Discrepancy of Effective Water Diffusivities Determined from Dynamic Vapor Sorption Measurements with Different Relative Humidity Step Sizes: Observations from Cereal Materials
Abstract
1. Introduction
2. Diffusion Models
2.1. For a Finite Cylinder
2.2. For a Sphere
2.3. For an Infinite Plate
2.4. Chemical Potential Gradient as a Driving Force
3. Materials and Methods
3.1. Materials
3.2. DVS Measurements
3.2.1. DVS of Glutinous Rice Grians
3.2.2. DVS of Glutinous Rice Flour
3.2.3. DVS of Dough Films
3.2.4. Water Concentration Calculation
3.3. Model Implementation and Parameter Estimation
4. Results and Analysis
4.1. Isotherm
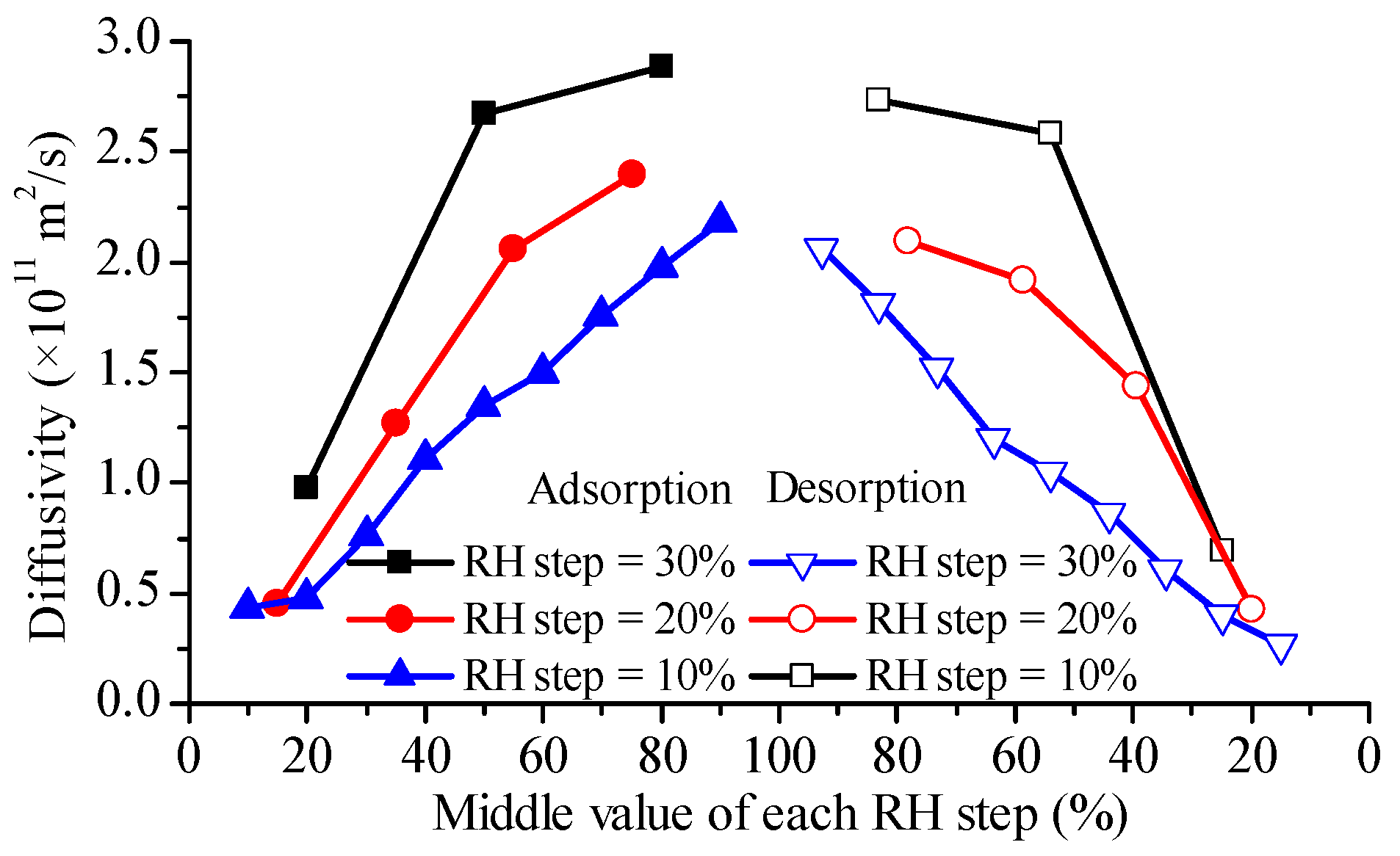
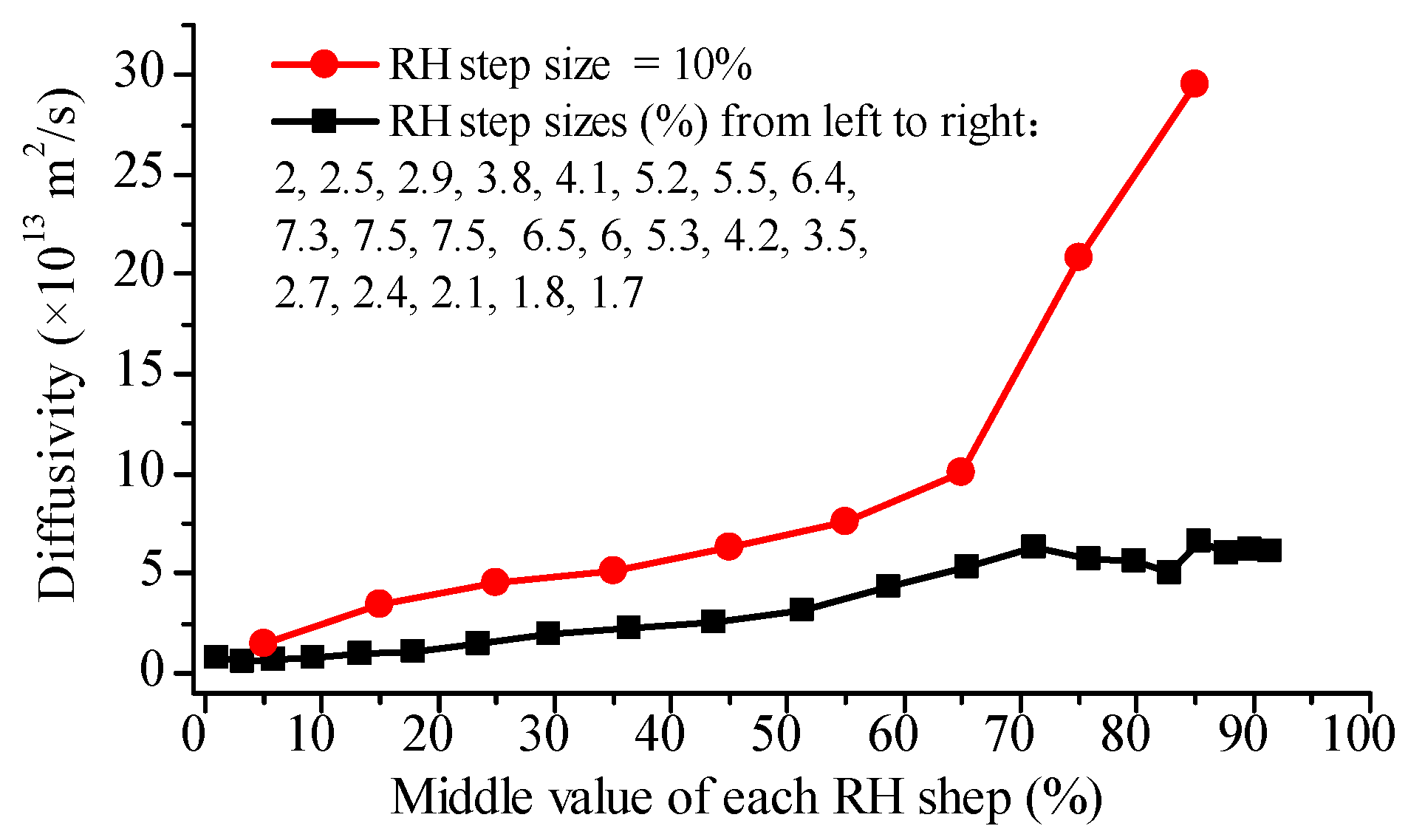
4.2. Observation from Glutinous Rice Grains
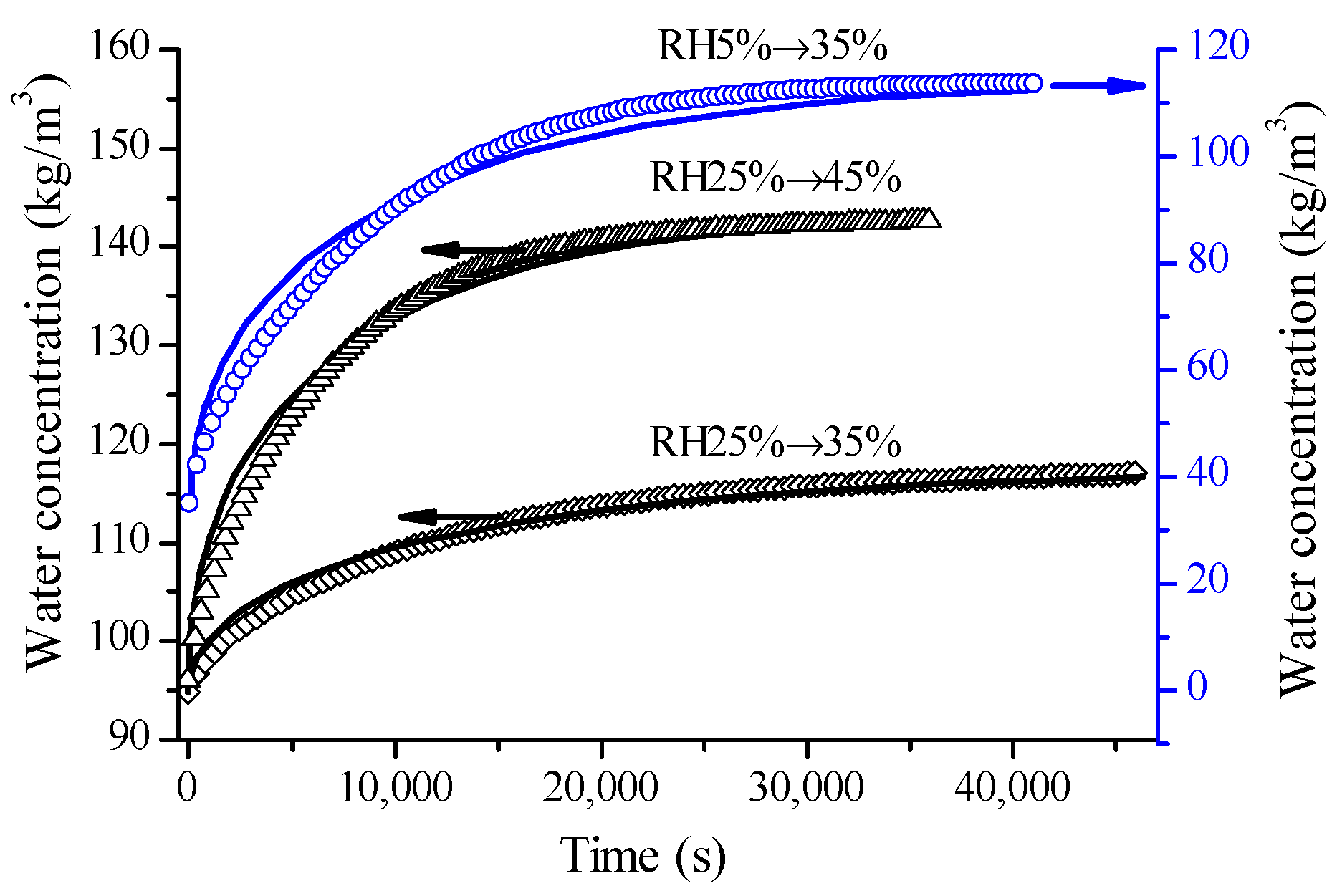
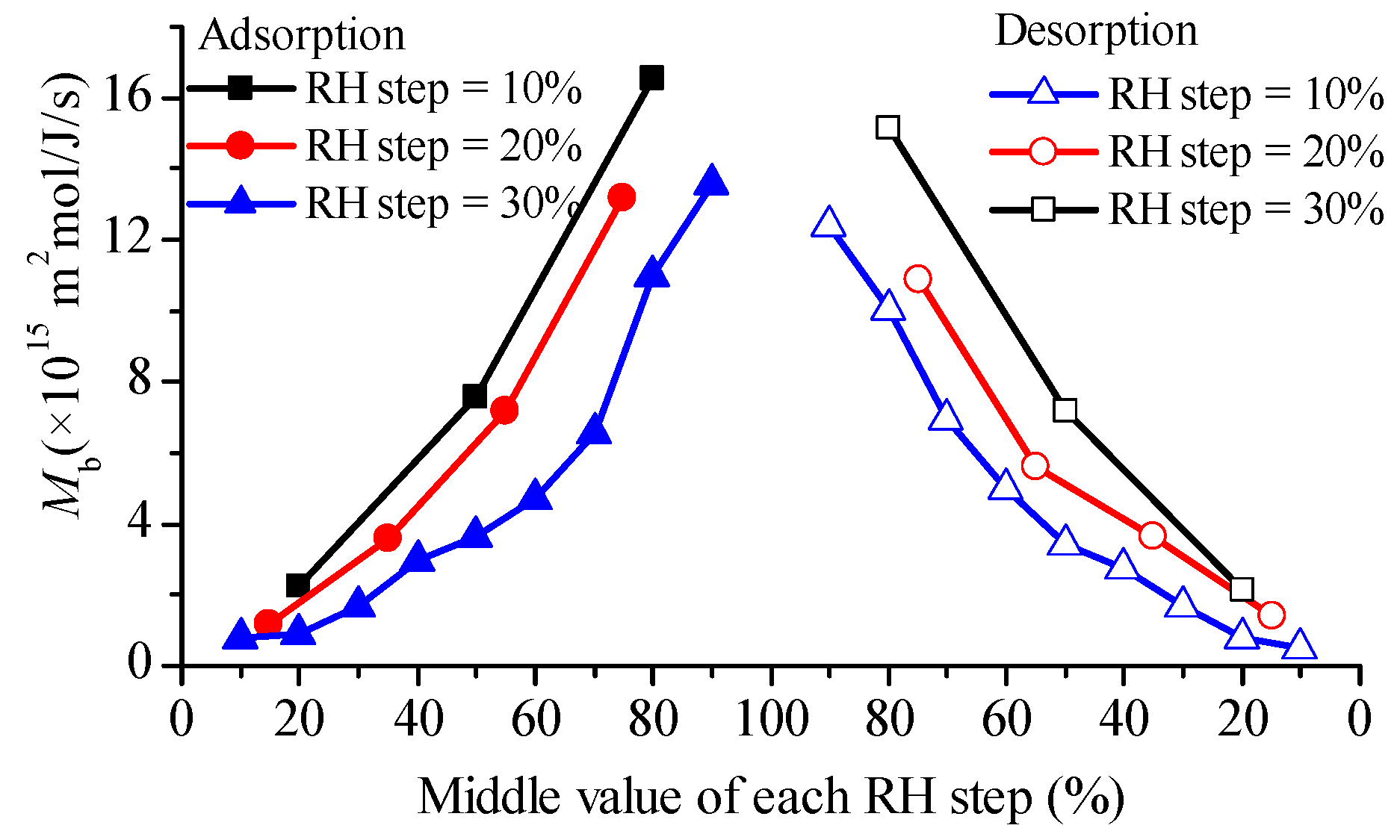
4.3. Observation from Glutinous Rice Flour
4.4. Observation from Wheat Flour Dough Films
4.5. Observation When Chemical Potential Gradient Was Considered as a Driving Force
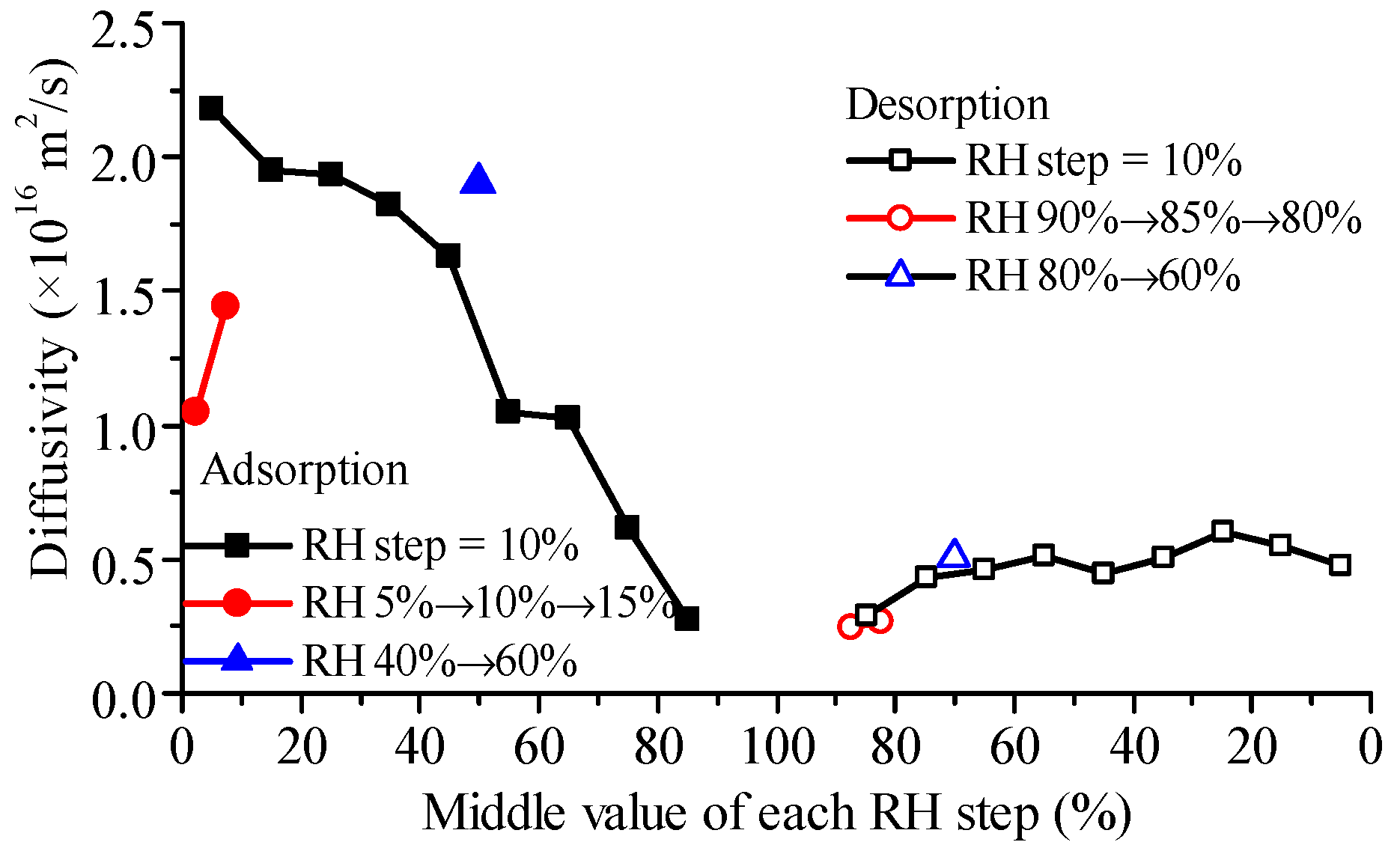
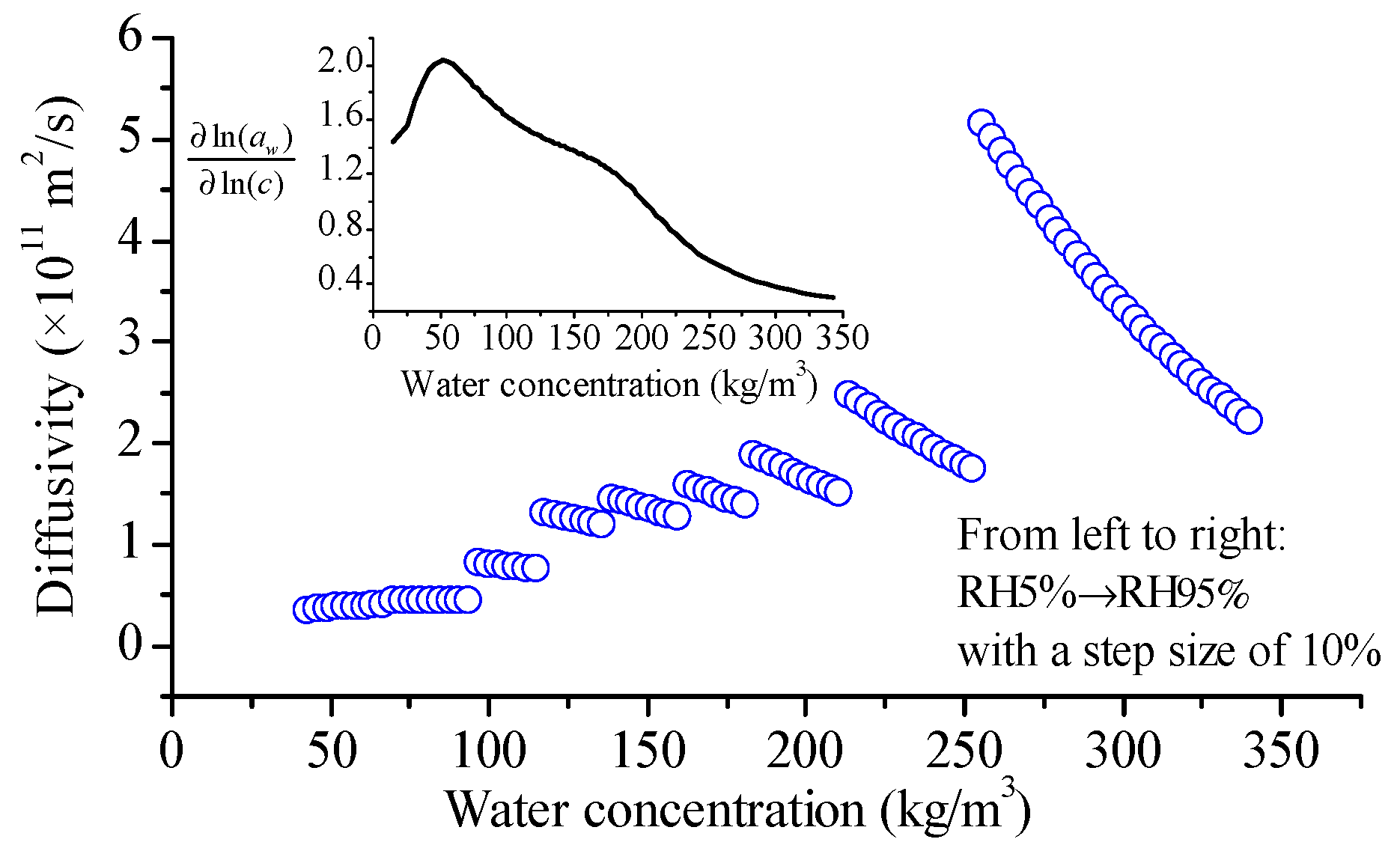
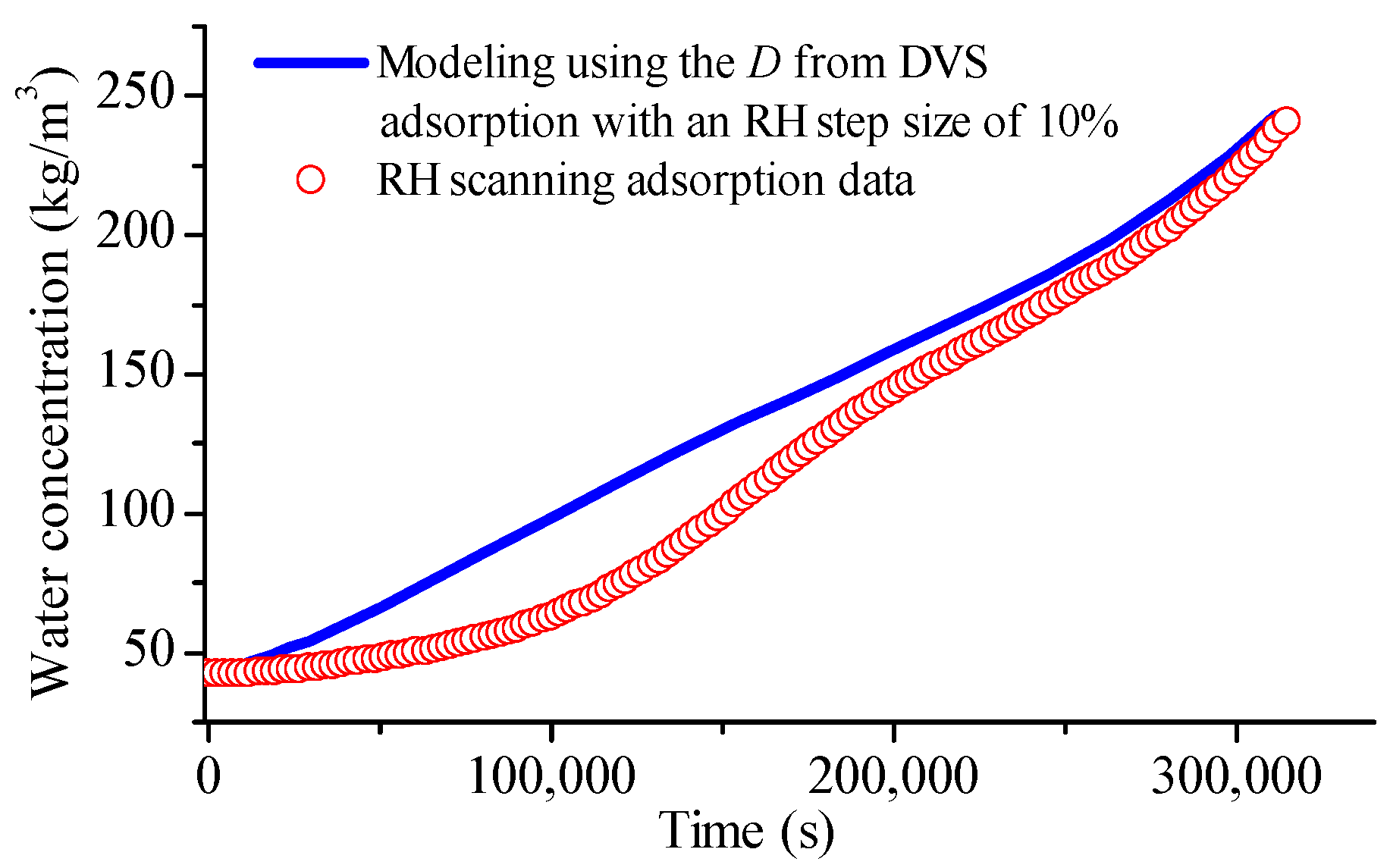
4.6. Observation from RH Scanning DVS Measurement
5. Discussions
5.1. Measurement Condition-Dependence and beyond of Water Diffusivity
5.2. Future Research Suggestions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jian, F.; Jayas, D.S. Grains Engineering Fundamentals of Drying and Storage; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Saravacos, G.D.; Krokida, M. Chapter 10 mass transfer properties of foods. In Engineering Properties of Foods, 4th ed.; Rao, M.A., Rizv, S.S.H., Datta, A.K., Ahmed, J., Eds.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Chitsuthipakorn, K.; Thanapornpoonpong, S. Effect of large-scale paddy rice drying process using hot air combined with radio frequency heating on milling and cooking qualities of milled rice. Foods 2022, 11, 519. [Google Scholar] [CrossRef]
