Effect of CSN3 Gene Polymorphism on the Formation of Milk Gels Induced by Physical, Chemical, and Biotechnological Factors
Abstract
:1. Introduction
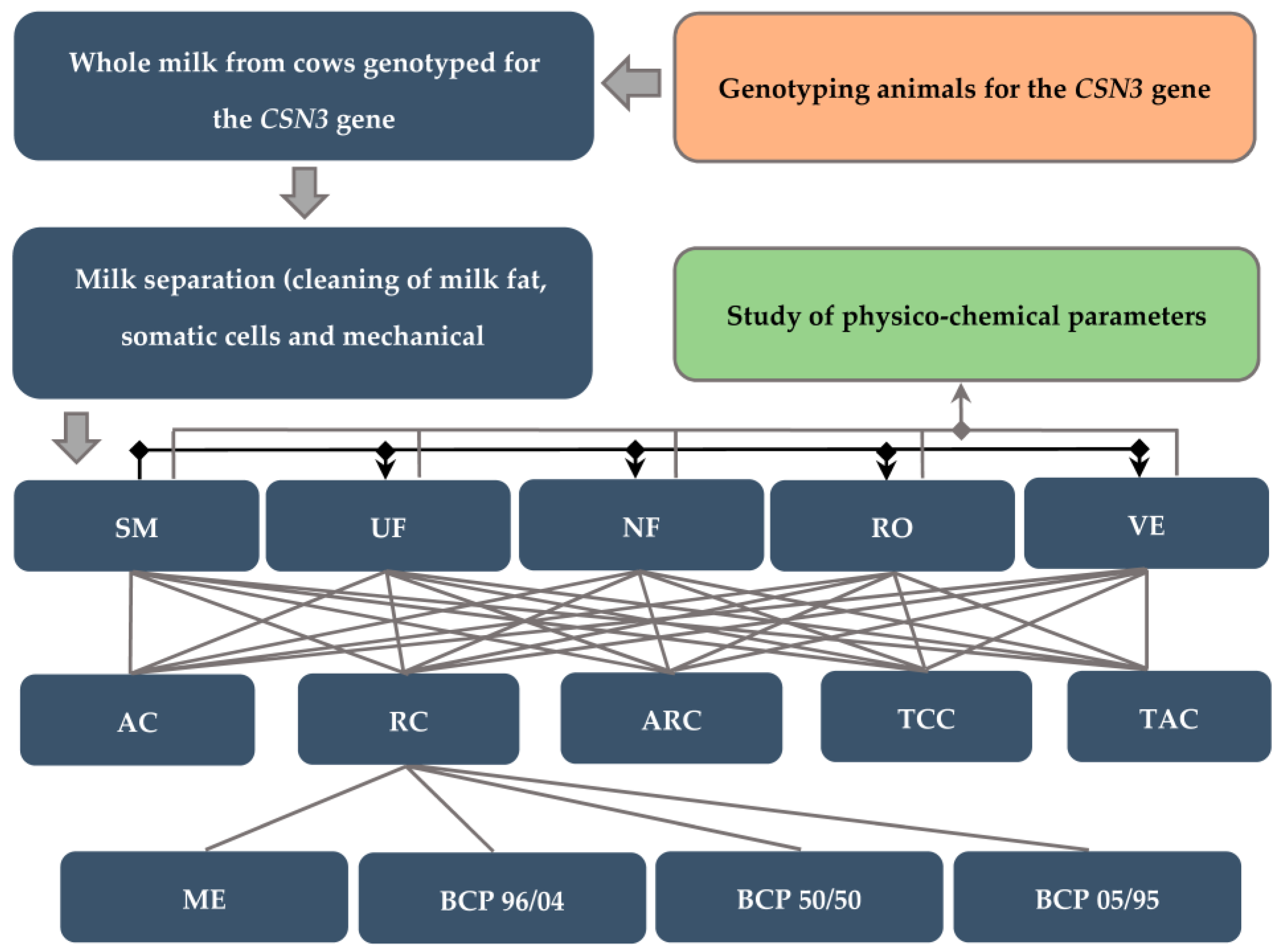
2. Materials and Methods
2.1. Design of the Experiment
2.2. Milk Samples
2.3. Preliminary Milk Preparation
2.4. Protein Standardization
2.5. Samples of Coagulating Enzymes
- -
- Milk-clotting enzyme of microbial origin (chymosin) Microclerici 2400 IMCU g−1 (Sacco Sistem, Cadorago, Italy), obtained in the process of controlled cultivation of the strains Rhizomucor miehei—ME;
- -
- A mixture of beef chymosin with pepsin Clerici 96/04 (Sacco Sistem, Cadorago, Italy) with an activity of 2400 IMCU g−1—BCP 96/04;
- -
- A mixture of beef chymosin with pepsin Clerici 50/50 (Sacco Sistem, Cadorago, Italy) with an activity of 800 IMCU g−1—BCP 50/50;
- -
- A mixture of beef chymosin with pepsin 05/95 (LLC “Modern Technologies”, Moscow, Russia) activity 800 IMCU g−1—BCP 05/95.
