Efficacy of Chitosan Oligosaccharide Combined with Cold Atmospheric Plasma for Controlling Quality Deterioration and Spoilage Bacterial Growth of Chilled Pacific White Shrimp (Litopenaeus vannamei)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sensory and Color Analysis
2.3. Moisture Content and Water Activity (AW) Analysis
2.4. Determination of TVC, TVB-N, and pH Value Analysis
2.5. Histological Analysis
2.6. Microbiota Composition Analysis Based on Illumina-MiSeq Sequencing
2.7. Data Analysis
3. Results and Discussions
3.1. Microbial Analysis
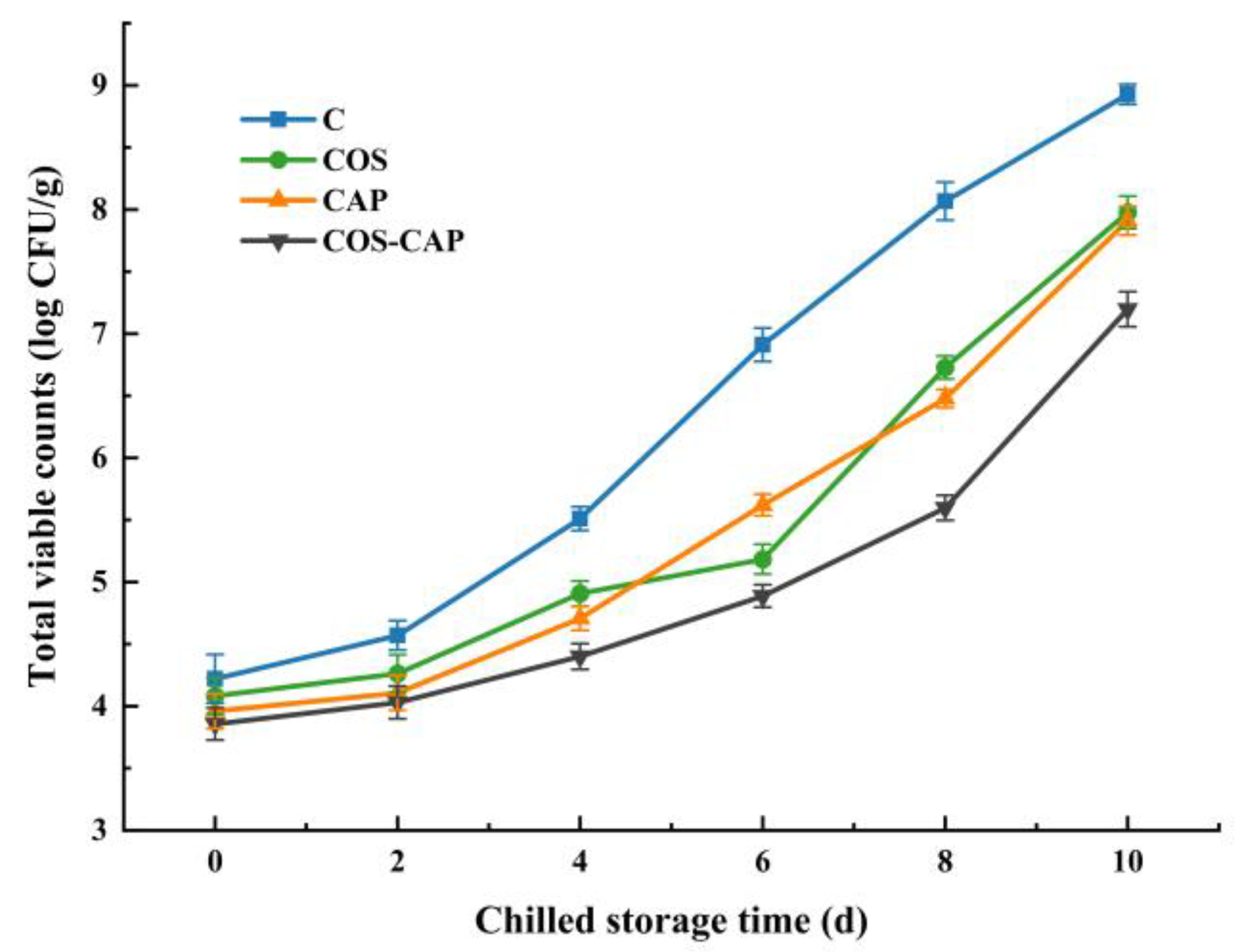
3.1.1. Total Viable Counts (TVC) Analysis
3.1.2. Richness and Diversity of the Microbial Community
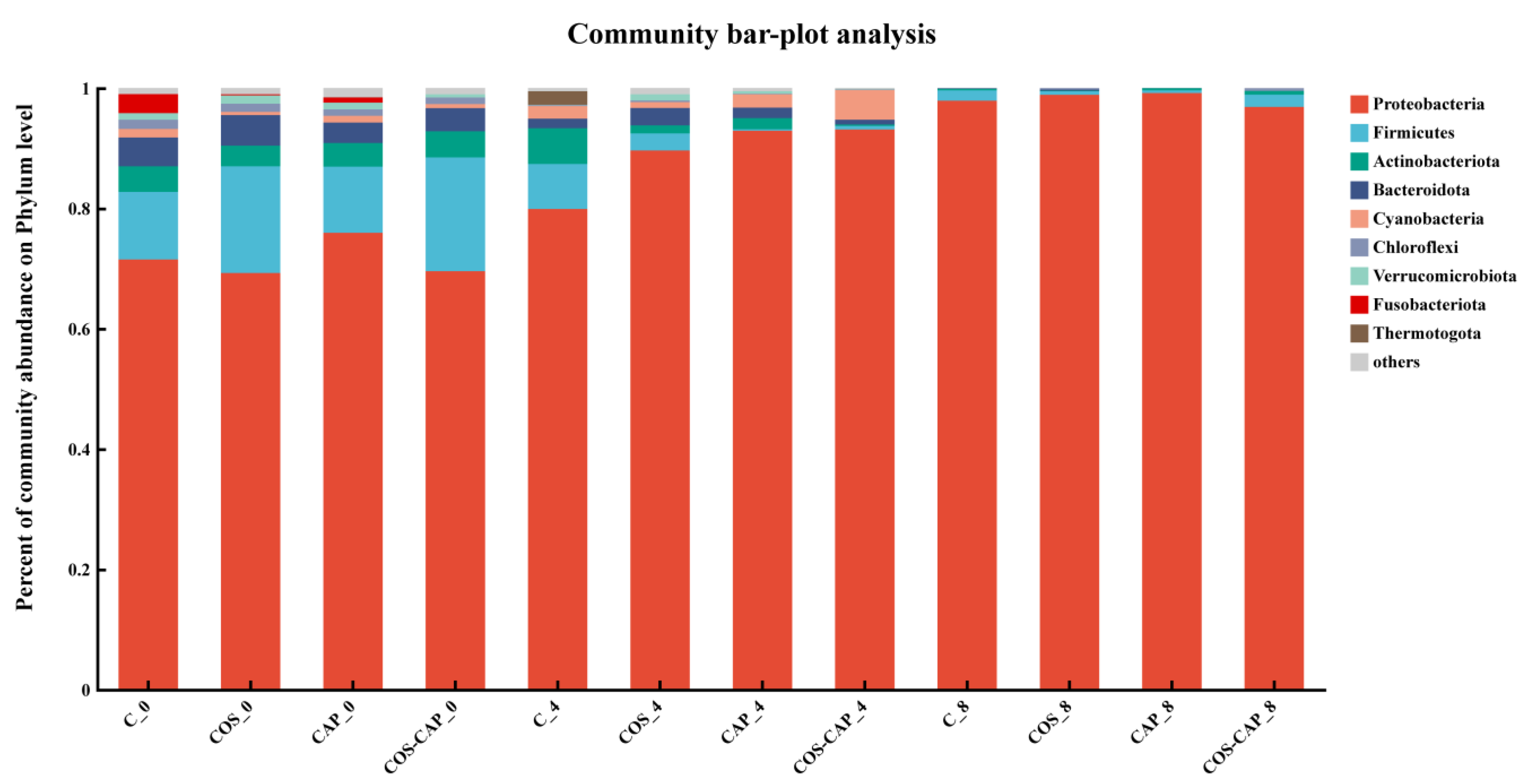
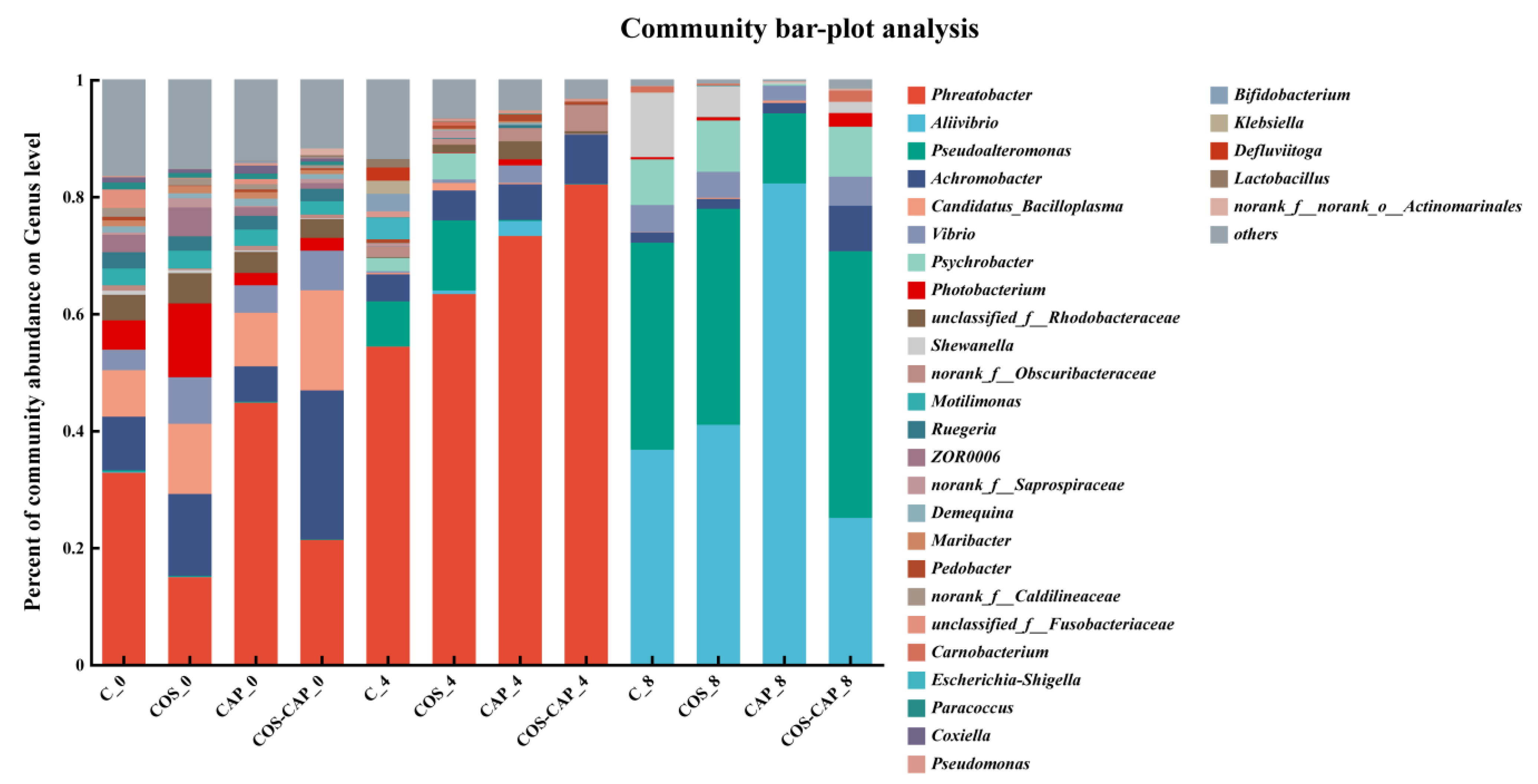
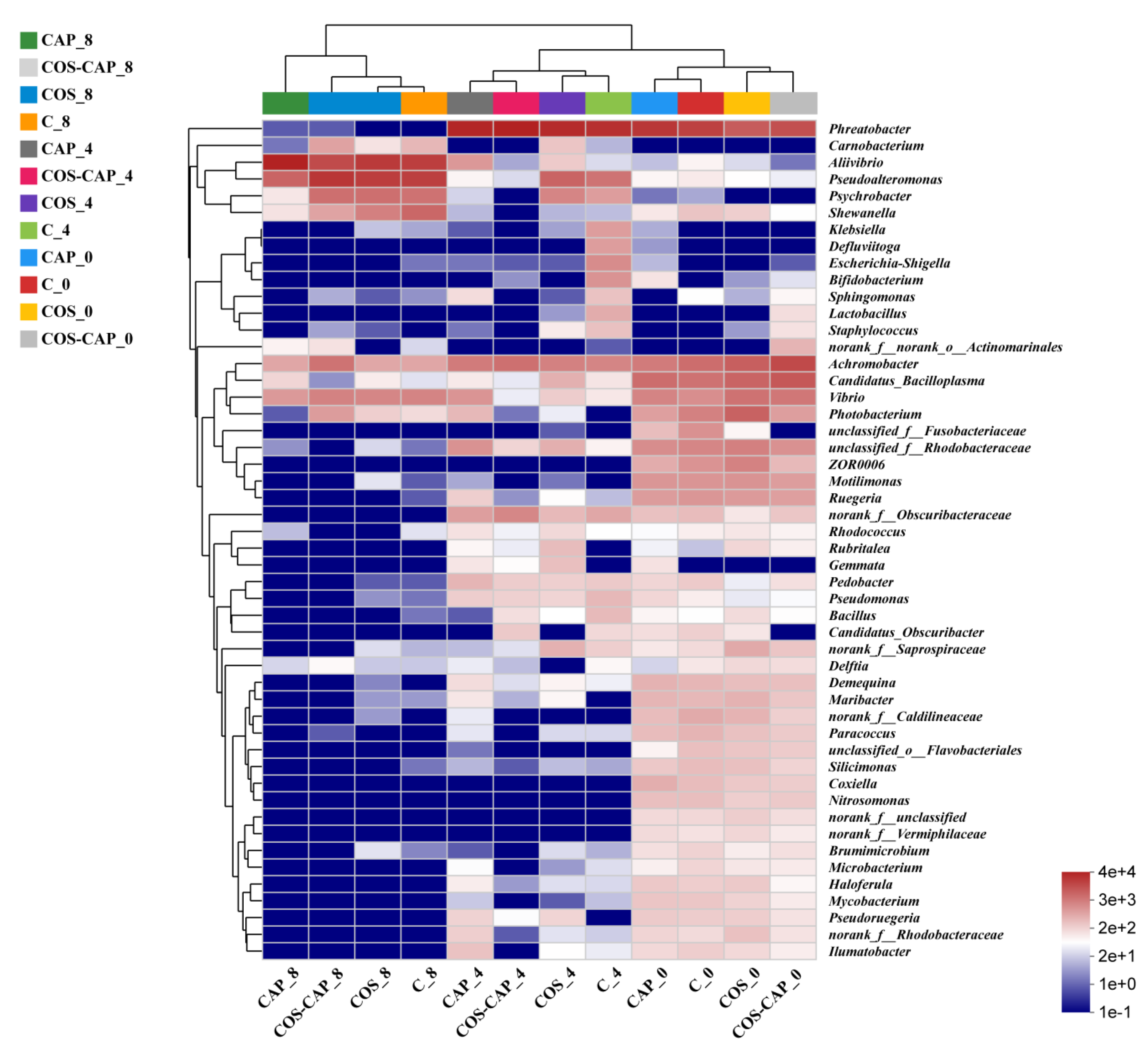
3.1.3. Microbial Community Structures
3.2. Physicochemical Analysis
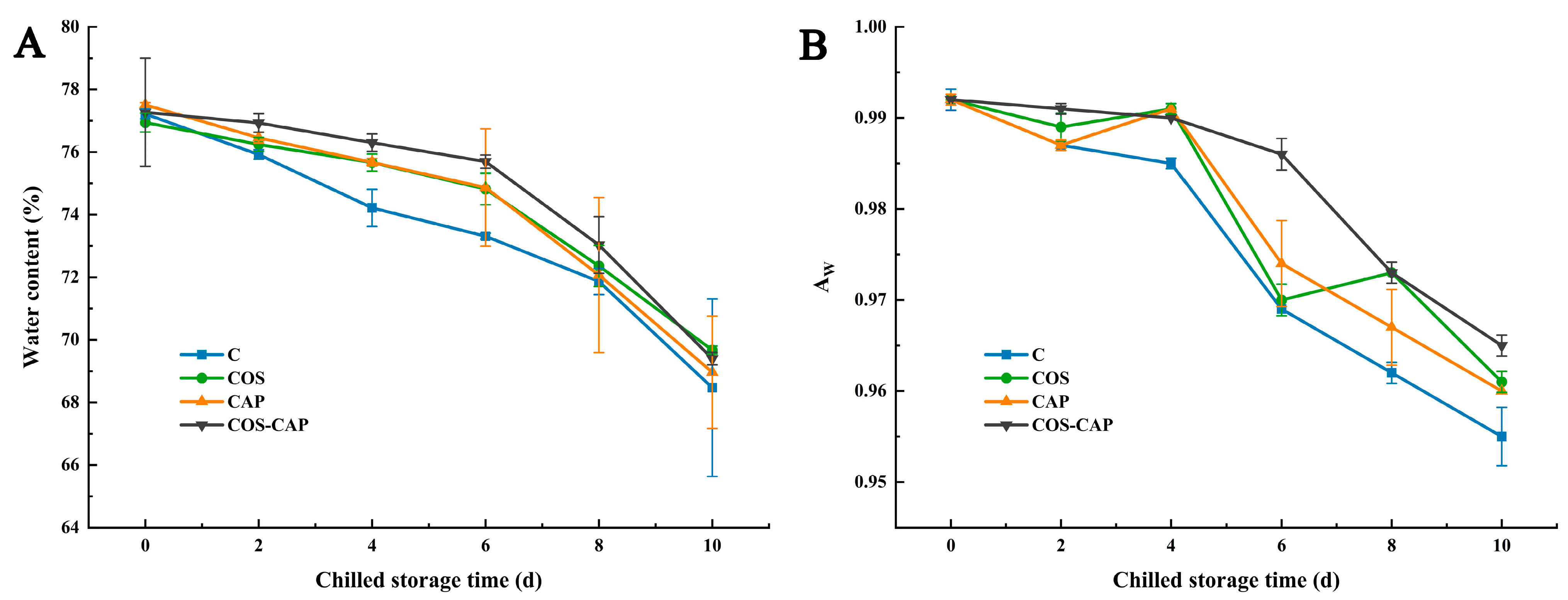
3.2.1. Water Content and Water Activity (AW) analysis
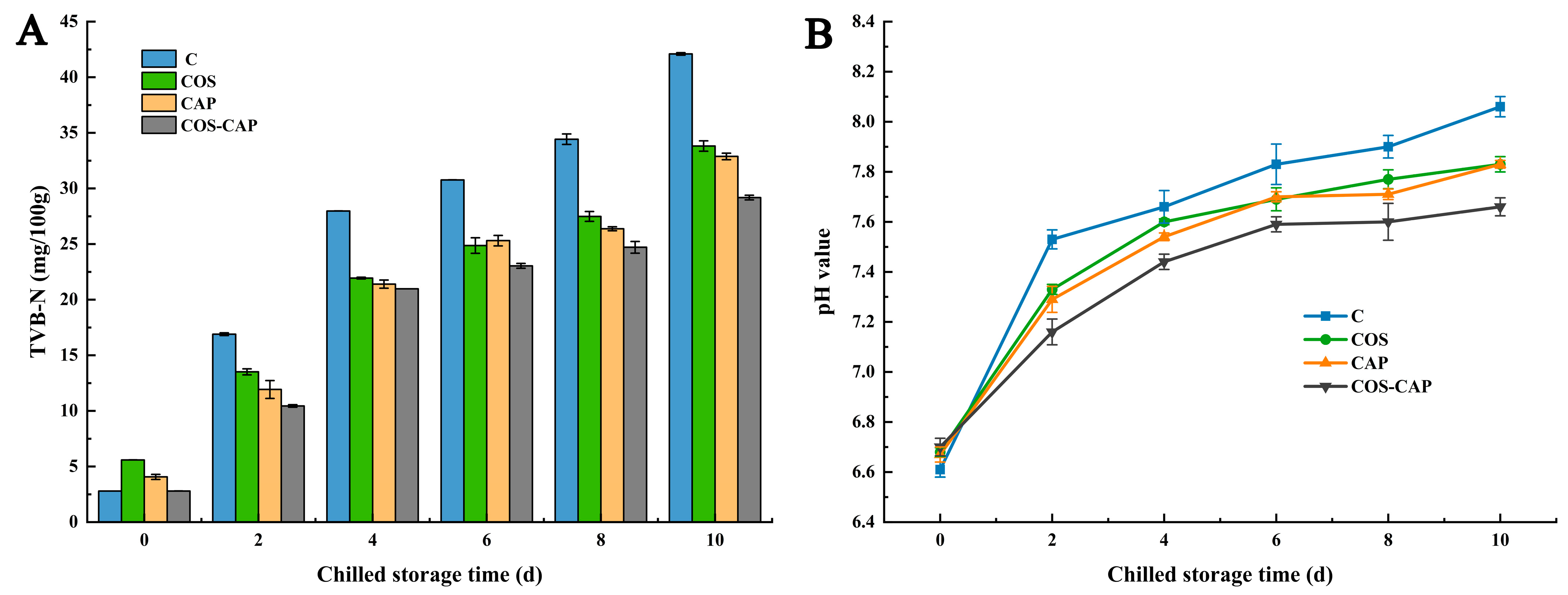
3.2.2. TVB-N and pH Value Analysis
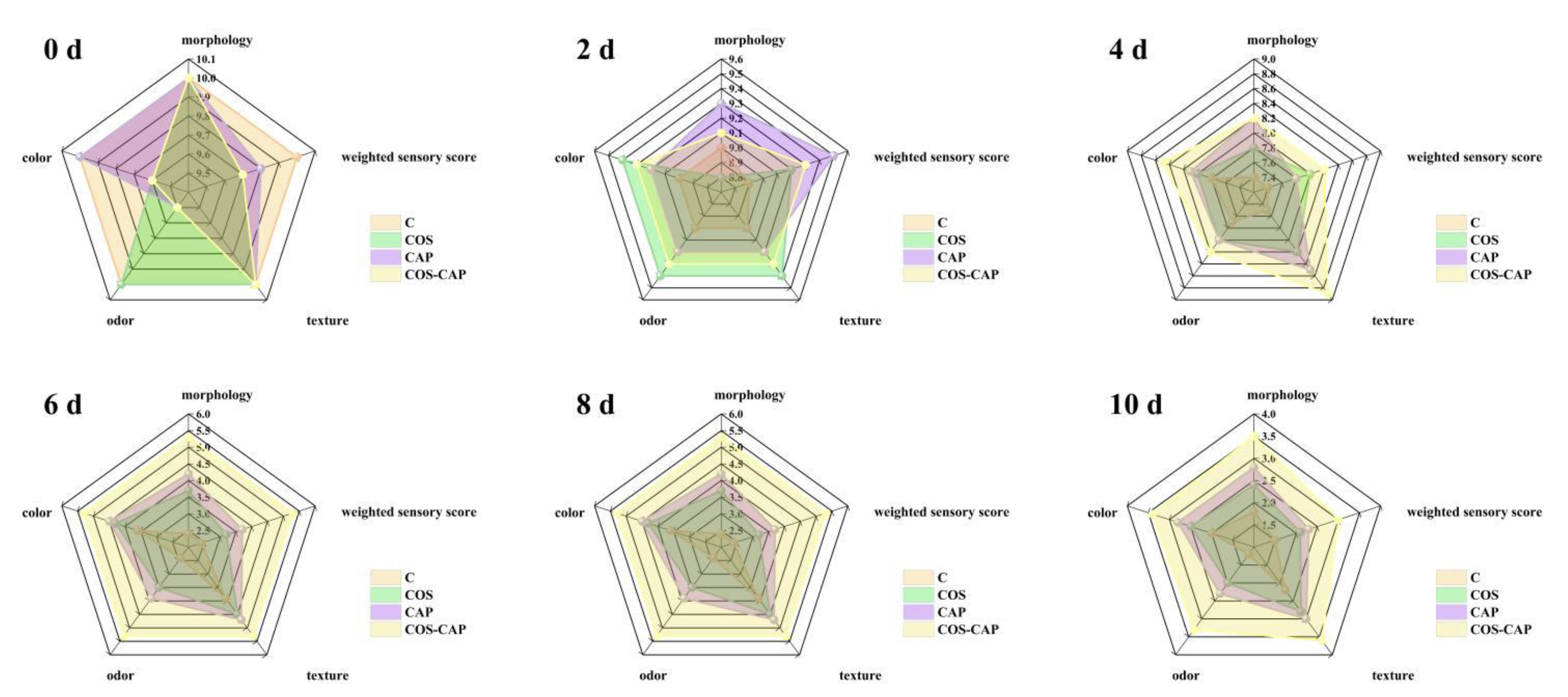
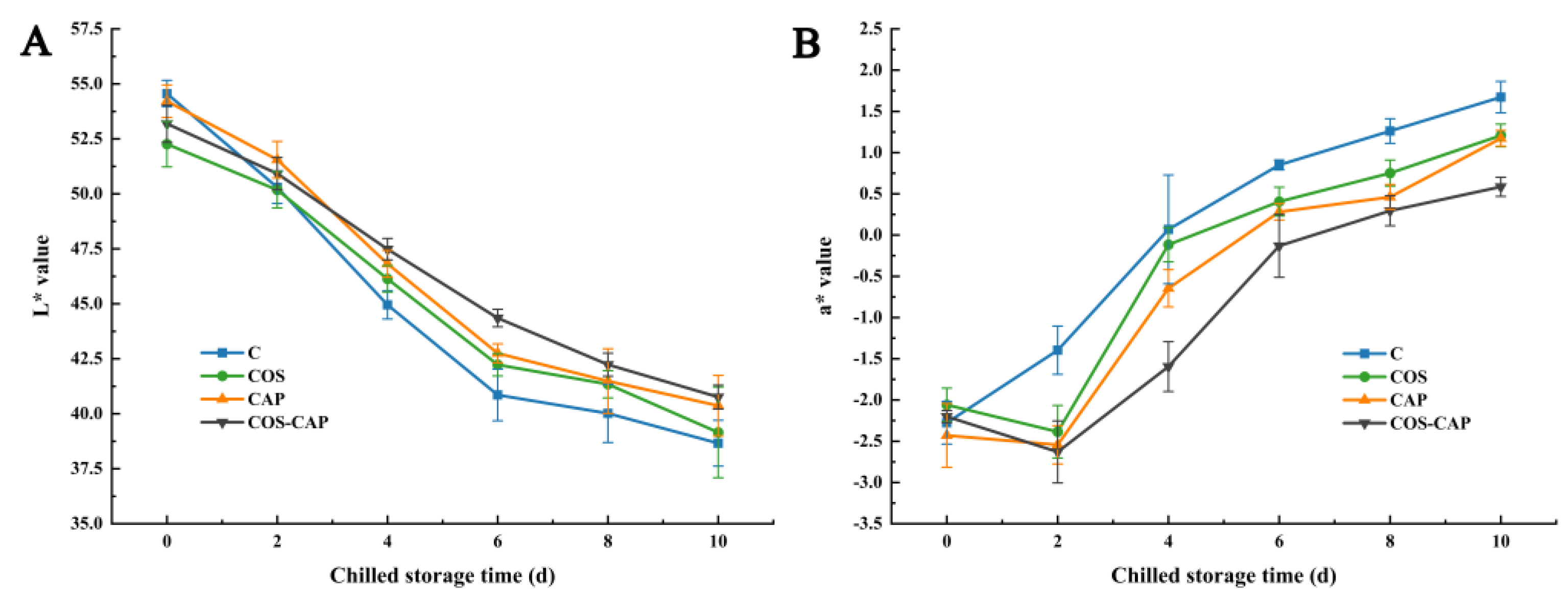
3.3. Sensory and Color Analysis
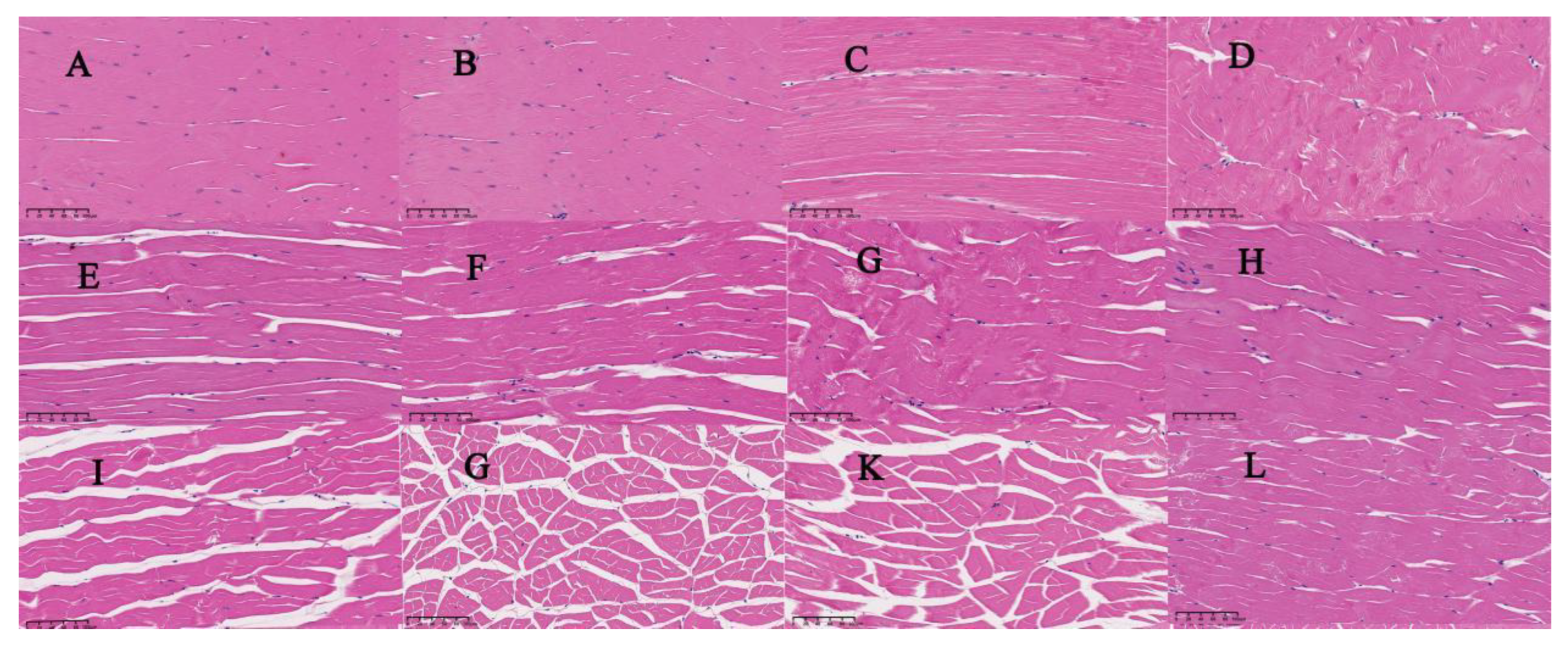
3.4. Histological Changes
3.5. Correlation Analysis between Physicochemical Changes and Microbiota
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Udayasoorian, L.; Peter, M.; Sabina, K.; Indumathi, C.; Muthusamy, S. Comparative evaluation on shelf life extension of MAP packed Litopenaeus vannamei shrimp treated with natural extracts. LWT Food Sci. Technol. 2017, 77, 217–224. [Google Scholar] [CrossRef]