- Prakash, B.; Bingol, G.; Pan, Z. Moisture diffusivity in rice components during absorption and desorption. Dry. Technol. 2011, 29, 939–945. [Google Scholar] [CrossRef]
- Roca, E.; Guillard, V.; Broyart, B.; Guilbert, S.; Gontard, N. Effective moisture diffusivity modelling versus food structure and hygroscopicity. Food Chem. 2008, 106, 1428–1437. [Google Scholar] [CrossRef]
- Besbes, E.; Jury, V.; Monteau, J.-Y.; Bail, A.L. Water vapor transport properties during staling of bread crumb and crust as affected by heating rate. Food Res. Int. 2013, 50, 10–19. [Google Scholar] [CrossRef]
- Oliver, L.; Meinders, M.B.J. Dynamic water vapour sorption in gluten and starch films. J. Cereal Sci. 2011, 54, 409–416. [Google Scholar] [CrossRef]
- Aravindakshan, S.; Nguyen, T.H.A.; Kyomugasho, C.; Buvé, C.; Dewettinck, K.; Loey, A.V.; Hendrickx, M.E. The impact of drying and rehydration on the structural properties and quality attributes of pre-cooked dried beans. Foods 2021, 10, 1665. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Clarendon: Oxford, UK, 1975. [Google Scholar]
- Ferreira, J.P.L.; Silva, W.P.; Queiroz, A.J.M.; Figueirêdo, R.M.F.; Gomes, J.P.; Melo, B.A.; Santos, D.C.; Lima, T.L.B.; Branco, R.R.C.; Hamawand, I.; et al. Description of Cumbeba (Tacinga inamoena) waste drying at different temperatures using diffusion models. Foods 2020, 9, 1818. [Google Scholar] [CrossRef]
- Elbert, G.; Tolaba, M.P.; Aguerre, R.J.; Suarez, C. A diffusion model with a moisture dependent diffusion coefficient for parboiled rice. Dry. Technol. 2001, 19, 155–166. [Google Scholar] [CrossRef]
- Gaston, A.L.; Abalone, R.M.; Giner, S.A.; Bruce, D.M. Effect of modelling assumptions on the effective water diffusivity in wheat. Biosyst. Eng. 2004, 88, 175–185. [Google Scholar] [CrossRef]
- Perdana, J.; van der Sman, R.G.M.; Fox, M.B.; Boom, R.M.; Schutyser, M.A.I. Measuring and modelling of diffusivities in carbohydrate-rich matrices during thin film drying. J. Food Eng. 2014, 122, 38–47. [Google Scholar] [CrossRef]
