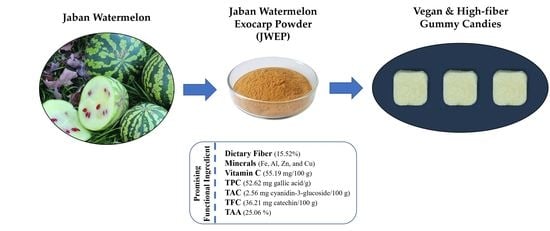
Physicochemical and Sensory Properties of Vegan Gummy Candies Enriched with High-Fiber Jaban Watermelon Exocarp Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. JWE Powder Preparation
2.3. JWE Powder Characterization
2.3.1. Vitamin C Content
2.3.2. Total Phenolic Compounds Content
2.3.3. Total Flavonoid
2.3.4. DPPH Radical-Scavenging Activity
2.3.5. Total Anthocyanin Content
2.4. GCs Production
2.5. GCs Characterization
2.5.1. Determination of Moisture Content and Water Activity
2.5.2. Determination of pH Value
2.5.3. Analysis of Color
2.5.4. Viscosity Properties
2.5.5. Textural Properties
2.5.6. Sensory Acceptability
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition and Antioxidant Activity of JWE Powder
3.2. Physicochemical Properties of GCs
3.2.1. Moisture Content and Water Activity
3.2.2. pH Value
3.2.3. Color
3.2.4. Viscosity
3.2.5. Textural Properties
3.2.6. Sensory Properties of GCs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gok, S.; Toker, O.S.; Palabiyik, I.; Konar, N. Usage possibility of mannitol and soluble wheat fiber in low calorie gummy candies. LWT 2020, 128, 109531. [Google Scholar] [CrossRef]
- Mandura, A.; Šeremet, D.; Ščetar, M.; Vojvodić Cebin, A.; Belščak-Cvitanović, A.; Komes, D. Physico-chemical, bioactive, and sensory assessment of white tea-based candies during 4-months storage. J. Food Process. Preserv. 2020, 44, e14628. [Google Scholar] [CrossRef]
- Šeremet, D.; Mandura, A.; Cebin, A.V.; Martinić, A.; Galić, K.; Komes, D. Challenges in confectionery industry: Development and storage stability of innovative white tea-based candies. J. Food Sci. 2020, 85, 2060–2068. [Google Scholar] [CrossRef]
- Silva, J.R.; da Silva, J.B.; Costa, G.N.; dos Santos, J.S.; Castro-Gomez, R.J.H. Probiotic gummy candy with xylitol: Development and potential inhibition of Streptococcus mutans UA 159. Res. Soc. Dev. 2020, 9, e7369108942. [Google Scholar] [CrossRef]
- Otálora, M.C.; de Jesús Barbosa, H.; Perilla, J.E.; Osorio, C.; Nazareno, M.A. Encapsulated betalains (Opuntia ficus-indica) as natural colorants. Case study: Gummy candies. LWT 2019, 103, 222–227. [Google Scholar] [CrossRef]
- Zhang, Y.; Barringer, S. Effect of hydrocolloids, sugar, and citric acid on strawberry volatiles in a gummy candy. J. Food Process. Preserv. 2018, 42, e13327. [Google Scholar] [CrossRef]
- Ali, M.R.; Mohamed, R.M.; Abedelmaksoud, T.G. Functional strawberry and red beetroot jelly candies rich in fibers and phenolic compounds. Food Syst. 2021, 4, 82–88. [Google Scholar] [CrossRef]
- Cappa, C.; Lavelli, V.; Mariotti, M. Fruit candies enriched with grape skin powders: Physicochemical properties. LWT 2015, 62, 569–575. [Google Scholar] [CrossRef]