- Sriket, P.; Benjakul, S.; Visessanguan, W.; Kijroongrojana, K. Comparative studies on chemical composition and thermal properties of black tiger shrimp (Penaeus monodon) and white shrimp (Penaeus vannamei) meats. Food Chem. 2007, 103, 1199–1207. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Benjakul, S. Effect of ferulic acid on inhibition of polyphenoloxidase and quality changes of Pacific white shrimp (Litopenaeus vannamei) during iced storage. Food Chem. 2009, 116, 323–331. [Google Scholar] [CrossRef]
- Peng, S.Y.; Wei, H.M.; Zhan, S.N.; Yang, W.G.; Lou, Q.M.; Deng, S.G.; Yu, X.X.; Huang, T. Spoilage mechanism and preservation technologies on the quality of shrimp: An overview. Trends Food Sci. Technol. 2022, 129, 233–243. [Google Scholar] [CrossRef]
- Dharini, M.; Jaspin, S.; Mahendran, R. Cold plasma reactive species: Generation, properties, and interaction with food biomolecules. Food Chem. 2023, 405, 134746. [Google Scholar] [CrossRef]
- Sruthi, N.U.; Josna, K.; Pandiselvam, R.; Kothakota, A.; Gavahian, M.; Mousavi Khaneghah, A. Impacts of cold plasma treatment on physicochemical, functional, bioactive, textural, and sensory attributes of food: A comprehensive review. Food Chem. 2022, 368, 130809. [Google Scholar] [CrossRef]
- Koddy, J.K.; Miao, W.H.; Hatab, S.; Tang, L.L.; Xu, H.I.; Nyaisaba, B.M.; Chen, M.; Deng, S. Understanding the role of atmospheric cold plasma (ACP) in maintaining the quality of hairtail (Trichiurus lepturus). Food Chem. 2021, 343, 128418. [Google Scholar] [CrossRef]
- Segat, A.; Misra, N.N.; Cullen, P.J.; Innocente, N. Atmospheric pressure cold plasma (ACP) treatment of whey protein isolate model solution. Innov. Food Sci. Emerg. Technol. 2015, 29, 247–254. [Google Scholar] [CrossRef]
- Zhou, R.W.; Rezaeimotlagh, A.; Zhou, R.S.; Zhang, T.Q.; Wang, P.Y.; Hong, J.M.; Soltani, B.; Mai-Prochnow, A.; Liao, X.Y.; Ding, T.; et al. In-package plasma: From reactive chemistry to innovative food preservation technologies. Trends Food Sci. Technol. 2022, 120, 59–74. [Google Scholar] [CrossRef]