- Yamamoto, S.; Hoshika, M.; Sano, Y. Determination of concentration dependent diffusion coefficient from drying rate. In Drying’85; Toei, R., Mujumdar, A.S., Eds.; Springer: Berlin/Heidelberg, Germany, 1985. [Google Scholar]
- Vera, C.M.; Mendoza, M.G.V. Concentration-dependent moisture diffusion coefficient estimation in peas drying considering shrinkage: An observer approach. Biosyst. Eng. 2022, 218, 256–273. [Google Scholar] [CrossRef]
- Jian, F.; Jayas, D.S. Characterization of isotherms and thin-layer drying of red kidney beans, Part II: Three-dimensional finite element models to estimate transient mass and heat transfer coefficients and water diffusivity. Dry. Technol. 2018, 36, 1707–1718. [Google Scholar] [CrossRef]
- Murrieta-Pazos, I.; Galet, L.; Patry, S.; Gaiani, C.; Scher, J. Evolution of particle structure during water sorption observed on different size fractions of durum wheat semolina. Powder Technol. 2014, 255, 66–73. [Google Scholar] [CrossRef]
- Meinders, M.B.J.; van Vliet, T. Modeling water sorption dynamics of cellular solid food systems using free volume theory. Food Hydrocoll. 2009, 23, 2234–2242. [Google Scholar] [CrossRef]
- Roman-Gutierrez, A.D.; Mabille, F.; Guilbert, S.; Cuq, B. Contribution of specific flour components to water vapor adsorption properties of wheat flours. Cereal Chem. 2003, 80, 558–563. [Google Scholar] [CrossRef]
- Thorell, A.; Wads, L. Determination of external mass transfer coefficients in dynamic sorption (DVS) measurements. Dry. Technol. 2018, 36, 332–340. [Google Scholar] [CrossRef]
- Masaro, L.; Zhu, X.X. Physical models of diffusion for polymer solutions, gels and solids. Prog. Polym. Sci. 1999, 24, 731–775. [Google Scholar] [CrossRef]