- Jallinoja, P.; Vinnari, M.V.; Niva, M. Veganism and plant-based eating: Analysis of interplay between discursive strategies and lifestyle political consumerism. In Oxford Handbook of Political Consumerism; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Saari, U.A.; Herstatt, C.; Tiwari, R.; Dedehayir, O.; Mäkinen, S.J. The vegan trend and the microfoundations of institutional change: A commentary on food producers’ sustainable innovation journeys in Europe. Trends Food Sci. Technol. 2021, 107, 161–167. [Google Scholar] [CrossRef]
- Tarahi, M.; Hedayati, S.; Shahidi, F. Effects of mung bean (Vigna radiata) protein isolate on rheological, textural, and structural properties of native corn starch. Polymers 2022, 14, 3012. [Google Scholar] [CrossRef]
- Sebastiani, G.; Herranz Barbero, A.; Borrás-Novell, C.; Alsina Casanova, M.; Aldecoa-Bilbao, V.; Andreu-Fernández, V.; Pascual Tutusaus, M.; Ferrero Martínez, S.; Gómez Roig, M.D.; García-Algar, O. The effects of vegetarian and vegan diet during pregnancy on the health of mothers and offspring. Nutrients 2019, 11, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarahi, M.; Shahidi, F.; Hedayati, S. Physicochemical, pasting, and thermal properties of native corn starch–mung bean protein isolate composites. Gels 2022, 8, 693. [Google Scholar] [CrossRef] [PubMed]
- Altınok, E.; Palabiyik, I.; Gunes, R.; Toker, O.S.; Konar, N.; Kurultay, S. Valorisation of grape by-products as a bulking agent in soft candies: Effect of particle size. LWT 2020, 118, 108776. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Antioxidant dietary fiber product: A new concept and a potential food ingredient. J. Agric. Food Chem. 1998, 46, 4303–4306. [Google Scholar] [CrossRef] [Green Version]
- Feizy, J.; Jahani, M.; Ahmadi, S. Antioxidant activity and mineral content of watermelon peel. J. Food Bioprocess Eng. 2020, 3, 35–40. [Google Scholar]
- Hasanin, M.S.; Hashem, A.H. Eco-friendly, economic fungal universal medium from watermelon peel waste. J. Microbiol. Methods 2020, 168, 105802. [Google Scholar] [CrossRef]
- Tarazona-Díaz, M.P.; Viegas, J.; Moldao-Martins, M.; Aguayo, E. Bioactive compounds from flesh and by-product of fresh-cut watermelon cultivars. J. Sci. Food Agric. 2011, 91, 805–812. [Google Scholar] [CrossRef]
- Ghaedrahmati, S.; Shahidi, F.; Roshanak, S.; Nassiri Mahallati, M. Application of jaban watermelon exocarp powder in low-calorie ice cream formulation and evaluation of its physicochemical, rheological, and sensory properties. J. Food Process. Preserv. 2021, 45, e15768. [Google Scholar] [CrossRef]
- Romo-Zamarrón, K.F.; Pérez-Cabrera, L.E.; Tecante, A. Physicochemical and sensory properties of gummy candies enriched with pineapple and papaya peel powders. Food Nutr. Sci. 2019, 10, 1300–1312. [Google Scholar] [CrossRef] [Green Version]
- Ho, L.H.; Suhaimi, M.A.; Ismail, I.; Mustafa, K.A. Effect of different drying conditions on proximate compositions of red-and yellow-fleshed watermelon rind powders. J. Agrobiotechnology 2016, 7, 1–12. [Google Scholar]
- Horwitz, W.; Chichilo, P.; Reynolds, H. Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Washington, DC, USA, 1970. [Google Scholar]