- Gupta, T.T.; Ayan, H. Application of Non-Thermal Plasma on Biofilm: A Review. Appl. Sci. 2019, 9, 3548. [Google Scholar] [CrossRef] [Green Version]
- Mourya, V.K.; Choudhari, N. Chitooligosaccharides: Synthesis, characterization and applications. Polym. Sci. Ser. A 2011, 53, 583–612. [Google Scholar] [CrossRef]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Wei, X.Y.; Xie, Y.Y.; Zhou, T. A novel chitosan oligosaccharide derivative: Synthesis, antioxidant and antibacterial properties. Carbohydr. Polym. 2022, 291, 119608. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Yang, X.; Wang, J.; Li, Y.F.; Yu, H.L.; Zhang, Y.X.; Liu, G.Y. Advances in characterisation and biological activities of chitosan and chitosan oligosaccharides. Food Chem. 2016, 190, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.K.; Kim, K.Y.; Yoo, Y.J.; Oh, S.J.; Choi, J.H.; Kim, C.Y. In vitro antimicrobial activity of a chitooligosaccharide mixture against Actinobacillus actinomycetemcomitans and Streptococcus mutans. Int. J. Antimicrob. Agents 2001, 18, 553–557. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.K.; Lee, T.S.; Park, W.H. Synthesis of chitooligosaccharide derivative with quaternary ammonium group and its antimicrobial activity against Streptococcus mutans. Int. J. Biol. Macromol. 2003, 32, 23–27. [Google Scholar] [CrossRef]
- Chen, H.; Guo, X.N.; Zhu, K.X. The effect of chitosan oligosaccharides on the shelf-life and quality of fresh wet noodles. Carbohydr. Polym. 2023, 309, 120704. [Google Scholar] [CrossRef]
- Sun, T.; Yao, Q.; Zhou, D.X.; Mao, F. Antioxidant activity of N-carboxymethyl chitosan oligosaccharides. Bioorganic. Med. Chem. Lett. 2008, 18, 5774–5776. [Google Scholar] [CrossRef]
