Chem. Soc. 2014, 91, 1773–1782. [Google Scholar] [CrossRef]
- Yong, H.; Wang, X.; Zhang, X.; Liu, Y.; Qin, Y.; Liu, J. Effects of anthocyanin-rich purple and black eggplant extracts on the physical, antioxidant, and pH-sensitive properties of chitosan film. Food Hydrocoll. 2019, 94, 93–104. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein-based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Henning, F.G.; Ito, V.C.; Demiate, I.M. Non-conventional starches for biodegradable films: A review focussing on characterisation and recent applications in food packaging. Carboidr. Polim. Tecnol. Appl. 2022, 4, 100157. [Google Scholar] [CrossRef]
- Rigueto, C.V.T.; Rosseto, M.; Alessandretti, I.; Oliveira, R.; Wohlmuth, D.A.R.; Menezes, J.F.; Loss, R.A.; Dettmer, A.; Pizzutti, I.R. Gelatin films from wastes: A review of production, characterization, and application trends in Food Preservation and Agriculture. Food Res. Int. 2022, 162, 112114. [Google Scholar] [CrossRef] [PubMed]
- Abdelhedi, O.; Salem, A.; Nasri, R.; Nasri, M.; Jridi, M. Food applications of bioactive marine gelatin films. Curr. Opin. Food Sci. 2022, 43, 206–215. [Google Scholar] [CrossRef]
- Bitencourt, C.M.; Fávaro-Trindade, C.S.; Sobral, P.J.A.; Carvalho, R.A. Gelatin-based films additive with curcuma ethanol extract: Antioxidant activity and physical properties of films. Food Hydrocoll. 2014, 40, 145–152. [Google Scholar] [CrossRef]
- Silva-Weiss, A.; Bifani, V.; Ihl, M.; Sobral, P.J.A.; Gómez-Guillén, M.C. Structural properties of films and rheology of film-forming solutions based on chitosan and chitosan-starch blend enriched with murta leaf extract. Food Hydrocoll. 2013, 31, 458–466. [Google Scholar] [CrossRef]
- Roesler, R.; Malta, L.G.; Carrasco, L.C.; Holanda, R.B.; Sousa, C.A.S.; Pastore, G.M. Atividade antioxidante de frutas do cerrado. Food Sci. Technol. 2007, 27, 53–60. [Google Scholar] [CrossRef]
- Malherbi, N.M.; Schmitz, A.C.; Grando, R.C.; Bilck, A.P.; Yamashita, F.; Tormen, L.; Fakhouri, F.M.; Velasco, J.I.; Bertan, L.C. Corn starch and gelatin-based films added with guabiroba pulp for application in food packaging. Food Packag. Shelf Life 2019, 19, 140–146. [Google Scholar] [CrossRef]
- ASTM—Standard test method for tensile properties of thin plastic sheeting. D882-97. In Annual Book of American Standard Testing Methods; ASTM: West Conshohocken, PA, USA, 1997.
- ASTM—Standard test method for water vapour transmission of materials. E96-80. In Annual Book of American Standard Testing Methods; ASTM: West Conshohocken, PA, USA, 1980.
- Mchugh, T.H.; Avena-Bustillos, R.; Krochta, J.M. Hydrophilic edible films: Modified procedure for water vapour permeability and explanation of thickness effects. J. Food Sci. 1993, 58, 899–903. [Google Scholar] [CrossRef]
- Peña, C.; Caba, K.d.L.; Eceiza, A.; Ruseckaite, R.; Mondragon, I. Enhancing water repellence and mechanical properties of gelatin films by tannin addition. Bioresour. Technol. 2010, 101, 6836–6868. [Google Scholar] [CrossRef]
- Filipini, G.S.; Romani, V.P.; Martins, V.G. Biodegradable and active-intelligent films based on methylcellulose and jambolão (Syzygium cumini) skins extract for food packaging. Food Hydrocoll. 2020, 109, 106139. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B. Physical properties of chitosan films incorporated with natural antioxidants. Ind. Crop. Prod. 2017, 107, 565–572. [Google Scholar] [CrossRef]
- Shahbazi, Y. The properties of chitosan and gelatin films incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil as biodegradable materials for active food packaging. Int. J. Biol. Macromol. 2017, 99, 746–753. [Google Scholar] [CrossRef]
- Boeira, C.P.; Flores, D.B.; Alves, J.S.; Moura, M.R.; Melo, P.T.S.; Rolim, C.M.B.; Libreloto, D.R.; Rosa, C.S. Effect of corn stigma extract on physical and antioxidant properties of biodegradable and edible gelatin and corn starch films. Int. J. Biol. Macromol. 2022, 208, 698–706. [Google Scholar] [CrossRef]
- Maciel, L.G.; Ribeiro, F.L.; Teixeira, G.L.; Molognoni, L.; dos Santos, J.N.; Nunes, I.L.; Block, J.M. The potential of the pecan nut cake as an ingredient for the food industry. Food Res. Int. 2020, 127, 108718. [Google Scholar] [CrossRef] [PubMed]