- Pinelo, M.; Rubilar, M.; Sineiro, J.; Nunez, M. Extraction of antioxidant phenolics from almond hulls (Prunus amygdalus) and pine sawdust (Pinus pinaster). Food Chem. 2004, 85, 267–273. [Google Scholar] [CrossRef]
- Roshanak, S.; Rahimmalek, M.; Goli, S.A.H. Evaluation of seven different drying treatments in respect to total flavonoid, phenolic, vitamin C content, chlorophyll, antioxidant activity and color of green tea (Camellia sinensis or C. assamica) leaves. J. Food Sci. Technol. 2016, 53, 721–729. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Li, T.; Fu, X.; Abbasi, A.M.; Zheng, B.; Liu, R.H. Phenolics content, antioxidant and antiproliferative activities of dehulled highland barley (Hordeum vulgare L.). J. Funct. Foods 2015, 19, 439–450. [Google Scholar] [CrossRef]
- Lin, C.-W.; Yu, C.-W.; Wu, S.-C.; Yih, K.-H. DPPH Free-Radical Scavenging Activity, Total Phenolic Contents and Chemical Composition Analysis of Forty-Two Kinds of Essential Oils. J. Food Drug Anal. 2009, 17. [Google Scholar] [CrossRef]
- Yang, L.; Rong-Rong, C.; Ji-Li, F.; Ke, Y. Total anthocyanins and cyanidin-3-O-glucoside contents and antioxidant activities of purified extracts from eight different pigmented plants. Pharmacogn. Mag. 2019, 15, 124. [Google Scholar]
- Camelo-Méndez, G.; Ragazzo-Sánchez, J.; Jimenez-Aparicio, A.R.; Vanegas-Espinoza, P.E.; Paredes-López, O.; Villar-Martínez, D. Comparative study of anthocyanin and volatile compounds content of four varieties of Mexican roselle (Hibiscus sabdariffa L.) by multivariable analysis. Plant Foods Hum. Nutr. 2013, 68, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Blanco, D.; Oria, R.; Sánchez-Gimeno, A.C. White guava fruit and purees: Textural and rheological properties and effect of the temperature. J. Texture Stud. 2009, 40, 334–345. [Google Scholar] [CrossRef]
- Amjadi, S.; Ghorbani, M.; Hamishehkar, H.; Roufegarinejad, L. Improvement in the stability of betanin by liposomal nanocarriers: Its application in gummy candy as a food model. Food Chem. 2018, 256, 156–162. [Google Scholar] [CrossRef]
- Haggag, E.G.; Kamal, A.M.; Abdelhady, M.I.; El-Sayed, M.M.; El-Wakil, E.A.; Abd-El-hamed, S.S. Antioxidant and cytotoxic activity of polyphenolic compounds isolated from the leaves of Leucenia leucocephala. Pharm. Biol. 2011, 49, 1103–1113. [Google Scholar] [CrossRef]
- Jayaram, S.; Dharmesh, S.M. Assessment of Antioxidant Potentials of Free and Bound Phenolics of Hemidesmus indicus (L) R. Br Against Oxidative Damage Smitha Jayaram. Pharmacogn. Res. 2011, 3. [Google Scholar]
- Volden, J.; Bengtsson, G.B.; Wicklund, T. Glucosinolates, L-ascorbic acid, total phenols, anthocyanins, antioxidant capacities and colour in cauliflower (Brassica oleracea L. ssp. botrytis); effects of long-term freezer storage. Food Chem. 2009, 112, 967–976. [Google Scholar] [CrossRef]
- Ghaznavi, R.; Kadkhodaee, M.; Khastar, H.; Zahmatkesh, M. Renal oxidative stress status and histology in gentamicin nephrotoxicity: The effects of antioxidant vitamins. Tehran Univ. Med. J. TUMS Publ. 2006, 64, 15–22. [Google Scholar]