- Tang, L.L.; Hatab, S.; Yan, J.H.; Miao, W.H.; Nyaisaba, B.M.; Piao, X.Y.; Zheng, B.; Deng, S.G. Changes in Biochemical Properties and Activity of Trypsin-like Protease (Litopenaeus vannamei) Treated by Atmospheric Cold Plasma (ACP). Foods 2022, 11, 1277. [Google Scholar] [CrossRef]
- Xu, H.Q.; Miao, W.H.; Zheng, B.; Deng, S.G.; Hatab, S.M. Assessment of the Effect of Cold Atmospheric Plasma (CAP) on the Hairtail (Trichiurus lepturus) Quality under Cold Storage Conditions. Foods 2022, 11, 3683. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Lan, W.Q.; Shen, J.L.; Xu, Z.F.; Xie, J. Combining ozone and slurry ice treatment to prolong the shelf-life and quality of large yellow croaker (Pseudosciaena crocea). LWT 2022, 154, 112615. [Google Scholar] [CrossRef]
- Jiao, L.; Tu, C.H.; Mao, J.L.; Benjakul, S.; Zhang, B. Impact of theaflavin soaking pretreatment on oxidative stabilities and physicochemical properties of semi-dried large yellow croaker (Pseudosciaena crocea) fillets during storage. Food Packag. Shelf Life 2022, 32, 100852. [Google Scholar] [CrossRef]
- Jia, S.L.; Liu, Y.M.; Zhuang, S.; Sun, X.H.; Li, Y.; Hong, H.; Lv, Y.M.; Luo, Y.K. Effect of ε-polylysine and ice storage on microbiota composition and quality of Pacific white shrimp (Litopenaeus vannamei) stored at 0 °C. Food Microbiol. 2019, 83, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cao, H.J.; Wei, W.Y.; Ying, X.G. Influence of temperature fluctuations on growth and recrystallization of ice crystals in frozen peeled shrimp (Litopenaeus vannamei) pre-soaked with carrageenan oligosaccharide and xylooligosaccharide. Food Chem. 2020, 306, 125641. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.S.; Zhao, D.F.; Ma, W.J.; Guo, Y.D.; Wang, A.J.; Wang, Q.L.; Lee, D.J. Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp. Appl. Microbiol. Biotechnol. 2016, 100, 1421–1426. [Google Scholar] [CrossRef]