- William, J.K.; Steven, K.B.; Zhang, C. Polymer transport properties. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Zhao, X.; Li, W.; Zhang, H.; Li, X.; Fan, W. Reaction–diffusion approach to modeling water diffusion in glutinous rice flour particles during dynamic vapor adsorption. J. Food Sci. Technol. 2019, 56, 4605–4615. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, H.; Li, X.; Fan, W. Extended dynamic vapor sorption of glutinous rice flour observed in logarithmic time scale and its modeling as a process with varying activation energy. J. Food Process Eng. 2020, 43, e13572. [Google Scholar] [CrossRef]
- Bingol, G.; Prakash, B.; Pan, Z. Dynamic vapor sorption isotherms of medium grain rice varieties. LWT—Food Sci. Technol. 2012, 48, 156–163. [Google Scholar] [CrossRef]
- Steffe, J.F.; Singh, R.P. Liquid diffusivity of rough rice components. Trans. ASAE 1980, 23, 767–774, 782. [Google Scholar] [CrossRef]
- Steffe, J.F.; Singh, R.P. Diffusivity of starchy endosperm and bran of fresh and rewetted rice. J. Food Sci. 1980, 45, 356–361. [Google Scholar] [CrossRef]
- Sarker, N.N.; Kunze, O.R.; Strouboulis, T. Finite element simulation of rough rice drying. Dry. Technol. 1994, 12, 761–775. [Google Scholar] [CrossRef]
- Lu, R.; Siebenmorgen, T.J. Moisture diffusivity of long-grain rice components. Trans. ASAE 1992, 35, 1955–1961. [Google Scholar] [CrossRef]
- Engels, C.; Hendrickx, M.; De Sarnblanx, S.; De Gryze, I.; Tobback, P. Modelling water diffusion during long-grain rice soaking. J. Food Eng. 1986, 5, 55–73. [Google Scholar] [CrossRef]
- Kashaninejad, M.; Maghsoudlou, Y.; Rafiee, S.; Khomeiri, M. Study of hydration kinetics and density changes of rice (Tarom Mahali) during hydrothermal processing. J. Food Eng. 2007, 79, 1383–1390. [Google Scholar] [CrossRef]
- Dutta, A.; Chanda, A.; Chakraborty, R. A linear driving force (LDF) approximation of moisture diffusion kinetics in white rice. Int. J. Food Eng. 2008, 4, 203–312. [Google Scholar] [CrossRef]
- Tang, X.; De Rooij, M.R.; Van Duynhoven, J.; Van Breugdel, K. Dynamic volume change measurements of cereal materials by environmental scanning electron microscopy and videomicroscopy. J. Microsc. 2008, 230, 100–107. [Google Scholar] [CrossRef]