2.6. Chemical and Physical Analyzes
2.7. Determination of the Size of Casein Micelles
2.8. Determination of Coagulation Ability
2.9. Methods of Milk Protein Coagulation
- -
- Acid coagulation (AC)—a decrease in the pH value below the isoelectric point of the casein complex (4.6–4.7) due to lactic acid formed as a result of fermentation of lactose during fermentation of skimmed milk or retentates or concentrates by mesophilic and thermophilic lactic acid microorganisms (Lactococcus lactis strains 795, 7910, 7913 and Streptococcus thermophilus strain 6 kb) in an amount of 5% of the mass of fermented milk;
- -
- Rennet coagulation (RC)—protein coagulation exposed to 16.7 mL of 1% solution of one of the enzyme preparations (ME, BCP 96/04, BCP 50/50, BCP 05/95), the activity of which was recalculated to 800 IMCU g−1;
- -
- Acid–rennet coagulation (ARC)—combined protein coagulation exposed to 16.7 mL of a 1% solution of an enzyme preparation (BCP 96/04) and lactic acid formed as a result of fermentation of dairy systems by mesophilic and thermophilic lactic acid microorganisms (Lactococcus lactis strains 795, 7910, 7913 and Streptococcus thermophilus strain 6 kb) in an amount of 5% of the mass of fermented milk;
- -
- Heat- and calcium-induced coagulation (TCC)—protein coagulation under the action of a 15% solution of strong electrolyte CaCl2 in combination with high-temperature treatment 92 ± 2 °C for 5.0 ± 0.1 min;
- -
- Heat- and acid-induced coagulation (TAC)—protein coagulation under the action of 80% lactic acid (up to pH 5.4) in combination with high-temperature treatment 92 ± 2 °C.
2.10. Statistical Analysis
3. Results and Discussion
3.1. Alleles and the Genotype Frequency for the CSN3 Locus
3.2. Quantitative and Qualitative Composition of Skim Milk, Retentates and Concentrates Depending on the Polymorphism of the CSN3 Gene
3.3. The Effect of Enzyme Preparations on the Milk Protein Cluster
3.4. The Effect of CSN3 Polymorphisms on the Technological Properties of Biotechnologically and Chemically Induced Milk Gels
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Almeida, C.M.; Simões, I. Cardoon-based rennets for cheese production. Appl. Microbiol. Biotechnol. 2018, 102, 4675–4686. [Google Scholar] [CrossRef] [PubMed]
- Deshwal, G.K.; Panjagari, N.R. Active and Intelligent Packaging of Cheese: Developments and Future Scope. In Food Packaging; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar] [CrossRef]
- Bansal, V.; Veena, N. Understanding the role of pH in cheese manufacturing: General aspects of cheese quality and safety. J. Food Sci. Technol. 2022, 1–11. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Fundamentals of Cheese Science; Springer: New York, NY, USA, 2017; p. 799. [Google Scholar] [CrossRef]
- Cipolat-Gotet, C.; Cecchinato, A.; Malacarne, M.; Bittante, G.; Summer, A. Variations in milk protein fractions affect the efficiency of the cheese-making process. J. Dairy Sci. 2018, 101, 8788–8804. [Google Scholar] [CrossRef] [PubMed]
- Amalfitano, N.; Cipolat-Gotet, C.; Cecchinato, A.; Malacarne, M.; Summer, A.; Bittante, G. Milk protein fractions strongly affect the patterns of coagulation, curd firming, and syneresis. J. Dairy Sci. 2019, 102, 2903–2917. [Google Scholar] [CrossRef]
- Corredig, M.; Nair, P.K.; Li, Y.; Eshpari, H.; Zhao, Z. Invited review: Understanding the behavior of caseins in milk concentrates. J. Dairy Sci. 2019, 102, 4772–4782. [Google Scholar] [CrossRef] [PubMed]