- Chen, S.F.; Zhou, Y.Q.; Chen, Y.R.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef]
- Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Bacteriol 1994, 44, 846–849. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Lan, W.Q.; Sun, Y.Q.; Liu, S.C.; Guan, Y.; Zhu, S.Y.; Xie, J. Effects of ultrasound-assisted chitosan grafted caffeic acid coating on the quality and microbial composition of pompano during ice storage. Ultrason. Sonochemistry 2022, 86, 106032. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.B.; Wang, M.Y.; Yang, C.F.; Wan, X.Z.; Ding, H.H.H.; Shi, Y.Z.; Zhao, C. Bacterial spoilage profiles in the gills of Pacific oysters (Crassostrea gigas) and Eastern oysters (C. virginica) during refrigerated storage. Food Microbiol. 2019, 82, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Poirier, S.; Rue, O.; Peguilhan, R.; Coeuret, G.; Zagorec, M.; Champomier-Verges, M.C.; Loux, V.; Chaillou, S. Deciphering intra-species bacterial diversity of meat and seafood spoilage microbiota using gyrB amplicon sequencing: A comparative analysis with 16S rDNA V3-V4 amplicon sequencing. PLoS ONE 2018, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Yao, Y.J.; Gao, L.F.; Wang, Z.P.; Xu, B.C. Characterization of a microbial community developing during refrigerated storage of vacuum packed Yao meat, a Chinese traditional food. LWT Food Sci. Technol. 2018, 90, 562–569. [Google Scholar] [CrossRef]