- Krinsky, N.I.; Beecher, G.; Burk, R.; Chan, A.; Erdman, J.; Jacob, R.; Jialal, I.; Kolonel, L.; Marshall, J.; Taylor Mayne, P. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Inst. Med. 2000, 19, 95–185. [Google Scholar]
- Al-Sayed, H.M.; Ahmed, A.R. Utilization of watermelon rinds and sharlyn melon peels as a natural source of dietary fiber and antioxidants in cake. Ann. Agric. Sci. 2013, 58, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Abdulazeez, A.; Usman, A.; Audu, S.; Ibrahim, I.L.; Kwokwu, S.I.; Umar, M.T.; Babatunde, J.; Uthman, A. Antioxidant assay and flavonoids of rind and seed of Citrullus lanatusl linn (water melon). Commun. Phys. Sci. 2020, 5, 29–33. [Google Scholar]
- Kistriyani, L.; Ramadhani, A.A.; Resphaty, D.P. Encapsulation of Anthocyanin and Flavonoid from Watermelon Rind (Citrullus lanatus) as a Natural Food Preservative. Key Eng. Mater. 2019, 818, 50–55. [Google Scholar] [CrossRef]
- Ergun, R.; Lietha, R.; Hartel, R.W. Moisture and shelf life in sugar confections. Crit. Rev. Food Sci. Nutr. 2010, 50, 162–192. [Google Scholar] [CrossRef]
- Buerman, E.C.; Worobo, R.W.; Padilla-Zakour, O.I. Thermal resistance of xerophilic fungi in Low-Water-Activity (0.70 to 0.80) confectionery model foods. J. Food Prot. 2019, 82, 390–394. [Google Scholar] [CrossRef]
- Miranda, J.S.; Costa, B.V.; de Oliveira, I.V.; de Lima, D.C.N.; Martins, E.M.F.; de Castro Leite Júnior, B.R.; do Nascimento Benevenuto, W.C.A.; de Queiroz, I.C.; da Silva, R.R.; Martins, M.L. Probiotic jelly candies enriched with native Atlantic Forest fruits and Bacillus coagulans GBI-30 6086. LWT 2020, 126, 109275. [Google Scholar] [CrossRef]
- Delgado, P.; Bañón, S. Effects of replacing starch by inulin on the physicochemical, texture and sensory characteristics of gummy jellies. CyTA J. Food 2018, 16, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Moghaddas Kia, E.; Ghaderzadeh, S.l.; Mojaddar Langroodi, A.; Ghasempour, Z.; Ehsani, A. Red beet extract usage in gelatin/gellan based gummy candy formulation introducing Salix Aegyptiaca distillate as a flavouring agent. J. Food Sci. Technol. 2020, 57, 3355–3362. [Google Scholar] [CrossRef] [PubMed]
- DeMars, L.L.; Ziegler, G.R. Texture and structure of gelatin/pectin-based gummy confections. Food Hydrocoll. 2001, 15, 643–653. [Google Scholar] [CrossRef]
- Hasani, M.; Yazdanpanah, S. The Effects of Gum Cordia on the Physicochemical, Textural, Rheological, Microstructural, and Sensorial Properties of Apple Jelly. J. Food Qual. 2020, 2020. [Google Scholar] [CrossRef]
- Hartel, R.W.; Firoozmand, H. Emulsifiers in confectionery. In Food Emulsifiers and Their Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 323–346. [Google Scholar]
- Yu, Z.; Zhan, J.; Wang, H.; Zheng, H.; Xie, J.; Wang, X. Analysis of influencing factors on viscosity of agar solution for capsules. J. Phys. Conf. Ser. 2020, 1653, 012059. [Google Scholar] [CrossRef]