- Gai, N.; Uniacke-lowe, T.; O’regan, J.; Faulkner, H.; Kelly, A.L. Effect of Protein Genotypes on Physicochemical Properties and Protein Functionality of Bovine Milk: A Review. Foods 2021, 10, 2409. [Google Scholar] [CrossRef]
- Ketto, I.A.; Knutsen, T.M.; Øyaas, J.; Heringstad, B.; Ådnøy, T.; Devold, T.G.; Skeie, S.B. Effects of milk protein polymorphism and composition, casein micelle size and salt distribution on the milk coagulation properties in Norwegian Red cattle. Int. Dairy J. 2017, 70, 55–64. [Google Scholar] [CrossRef]
- Oštarić, F.; Antunac, N.; Cubric-curik, V.; Curik, I.; Jurić, S.; Kazazić, S.; Kiš, M.; Vinceković, M.; Zdolec, N.; Špoljarić, J.; et al. Challenging Sustainable and Innovative Technologies in Cheese Production: A Review. Processes 2022, 10, 529. [Google Scholar] [CrossRef]
- Gurses, M.; Yuce, H.; Etem, E.O.; Patir, B. Polymorphisms of kappa-casein gene and their effects on milk production traits in Holstein, Jersey and Brown Swiss cattle. Anim. Prod. Sci. 2016, 58, 778–784. [Google Scholar] [CrossRef]
- Mahmoudi, P.; Rostamzadeh, J.; Rashidi, A.; Zergani, E.; Razmkabir, M. A meta-analysis on association between CSN3 gene variants and milk yield and composition in cattle. Anim. Genet. 2020, 51, 369–381. [Google Scholar] [CrossRef]
- Pazzola, M.; Vacca, G.M.; Noce, A.; Porcedda, M.; Onnis, M.; Manca, N.; Dettori, M.L. Exploring the Genotype at CSN3 Gene, Milk Composition, Coagulation and Cheese-Yield Traits of the Sardo-Modicana, an Autochthonous Cattle Breed from the Sardinia Region, Italy. Animals 2020, 10, 1995. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Milk Proteins. In Dairy Chemistry and Biochemistry; Springer International Publishing: Cham, Switzerland, 2015; Chapter 4; pp. 145–239. [Google Scholar] [CrossRef]
- Bauland, J.; Famelart, M.H.; Faiveley, M.; Croguennec, T. Rheological properties of enzymatic milk gel: Effect of ion partitioning in casein micelles. Food Hydrocoll. 2022, 130, 107739. [Google Scholar] [CrossRef]
- Töpel, A. Chemie und Physik der Milch; Behr’s Verlag: Hamburg, Germany, 2015; 737p. [Google Scholar]
- Malchiodi, F.; Cecchinato, A.; Penasa, M.; Cipolat-Gotet, C.; Bittante, G. Milk quality, coagulation properties, and curd firmness modeling of purebred Holsteins and first-and second-generation crossbred cows from Swedish Red, Montbéliarde, and Brown Swiss bulls. J. Dairy Sci. 2014, 97, 4530–4541. [Google Scholar] [CrossRef] [PubMed]
- Djedović, R.; Bogdanović, V.; Perišić, P.; Stanojević, D.; Popović, J.; Brka, M.; Bogdanović, V.; Perišić, P.; Stanojević, D.; Popović, J.; et al. Relationship between genetic polymorphism of κ-casein and quantitative milk yield traits in cattle breeds and crossbreds in Serbia. Genetika 2015, 47, 23–32. [Google Scholar] [CrossRef]
- Awad, A.; el Araby, I.E.; El-Bayomi, K.M.; Zaglool, A.W. Association of polymorphisms in kappa casein gene with milk traits in Holstein Friesian cattle. Jpn. J. Vet. Res. 2016, 64 (Suppl. S2), S39–S43. [Google Scholar]
- Bijl, E.; de Vries, R.; van Valenberg, H.; Huppertz, T.; van Hooijdonk, T. Factors influencing casein micelle size in milk of individual cows: Genetic variants and glycosylation of κ-casein. Int. Dairy J. 2014, 34, 135–141. [Google Scholar] [CrossRef]