- Haque, M.A.; Shimizu, N.; Kimura, T.; Bala, B.K. Net isosteric heats of adsorption and desorption for different forms of hybrid rice. Int. J. Food Prop. 2007, 10, 25–37. [Google Scholar] [CrossRef]
- Li, X.; Cao, Z.; Wei, Z.; Feng, Q.; Wang, J. Equilibrium moisture content and sorption isosteric heats of five wheat varieties in China. J. Stored Prod. Res. 2011, 47, 39–47. [Google Scholar] [CrossRef]
- Hong, Y.-S.; Hong, K.S.; Lee, E.-S.; Cho, J.-H.; Lee, C.; Cheong, C.; Lee, C.-H. MR imaging and diffusion studies of soaked rice. Food Res. Int. 2009, 42, 237–245. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, H.; Duan, R.; Feng, Z. The states of water in glutinous rice flour characterized by interpreting desorption isotherm. J. Food Sci. Technol. 2017, 54, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Aguerre, R.J.; Suarez, C. Diffusion of bound water in starchy materials: Application to drying. J. Food Eng. 2004, 64, 389–395. [Google Scholar] [CrossRef]
- Carter, B.P.; Schmidt, S.J. Developments in glass transition determination in foods using moisture sorption isotherms. Food Chem. 2012, 132, 1693–1698. [Google Scholar] [CrossRef]
- Pierre, F.; Ayouz, M.; Perre, P. An original method for the determination of mass diffusivity in unsteady state. In Proceedings of the Eurodrying’2013, Paris, France, 2–4 October 2013. [Google Scholar]
- Srikiatden, J.; Roberts, J.S. Moisture transfer in solid food materials: A review of mechanisms, models, and measurements. Int. J. Food Prop. 2007, 10, 739–777. [Google Scholar] [CrossRef]
- Liu, X.; Chen, J.; Hou, H. Theoretical analysis of water diffusivity estimated by Crank’s equation. Chem. Eng. Proc. 2012, 55, 24–28. [Google Scholar] [CrossRef]
- Vogt, B.D.; Soles, C.L.; Lee, H.-J.; Lin, E.K.; Wu, W.-l. Moisture absorption and absorption kinetics in polyelectrolyte films: Influence of film thickness. Langmuir 2004, 20, 1453–1458. [Google Scholar] [CrossRef]