- Appel, H.M.; Govenor, H.L.; Ascenzo, E.S.; Schultz, J.C. Limitations of Folin assays of foliar phenolics in ecological studies. J. Chem. Ecol. 2001, 27, 761–778. [Google Scholar] [CrossRef]
- Galvão, A.C.; Bosch, R.; Coelho, K.A.; Machado, D.C.; Zuqui, V.; Robazza, W.S. Solubilidade do metanol, etanol e isopropanol em óleos vegetais a diferentes temperaturas e pressão atmosférica. Rev. Ciênc. Nat. 2013, 35, 311–317. [Google Scholar] [CrossRef]
- Dewi, S.R.; Stevens, L.A.; Person, A.E.; Ferrari, R.; Irvine, D.J.; Binner, E.R. Investigating the role of solvent type and microwave selective heating on the extraction of phenolic compounds from cacao (Theobroma cacao L.) pod husk. Food Bioprod. Process 2022, 134, 210–222. [Google Scholar] [CrossRef]
- Kiralan, S.S.; Kiralan, M.; Ozkan, G. Cold pressed pecan (Carya illinoinensis) oil. In Cold Pressed Oils: Green Technology, Bioactive Compounds, Functionality, and Applications, 1st ed.; Mohamed, F.R., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 515–524. [Google Scholar]
- Zhang, X.; Do, M.D.; Casey, P.; Sulistio, A.; Qiao, G.G.; Lundin, L.; Likkford, P.; Kosaraju, S. Chemical modification of gelatin by a natural phenolic cross-linker, tannic acid. J. Agric. Food Chem. 2010, 58, 6809–68015. [Google Scholar] [CrossRef]
- López, O.V.; Lecot, C.J.; Zaritzky, N.E.; García, M.A. Biodegradable packages development from starch based heat sealable films. J. Food Eng. 2011, 105, 254–263. [Google Scholar] [CrossRef]
- Hosseni, S.F.; Razaei, M.; Zandi, M.; Farahmandghavi, F. Fabrication of bio-nanocomposite films based on fish gelatin reinforced with quitosan nanoparticles. Food Hydrocoll. 2015, 44, 172–182. [Google Scholar] [CrossRef]
- Hosseni, S.F.; Razaei, M.; Zandi, M.; Farahmandghavi, F. Development of bioactive fish gelatin/chitosan nanoparticles composite films with antimicrobial properties. Food Chem. 2016, 194, 1266–1274. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S.; Prodpran, T.; Agustini, T.W. Physico-mechanical and antimicrobial properties of gelatin film from the skin of unicorn leatherjacket incorporated with essential oils. Food Hydrocoll. 2012, 28, 189–199. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Li, C.; Qin, Y.; Xiao, L.; Liu, J. Comparison of the structural, physical and functional properties of κ-carrageenan films incorporated with pomegranate flesh and peel extracts. Int. J. Biol. Macromol. 2020, 147, 1076–1088. [Google Scholar] [CrossRef]
- Brandsch, J.; Piringer, O. Characteristics of plastic materials. In Plastic Packaging: Interactions with Food and Pharmaceuticals, 2nd ed.; Piringer, O.G., Baner, A.L., Eds.; Wiley: Weinheim, Germany, 2008; pp. 15–60. [Google Scholar]
- Nur Amila Najwa, I.S.; Guerrero, P.; de La Caba, K.; Nur Hanani, Z.A. Physical and antioxidant properties of starch/gelatin films incorporated with Garcinia atroviridis leaves. Curr. Opin. Food Sci. 2020, 26, 100583. [Google Scholar] [CrossRef]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and characterisation of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef]
- Haghighi, H.; Biard, S.; Bigi, F.; De Leo, R.; Bedin, E.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Comprehensive characterisation of active chitosan-gelatin blend films enriched with different essential oils. Food Hydrocoll. 2019, 95, 33–42. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Bio-based composite edible films containing Origanum vulgare L. essential oil. Ind. Crop. Prod. 2015, 67, 403–413. [Google Scholar] [CrossRef]
- Xu, Y.; Rehmani, N.; Alsubaie, L.; Kim, C.; Sismour, E.; Scales, A. Tapioca starch active nanocomposite films and their antimicrobial effectiveness on ready-to-eat chicken meat. Food Packag. Shelf Life 2018, 16, 86–91. [Google Scholar] [CrossRef]
- Crizel, T.; Costa, T.M. Valorisation of food-grade industrial waste in obtaining active biodegradable films for packaging. Ind. Crop. Prod. 2016, 87, 218–228. [Google Scholar] [CrossRef]
- Albertos, I.; Avena-Bustillos, R.J.; Martín-Diana, A.B.; Wen-Xian, D.; Rico, D.; McHugh, T.H. Antimicrobial olive leaf gelatin films for enhancing the quality of cold-smoked salmon. Food Packag. Shelf Life 2017, 13, 49–55. [Google Scholar] [CrossRef]