- Qian, Y.F.; Cheng, Y.; Ye, J.X.; Zhao, Y.; Xie, J.; Yang, S.P. Targeting shrimp spoiler Shewanella putrefaciens: Application of ε-polylysine and oregano essential oil in Pacific white shrimp preservation. Food Control 2021, 123, 107702. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Combined effects of high voltage cold atmospheric plasma and antioxidants on the qualities and shelf-life of Asian sea bass slices. Innov. Food Sci. Emerg. Technol. 2019, 54, 113–122. [Google Scholar] [CrossRef]
- Ansari, A.; Parmar, K.; Shah, M. A comprehensive study on decontamination of food-borne microorganisms by cold plasma. Food Chem. Mol. Sci. 2022, 4, 100098. [Google Scholar] [CrossRef]
- Laokuldilok, T.; Potivas, T.; Kanha, N.; Surawang, S.; Seesuriyachan, P.; Wangtueai, S.; Phimolsiripol, Y.; Regenstein, J.M. Physicochemical, antioxidant, and antimicrobial properties of chitooligosaccharides produced using three different enzyme treatments. Food Biosci. 2017, 18, 28–33. [Google Scholar] [CrossRef]
- Cen, S.J.; Fang, Q.; Tong, L.; Yang, W.G.; Zhang, J.J.; Lou, Q.M.; Huang, T. Effects of chitosan-sodium alginate-nisin preservatives on the quality and spoilage microbiota of Penaeus vannamei shrimp during cold storage. Int. J. Food Microbiol. 2021, 349, 109227. [Google Scholar] [CrossRef]
- Françoise, L. Occurrence and role of lactic acid bacteria in seafood products. Food Microbiol. 2010, 27, 698–709. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.J.; Xie, J.; Li, L.; Xue, B.; Li, X.H.; Gan, J.H.; Shao, Z.H.; Sun, T. Properties of phenolic acid-chitosan composite films and preservative effect on Penaeus vannamei. J. Mol. Struct. 2021, 1239, 130531. [Google Scholar] [CrossRef]
- LI, Y.; Fang, Y.D.; Luo, Y.K. Effect of ice coating on the quality of frozen shrimp (Litopenaeus vannamei). Meat Res. 2018, 32, 39–44. [Google Scholar]