- Liu, S.; Bettelli, M.A.; Wei, X.; Capezza, A.J.; Sochor, B.; Nilsson, F.; Olsson, R.T.; Johansson, E.; Roth, S.V.; Hedenqvist, M.S. Design of hygroscopic bioplastic products stable in varying humidities. Macromol. Mater. Eng. 2023, 308, 2200630. [Google Scholar] [CrossRef]
- Buss, F.; Gocke, J.; Scharfer, P.; Schabel, W. From micro to nano thin polymer layers: Thickness and concentration dependence of sorption and the solvent diffusion coefficient. Macromolecules 2015, 48, 8285–8293. [Google Scholar] [CrossRef]
- Chadha, K.; Martemianov, S.; Thomas, A. Estimation of the effective water diffusion coefficient in Nafion membrane by water balance measurements. Fuel Cells 2021, 21, 139–148. [Google Scholar] [CrossRef]
- Tas, O.; Ertugrul, U.; Grunin, L.; Oztop, M.H. Investigation of the hydration behavior of different sugars by time domain-NMR. Foods 2022, 11, 1148. [Google Scholar] [CrossRef]
- De Santis, F.; Gorrasi, G.; Pantani, R. A spectroscopic approach to assess transport properties of water vapor in PLA. Polym. Test. 2015, 44, 15–22. [Google Scholar] [CrossRef]
- Ketelaars, A.A.J.; Pel, L.; Coumans, W.J.; Kerkhof, P.J.A.M. Drying kinetics: A comparison of diffusion coefficients from moisture concentration profiles and drying curves. Chem. Eng. Sci. 1995, 50, 1187–1191. [Google Scholar] [CrossRef]
- Kruckels, W.W. On gradient dependent diffusivity. Chem. Eng. Sci. 1973, 28, 1565–1576. [Google Scholar] [CrossRef]
- Van Milligen, B.P.; Bons, P.D.; Carreras, B.A.; Sanchez, R. On the applicability of Fick’s law to diffusion in inhomogeneous systems. Eur. J. Phys. 2005, 26, 913–925. [Google Scholar] [CrossRef]
- Ying, R.; Li, T.; Wu, C.; Huang, M. Role of aleurone cell walls in water diffusion and distribution within cereal grains. J. Cereal Sci. 2020, 93, 102952. [Google Scholar] [CrossRef]
- Mudiyanselage, C.M.R.; Karunasena, H.C.P.; Gu, Y.T.; Guan, L.; Senadeera, W. Novel trends in numerical modelling of plant food tissues and their morphological changes during drying—A review. J. Food Eng. 2017, 194, 24–39. [Google Scholar] [CrossRef]