- Mali, S.; Karam, L.B.; Ramos, L.P.; Grossman, M.V. Relationships among the composition and physicochemical properties of starches with the characteristics of their films. J. Agric. Food Chem. 2004, 52, 7720–7725. [Google Scholar] [CrossRef] [PubMed]
- Susmitha, A.; Sasikumar, K.; Rajan, D.; Padmakumar, A.M.; Nampoothiri, K.M. Development and characterization of corn starch-gelatin based edible films incorporated with mango and pineapple for active packaging. Food Biosci. 2021, 41, 100977. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Fontes, L.C.B.; Gonçalves, P.V.M.; Milanez, C.R.; Steel, C.J.; Collares-Queiroz, F.P. Filmes e coberturas comestíveis compostas à base de amidos nativo e gelatina na conservação e aceitação sensorial de uvas Crimson. Food Sci. Technol. 2007, 27, 369–375. [Google Scholar] [CrossRef]
- Wang, K.; Wu, K.; Xiao, M.; Kuang, Y.; Corke, H.; Ni, X.; Jiang, F. Structural characterisation and properties of konjac glucomannan and zein blend films. Int. J. Biol. Macromol. 2017, 105, 1096–1104. [Google Scholar] [CrossRef]
- Syahida, N.; Fitry, I.; Zuriyati, A.; Hanani, N. Effects of palm wax on the physical, mechanical and water barrier properties of fish gelatin films for food packaging application. Food Packag. Shelf Life 2020, 23, 100437. [Google Scholar] [CrossRef]
- Kaya, M.; Ravikumar, P.; Ilk, S.; Mujtaba, M.; Akyuz, L.; Labidi, J.; Salaberria, A.M.; Cakmak, Y.S.; Erful, S.K. Production and characterisation of chitosan-based edible films from Berberis crataegina’s fruit extract and seed oil. Innov. Food Sci. Emerg. Technol. 2018, 45, 287–297. [Google Scholar] [CrossRef]
- Pirnia, M.; Shirani, K.; Yazdi, F.T.; Moratazavi, S.A.; Mohebbi, M. Characterization of antioxidant active biopolymer bilayer film based on gelatin-frankincense incorporated with ascorbic acid and Hyssopus officinalis essential oil. Food Chem. 2022, 14, 100300. [Google Scholar] [CrossRef] [PubMed]
- Kan, J.; Liu, J.; Yong, H.; Liu, Y.; Qin, Y.; Liu, J. Development of active packaging based on chitosan-gelatin blend films functionalised with Chinese hawthorn (Crataegus pinnatifida) fruit extract. Int. J. Biol. Macromol. 2019, 140, 384–392. [Google Scholar] [CrossRef]
- Li, J.H.; Miao, J.; Wu, J.L.; Chen, S.F.; Zhang, Q.Q. Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll. 2014, 37, 166–173. [Google Scholar] [CrossRef]
- Kakaei, S.; Shahbazi, Y. Effect of chitosan-gelatin film incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil on survival of Listeria monocytogenes and chemical, microbial and sensory properties of minced trout fillet. LWT-Food Sci. Technol. 2016, 72, 432–438. [Google Scholar] [CrossRef]
- Silva, V.D.M.; Macedo, M.C.C.; Rodrigues, C.G.; Santos, A.N.; Freitas, A.C.; Fante, C.A. Biodegradable edible films of ripe banana peel and starch enriched with extract of Eriobotrya japonica leaves. Food Biosci. 2020, 38, 100750. [Google Scholar] [CrossRef]
- De Carli, C.; Aylanc, V.; Mouffok, K.M.; Santamaria-Echart, A.; Barreiro, F.; Tomás, A.; Pereira, C.; Rodrigues, P.; Vilas-Boas, M.; Falcão, S.I. Production of chitosan-based biodegradable active films using bio-waste enriched with polyphenol própolis extract envisaging food packaging applications. Int. J. Biol. Macromol. 2022, 213, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Boeira, C.P.; Alves, J.S.; Flores, D.B.; Moura, M.R.; Melo, P.T.S.; Rosa, C.S. Antioxidant and antimicrobial effect of an innovative active film containing corn stigma residue extract for refrigerated meat conservation. J. Food Proc. Preserv. 2021, 45, e15721. [Google Scholar] [CrossRef]
- Flores, M.; Cazón, P.; Vázquez, M. Active packaging film of chitosan and Santalum album essential oil: Characterization and application as butter sachet to retard lipid oxidation. Food Packag. Shelf Life 2022, 34, 100938. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- EN 13432; Packaging—Requirements for Packaging Recoverable through Composting and Biodegradation—Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging. European Committee for Standardisation: Brussels, Brussels, 2000. Available online: https://www.en-standard.eu/bs (accessed on 10 April 2021).