- Hani, N.M.; Romli, S.R.; Ahmad, M. Influences of red pitaya fruit puree and gelling agents on the physico-mechanical properties and quality changes of gummy confections. Int. J. Food Sci. Technol. 2015, 50, 331–339. [Google Scholar] [CrossRef]
- da Costa, J.N.; Leal, A.R.; Nascimento, L.G.; Rodrigues, D.C.; Muniz, C.R.; Figueiredo, R.W.; Mata, P.; Noronha, J.P.; de Sousa, P.H.M. Texture, microstructure and volatile profile of structured guava using agar and gellan gum. Int. J. Gastron. Food Sci. 2020, 20, 100207. [Google Scholar] [CrossRef]
- Lubbers, S.; Guichard, E. The effects of sugars and pectin on flavour release from a fruit pastille model system. Food Chem. 2003, 81, 269–273. [Google Scholar] [CrossRef]

| Samples | JWE Powder | Citric Acid | Agar Gum | Pectin | Arabic Gum | Sucrose | Glucose | Starch | Water |
|---|---|---|---|---|---|---|---|---|---|
| GC 1 | 20 | 0.75 | 0.5 | 0.541 | 0.541 | 20 | 10 | 0.18 | 47.488 |
| GC 2 | 20 | 0.75 | 1 | 0.541 | 0.541 | 20 | 10 | 0.18 | 46.988 |
| GC 3 | 20 | 1 | 0.5 | 0.541 | 0.541 | 20 | 10 | 0.18 | 47.238 |
| GC 4 | 20 | 1 | 1 | 0.541 | 0.541 | 20 | 10 | 0.18 | 46.738 |
| GC 5 | 35 | 0.75 | 0.5 | 0.541 | 0.541 | 20 | 10 | 0.18 | 32.488 |
| GC 6 | 35 | 0.75 | 1 | 0.541 | 0.541 | 20 | 10 | 0.18 | 31.988 |
| GC 7 | 35 | 1 | 0.5 | 0.541 | 0.541 | 20 | 10 | 0.18 | 32.238 |
| GC 8 | 35 | 1 | 1 | 0.541 | 0.541 | 20 | 10 | 0.18 | 31.738 |
| GC 9 | 50 | 0.75 | 0.5 | 0.541 | 0.541 | 20 | 10 | 0.18 | 17.488 |
| GC 10 | 50 | 0.75 | 1 | 0.541 | 0.541 | 20 | 10 | 0.18 | 16.988 |
| GC 11 | 50 | 1 | 0.5 | 0.541 | 0.541 | 20 | 10 | 0.18 | 17.238 |
| GC 12 | 50 | 1 | 1 | 0.541 | 0.541 | 20 | 10 | 0.18 | 16.738 |
| Value | Measurement Index |
|---|---|
| Vitamin C (mg/100 g) | 55.19 ± 0.41 |
| TPC (mg gallic acid/g) | 52.62 ± 0.34 |
| TAC (mg cyanidin-3-glucoside/100 g) | 2.56 ± 0.03 |
| TFC (mg catechin/100 g) | 36.21 ± 0.12 |
| TAA (%) | 25.06 ± 0.27 |
| Samples | Moisture Content (g/100 g) | Water Activity | pH |
|---|---|---|---|
| GC 1 | 29.408 ± 0.354 a | 0.815 ± 0.006 a | 2.735 ± 0.078 bcd |
| GC 2 | 27.586 ± 1.087 a | 0.792 ± 0.004 ab | 2.725 ± 0.035 bcd |
| GC 3 | 27.820 ± 1.393 a | 0.777 ± 0.001 b | 2.415 ± 0.064 de |
| GC 4 | 28.580 ± 1.563 a | 0.793 ± 0.002 ab | 2.370 ± 0.085 e |
| GC 5 | 26.988 ± 0.178 abc | 0.763 ± 0.017 bc | 2.950 ± 0.014 abc |
| GC 6 | 26.607 ± 0.562 abc | 0.771 ± 0.001 b | 2.975 ± 0.007 abc |
| GC 7 | 27.457 ± 0.609 ab | 0.790 ± 0.011 ab | 2.680 ± 0.226 cde |
| GC 8 | 26.745 ± 0.147 abc | 0.774 ± 0.008 b | 2.790 ± 0.014 bc |
| GC 9 | 24.347 ± 0.245 cd | 0.732 ± 0.003 cd | 3.205 ± 0.071 a |
| GC 10 | 24.120 ± 0.035 cd | 0.768 ± 0.001 b | 3.150 ± 0.071 a |
| GC 11 | 24.613 ± 0.143 bcd | 0.725 ± 0.020 de | 2.985 ± 0.007 abc |
| GC 12 | 23.348 ± 0.470 d | 0.695 ± 0.010 e | 3.025 ± 0.035 ab |
| Samples | L* | a* | b* | Viscosity (Pa·s) |
|---|---|---|---|---|
| GC 1 | 70.86 ± 4.83 d | −12.81 ± 0.25 c | 14.99 ± 0.20 ef | 0.457 ± 0.002 k |
| GC 2 | 70.71 ± 2.77 d | −12.39 ± 0.04 c | 15.36 ± 0.98 e | 0.687 ± 0.005 i |