- Khan, M.I.H.; Joarddera, M.U.H.; Kumara, C.; Karim, M.A. Multiphase porous media modelling: A novel approach to predicting food processing performance. Crit. Rev. Food Sci. Nutr. 2018, 58, 528–546. [Google Scholar] [CrossRef]
- Aregawi, W.A.; Abera, M.K.; Fanta, S.W.; Verboven, P.; Nicolai, B. Prediction of water loss and viscoelastic deformation of apple tissue using a multiscale model. J. Phys. Condens. Matter 2014, 26, 464111. [Google Scholar] [CrossRef]
- Hiew, T.N.; Huang, R.; Popov, I.; Feldman, Y.; Heng, P.W.S. A study of moisture sorption and dielectric processes of starch and sodium starch glycolate. Pharm. Res. 2017, 34, 2675–2688. [Google Scholar] [CrossRef]
- Meinders, M.B.J.; Oliver, L. Viscoelastic sorption behavior of starch and gluten. In Water Stress in Biological, Chemical, Pharmaceutical and Food Systems; Gustavo, F.G.-L., Liliana, A.-B., María, D.P.B., Jorge, W.-C., Efrén, P.-A., Gustavo, V.B.-C., Eds.; Springer: New York, NY, USA, 2015. [Google Scholar]
- Baup, S.; Jaffre, C.; Wolbert, D.; Laplanche, A. Adsorption of pesticides onto granular activated carbon: Determination of surface diffusivities using simple batch experiments. Adsorption 2000, 6, 219–228. [Google Scholar] [CrossRef]
| Adsorption | Desorption | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cL | kL | kH | kc | n | R2 | cL | kL | kH | kc | n | R2 | |
| Glutinous rice grains | 46.12 | 39.94 | 218.44 | 172.21 | 10.15 | 0.9930 | 63.45 | 21.17 | 220.64 | 117.67 | 8.62 | 0.9968 |
| Glutinous rice flour | 72.58 | 12.91 | 188.17 | 164.20 | 6.85 | 0.9998 | 93.99 | 11.86 | 193.47 | 96.82 | 5.48 | 0.9988 |
| Wheat dough films | 51.84 | 11.01 | 197.96 | 295.39 | 11.28 | |||||||
| Experimental Method | Diffusion Model | Grain Type | Diffusivity (×1011 m2/s) | Notes | Ref. |
|---|---|---|---|---|---|
| Drying | Fick’s 2nd law + DBC | Short grain | 7.24 | MCi = 29.8% db; 40 °C RH of drying air = 68.6% | [26] |
| Drying | Fick’s 2nd law + DBC | Short grain | 6.27 | MCi = 34% db; 40 °C RH of drying air = 63.4% | [27] |