| Assay | Alcohol Content (%) | Time (min) | TPC (mg EAG/g of Sample) |
|---|---|---|---|
| T1 | 65 (−1) | 20 (−1) | 101.6 |
| T2 | 65 (−1) | 40 (+1) | 50.5 |
| T3 | 95 (+1) | 20 (−1) | 54.5 |
| T4 | 95 (+1) | 40 (+1) | 36.9 |
| T5 * | 80 (0) | 30 (0) | 40.9 |
| T6 * | 80 (0) | 30 (0) | 42.3 |
| T7 * | 80 (0) | 30 (0) | 39.5 |
| Effects | Standard Deviation | p-Value | |
|---|---|---|---|
| Total phenolic compounds | |||
| Interception | 52.3257 | 0.3156 | <0.01 * |
| (1) Alcohol content | −30.3450 | 0.8350 | <0.01 * |
| (2) Time | −34.3750 | 0.8350 | <0.01 * |
| 1 × 2 | 16.7350 | 0.8350 | 0.2235 |
| Sample | Thickness (mm) | TS (MPa) | EB (%) |
|---|---|---|---|
| GA | 0.039 ± <0.01 a | 62.99 ± 7.67 a | 3.53 ± 0.62 b |
| GA-10% | 0.036 ± <0.01 a | 58.82 ± 2.53 a | 4.42 ± 0.56 a |
| GA-20% | 0.039 ± <0.01 a | 62.87± 4.77 a | 4.18 ± 0.68 ab |
| Sample | WVP (g·m/m2·s·Pa) | Solubility (%) | Contact Angle (θ °) |
|---|---|---|---|
| GA | 1.03 × 10−10 ± <0.01 a | 69.10 ± 1.49 a | 70.11 ± 0.98 a |
| GA-10% | 1.10 × 10−10 ± <0.01 a | 67.28 ± 3.57 a | 64.90± 2.08 ab |
| GA-20% | 9.44 × 10−11 ± <0.01 a | 69.38 ± 3.35 a | 59.95 ± 5.90 b |
| Sample | Opacity (mm) | L* | C* | h° |
|---|---|---|---|---|
| GA | 1.32 ± 0.03 b | 90.59 ± 0.27 a | 6.03 ± 0.21 a | 287.35 ± 0.17 c |
| GA-10% | 1.81 ± 0.13 a | 89.70 ± 0.23 b | 5.95 ± 0.05 a | 292.56 ± 0.26 b |
| GA-20% | 1.96 ± 0.06 a | 88.79 ± 0.12 c | 4.94± 0.14 b | 298.33 ± 0.73 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, J.d.S.; Canabarro, N.I.; Boeira, C.P.; Melo, P.T.S.; Aouada, M.R.d.M.; da Rosa, C.S. Design of Biodegradable Films Using Pecan Nut Cake Extracts for Food Packing. Foods 2023, 12, 1405. https://doi.org/10.3390/foods12071405
Alves JdS, Canabarro NI, Boeira CP, Melo PTS, Aouada MRdM, da Rosa CS. Design of Biodegradable Films Using Pecan Nut Cake Extracts for Food Packing. Foods. 2023; 12(7):1405. https://doi.org/10.3390/foods12071405
Chicago/Turabian StyleAlves, Jamila dos Santos, Nicholas Islongo Canabarro, Caroline Pagnossim Boeira, Pamela Thais Sousa Melo, Marcia Regina de Moura Aouada, and Claudia Severo da Rosa. 2023. "Design of Biodegradable Films Using Pecan Nut Cake Extracts for Food Packing" Foods 12, no. 7: 1405. https://doi.org/10.3390/foods12071405
APA StyleAlves, J. d. S., Canabarro, N. I., Boeira, C. P., Melo, P. T. S., Aouada, M. R. d. M., & da Rosa, C. S. (2023). Design of Biodegradable Films Using Pecan Nut Cake Extracts for Food Packing. Foods, 12(7), 1405. https://doi.org/10.3390/foods12071405