- Cropotova, J.; Tappi, S.; Genovese, J.; Rocculi, P.; Dalla Rosa, M.; Rustad, T. The combined effect of pulsed electric field treatment and brine salting on changes in the oxidative stability of lipids and proteins and color characteristics of sea bass (Dicentrarchus labrax). Heliyon 2021, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Shiekh, K.A.; Zhou, P.; Benjakul, S. Combined effects of pulsed electric field, Chamuang leaf extract and cold plasma on quality and shelf-life of Litopenaeus vannamei. Food Biosci. 2021, 41, 100975. [Google Scholar] [CrossRef]
- Ucar, Y.; Ceylan, Z.; Durmus, M.; Tomar, O.; Cetinkaya, T. Application of cold plasma technology in the food industry and its combination with other emerging technologies. Trends Food Sci. Technol. 2021, 114, 355–371. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.Y.; Fu, Z.X.; Zhang, H.J.; Zhang, L.T.; Li, B.; Tan, Y.Q.; Hong, H.; Luo, Y.K. Uncovering quality changes of salted bighead carp fillets during frozen storage: The potential role of time-dependent protein denaturation and oxidation. Food Chem. 2023, 414, 135714. [Google Scholar] [CrossRef]

| Storage Time (d) | Sample | OTUs | Shannon | Simpson | Ace | Chao | Coverage |
|---|---|---|---|---|---|---|---|
| 0 | C | 150.67 ± 10.97 Aa | 2.96 ± 0.63 Aa | 0.19 ± 0.13 Ad | 154.78 ± 11.49 Aab | 156.03 ± 9.61 Aabc | 0.99 |
| COS | 156.67 ± 25.48 Aa | 3.05 ± 0.17 Aa | 0.14 ± 0.07 Ad | 159.75 ± 26.21 Aa | 160.07 ± 27.68 Aab | 0.99 | |
| CAP | 169.33 ± 6.66 Aa | 2.76 ± 0.17 Aab | 0.22 ± 0.05 Acd | 174.61 ± 7.09 Aa | 172.41 ± 7.23 Aa | 0.99 | |
| COS–CAP | 115.00 ± 7.81 Bb | 2.37 ± 0.54 Aabc | 0.26 ± 0.14 Acd | 120.25 ± 7.24 Bbc | 122.46 ± 10.20 Bbcd | 0.99 | |
| 4 | C | 104.67 ± 34.56 Abc | 2.14 ± 0.58 Abc | 0.32 ± 0.10 Bcd | 109.37 ± 34.74 Ac | 117.78 ± 43.49 Acd | 0.99 |
| COS | 95.33 ± 3.79 Abc | 1.74 ± 0.14 Acd | 0.42 ± 0.04 Bbc | 99.61 ± 7.45 Acd | 98.03 ± 6.16 ABde | 0.99 | |