| Drying | Fick’s 2nd law + CBC | Long grain | 14.10 | MCi = 19% db; 40 °C | [28] |
| Adsorption | Fick’s 2nd law + DBC | Long grain | ~2.2 | MCi = ~13.2% db RH = 84–90%; 40 °C | [29] |
| Adsorption | Fick’s 2nd law + CBC | Medium grain | 1.11, 0.19, 0.20, 0.80, 0.49 | RH 0% → 20% → 40% → 60% → 80% → 98%; 25 °C | [4] |
| Desorption | Fick’s 2nd law + DBC | Medium grain | 0.4, 0.80, 1.31, 1.51, 1.30 | RH 98% → 80% → 60% → 40% → 20% → 0%; 25 °C | [4] |
| Soaking | Fick’s 2nd law + CBC | Long grain | 6.67 | MCi = 14.5% db; 40 °C | [30] |
| Soaking | Fick’s 2nd law + DBC | Long grain | 16.20 | MCi = 17–22% wb 40 °C | [31] |
| Soaking | Fick’s 2nd law + DBC | Long grain | 8.62 | MCi = 16.5–22.5% wb 40 °C | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Wei, X.; Wang, H.; Liu, X.; Zhang, Y.; Zhang, H. Discrepancy of Effective Water Diffusivities Determined from Dynamic Vapor Sorption Measurements with Different Relative Humidity Step Sizes: Observations from Cereal Materials. Foods 2023, 12, 1470. https://doi.org/10.3390/foods12071470
Zhao X, Wei X, Wang H, Liu X, Zhang Y, Zhang H. Discrepancy of Effective Water Diffusivities Determined from Dynamic Vapor Sorption Measurements with Different Relative Humidity Step Sizes: Observations from Cereal Materials. Foods. 2023; 12(7):1470. https://doi.org/10.3390/foods12071470
Chicago/Turabian StyleZhao, Xuewei, Xiaoxiao Wei, Hongwei Wang, Xingli Liu, Yanyan Zhang, and Hua Zhang. 2023. "Discrepancy of Effective Water Diffusivities Determined from Dynamic Vapor Sorption Measurements with Different Relative Humidity Step Sizes: Observations from Cereal Materials" Foods 12, no. 7: 1470. https://doi.org/10.3390/foods12071470
APA StyleZhao, X., Wei, X., Wang, H., Liu, X., Zhang, Y., & Zhang, H. (2023). Discrepancy of Effective Water Diffusivities Determined from Dynamic Vapor Sorption Measurements with Different Relative Humidity Step Sizes: Observations from Cereal Materials. Foods, 12(7), 1470. https://doi.org/10.3390/foods12071470