| GC 3 | 70.87 ± 5.65 d | −12.09 ± 0.54 c | 13.43 ± 0.75 f | 0.810 ± 0.003 g |
| GC 4 | 72.05 ± 2.35 d | −12.40 ± 0.45 c | 14.36 ± 0.03 f | 0.795 ± 0.005 h |
| GC 5 | 83.18 ± 3.01 a | −10.22 ± 0.34 ab | 14.43 ± 0.29 f | 1.183 ± 0.003 c |
| GC 6 | 79.14 ± 4.40 b | −10.67 ± 0.85 ab | 18.43 ± 0.99 d | 0.848 ± 0.003 f |
| GC 7 | 82.62 ± 6.77 a | −11.03 ± 0.75 b | 17.90 ± 0.33 d | 0.543 ± 0.006 j |
| GC 8 | 79.81 ± 3.87 ab | −10.91 ± 0.26 ab | 19.52 ± 0.19 c | 1.018 ± 0.005 d |
| GC 9 | 82.56 ± 1.09 a | −10.15 ± 0.46 ab | 18.34 ± 0.46 d | 1.385 ± 0.008 b |
| GC 10 | 77.30 ± 2.86 bc | −10.27 ± 0.13 ab | 24.24 ± 0.40 a | 1.550 ± 0.008 a |
| GC 11 | 75.48 ± 4.09 c | −10.66 ± 0.92 ab | 24.55 ± 0.54 a | 0.940 ± 0.003 e |
| GC 12 | 78.43 ± 5.91 bc | −9.97 ± 0.52 a | 21.70 ± 0.64 b | 1.184 ± 0.003 c |
| Samples | Hardness (g) | Springiness (mm) | Cohesiveness | Gumminess (g) | Chewiness (gmm) |
|---|---|---|---|---|---|
| GC 1 | 1667 ± 120 d | 5.355 ± 0.021 a | 0.420 ± 0.003 e | 928.3 ± 157 e | 43.20 ± 1.21 e |
| GC 2 | 2101 ± 230 d | 4.085 ± 0.076 cde | 0.615 ± 0.007 bc | 1287.0 ± 345 de | 58.95 ± 1.87 de |
| GC 3 | 2253 ± 654 d | 3.875 ± 0.065 de | 0.670 ± 0.003 ab | 1283.5 ± 760 de | 46.65 ± 1.01 e |
| GC 4 | 3595 ± 435 bcd | 3.690 ± 0.023 e | 0.695 ± 0.001 ab | 2498.5 ± 125 ab | 72.70 ± 2.08 d |
| GC 5 | 3934 ± 128 bcd | 4.455 ± 0.012 bcd | 0.625 ± 0.010 b | 2451.0 ± 659 abc | 94.55 ± 1.02 c |
| GC 6 | 2446 ± 107 cd | 4.025 ± 0.010 de | 0.730 ± 0.011 a | 1775.5 ± 234 bcde | 67.35 ± 6.20 d |
| GC 7 | 2164 ± 802 d | 4.395 ± 0.062 bcd | 0.615 ± 0.009 bc | 1308.5 ± 222 cde | 56.45 ± 3.65 de |
| GC 8 | 4572 ± 700 bc | 4.830 ± 0.012 ab | 0.540 ± 0.005 cd | 2280.5 ± 896 abcd | 115.25 ± 2.51 ab |
| GC 9 | 4686 ± 203 bc | 4.790 ± 0.043 ab | 0.435 ± 0.001 e | 2232.0 ± 450 abcd | 103.60 ± 1.90 bc |
| GC 10 | 5779 ± 513 ab | 4.720 ± 0.019 abc | 0.470 ± 0.003 de | 2708.0 ± 65 ab | 125.35 ± 1.33 a |
| GC 11 | 4835 ± 129 b | 4.830 ± 0.011 ab | 0.475 ± 0.006 de | 2290.0 ± 102 abcd | 116.80 ± 2.23 ab |
| GC 12 | 7232 ± 345 a | 4.475 ± 0.029 bcd | 0.415 ± 0.002 e | 3023.5 ± 603 a | 124.20 ± 1.13 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarahi, M.; Mohamadzade Fakhr-davood, M.; Ghaedrahmati, S.; Roshanak, S.; Shahidi, F. Physicochemical and Sensory Properties of Vegan Gummy Candies Enriched with High-Fiber Jaban Watermelon Exocarp Powder. Foods 2023, 12, 1478. https://doi.org/10.3390/foods12071478
Tarahi M, Mohamadzade Fakhr-davood M, Ghaedrahmati S, Roshanak S, Shahidi F. Physicochemical and Sensory Properties of Vegan Gummy Candies Enriched with High-Fiber Jaban Watermelon Exocarp Powder. Foods. 2023; 12(7):1478. https://doi.org/10.3390/foods12071478
Chicago/Turabian StyleTarahi, Mohammad, Malihe Mohamadzade Fakhr-davood, Shiva Ghaedrahmati, Sahar Roshanak, and Fakhri Shahidi. 2023. "Physicochemical and Sensory Properties of Vegan Gummy Candies Enriched with High-Fiber Jaban Watermelon Exocarp Powder" Foods 12, no. 7: 1478. https://doi.org/10.3390/foods12071478