| CAP | 71.67 ± 17.79 ABcd | 1.37 ± 0.41 ABde | 0.55 ± 0.11 ABab | 72.70 ± 18.60 ABde | 72.37 ± 18.54 ABef | 0.99 | |
| COS–CAP | 38.00 ± 7.81 Be | 0.75 ± 0.36 Be | 0.70 ± 0.15 Aa | 39.73 ± 23.07 Bef | 41.83 ± 27.86 Bf | 0.99 | |
| 8 | C | 52.67 ± 4.16 Ade | 1.85 ± 0.11 Acd | 0.22 ± 0.02 Acd | 57.07 ± 7.89 Aef | 56.73 ± 8.40 Af | 0.99 |
| COS | 54.33 ± 15.04 Ade | 1.76 ± 0.15 Acd | 0.26 ± 0.04 Acd | 56.94 ± 17.03 Aef | 56.93 ± 17.42 Af | 0.99 | |
| CAP | 29.33 ± 4.04 Be | 0.72 ± 0.35 Be | 0.70 ± 0.17 Ba | 47.16 ± 11.93 Aef | 39.33 ± 7.51 ABf | 0.99 | |
| COS–CAP | 35.00 ± 2.65 Be | 1.93 ± 0.04 Acd | 0.21 ± 0.02 Ad | 35.63 ± 2.65 Af | 35.83 ± 2.75 Bf | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Ding, Y.; Du, Q.; Liao, Y.; Miao, W.; Deng, S.; Cullen, P.J.; Zhou, R. Efficacy of Chitosan Oligosaccharide Combined with Cold Atmospheric Plasma for Controlling Quality Deterioration and Spoilage Bacterial Growth of Chilled Pacific White Shrimp (Litopenaeus vannamei). Foods 2023, 12, 1763. https://doi.org/10.3390/foods12091763
Yu M, Ding Y, Du Q, Liao Y, Miao W, Deng S, Cullen PJ, Zhou R. Efficacy of Chitosan Oligosaccharide Combined with Cold Atmospheric Plasma for Controlling Quality Deterioration and Spoilage Bacterial Growth of Chilled Pacific White Shrimp (Litopenaeus vannamei). Foods. 2023; 12(9):1763. https://doi.org/10.3390/foods12091763
Chicago/Turabian StyleYu, Mijia, Yixuan Ding, Qi Du, Yueqin Liao, Wenhua Miao, Shanggui Deng, Patrick J. Cullen, and Rusen Zhou. 2023. "Efficacy of Chitosan Oligosaccharide Combined with Cold Atmospheric Plasma for Controlling Quality Deterioration and Spoilage Bacterial Growth of Chilled Pacific White Shrimp (Litopenaeus vannamei)" Foods 12, no. 9: 1763. https://doi.org/10.3390/foods12091763