- Jensen, H.B.; Pedersen, K.S.; Johansen, L.B.; Poulsen, N.A.; Bakman, M.; Chatterton, D.E.W.; Larsen, L.B. Genetic variation and posttranslational modification of bovine κ-casein: Effects on caseino-macropeptide release during renneting. J. Dairy Sci. 2015, 98, 747–758. [Google Scholar] [CrossRef]
- Marković, M.; Radonjić, D.; Đokić, M.; Kandić, A.; Marković, B. Allelic polymorphism of k-casein gene (CSN3) in three Montenegrin cattle breeds. Agric. For. 2021, 67, 61–70. [Google Scholar] [CrossRef]
- Comin, A.; Cassandro, M.; Chessa, S.; Ojala, M.; Dal Zotto, R.; de Marchi, M.; Carnier, P.; Gallo, L.; Pagnacco, G.; Bittante, G. Effects of Composite β- and κ-Casein Genotypes on Milk Coagulation, Quality, and Yield Traits in Italian Holstein Cows. J. Dairy Sci. 2008, 91, 4022–4027. [Google Scholar] [CrossRef]
- Neamt, R.I.; Saplacan, G.; Acatincai, S.; Cziszter, L.T.; Gavojdian, D.; Ilie, D.E. The influence of CSN3 and LGB polymorphisms on milk production and chemical composition in Romanian Simmental cattle. Acta Biochim. Pol. 2017, 64, 493–497. [Google Scholar] [CrossRef]
- Zambrano Burbano, G.L.; Eraso Cabrera, Y.M.; Solarte Portilla, C.E.; Rosero Galindo, C.Y. Kappa casein genotypes and curd yield in Holstein cows. Rev. Colomb. Cienc. Pecu. 2010, 23, 422–428. [Google Scholar]
- Frederiksen, P.D.; Andersen, K.K.; Hammershøj, M.; Poulsen, H.D.; Sørensen, J.; Bakman, M.; Qvist, K.B.; Larsen, L.B. Composition and effect of blending of noncoagulating, poorly coagulating, and well-coagulating bovine milk from individual Danish Holstein cows. J. Dairy Sci. 2011, 94, 4787–4799. [Google Scholar] [CrossRef] [PubMed]
- Tyulkin, S.V.; Vafin, R.R.; Zagidulin, L.R.; Akhmetov, T.M.; Petrov, A.N.; Diel, F. Technological properties of milk of cows with different genotypes of kappa-casein and beta-lactoglobulin. Foods Raw Mater. 2018, 6, 154–162. [Google Scholar] [CrossRef]
- Kruchinin, A.G.; Turovskaya, S.N.; Illarionova, E.E.; Bigaeva, A.V. Evaluation of the effect of κ-casein gene polymorphism in milk powder on the technological properties of acid-induced milk gels. Food Process. Tech. Technol. 2021, 51, 53–66. [Google Scholar] [CrossRef]
- Kumar, A.; Grover, S.; Sharma, J.; Batish, V.K. Chymosin and other milk coagulants: Sources and biotechnological interventions. Crit. Rev. Biotechnol. 2010, 30, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Mir Khan, U.; Selamoglu, Z. Use of Enzymes in Dairy Industry: A Review of Current Progress. Arch. Razi Inst. 2020, 75, 131. [Google Scholar] [CrossRef]
- Soodam, K.; Guinee, T.P. The case for milk protein standardisation using membrane filtration for improving cheese consistency and quality. Int. J. Dairy Technol. 2018, 71, 277–291. [Google Scholar] [CrossRef]
- Henriques, M.; Gomes, D.; Pereira, C. Liquid whey protein concentrates produced by ultrafiltration as primary raw materials for thermal dairy gels. Food Technol. Biotechnol. 2017, 55, 454–463. [Google Scholar] [CrossRef]
- Gilmanov, K.K.; Semipyatnyi, V.K.; Bigaeva, A.V.; Vafin, R.R.; Turovskaya, S.N. New determination method for the ratio of the relative proportions of?-casein alleles in milk powder. Food Process. Tech. Technol. 2020, 50, 525–535. [Google Scholar] [CrossRef]
- Sulimova, G.E.; Azari, M.A.; Rostamzadeh, J.; Mohammad Abadi, M.R.; Lazebny, O.E. κ-casein gene (CSN3) allelic polymorphism in Russian cattle breeds and its information value as a genetic marker. Russ. J. Genet. 2007, 43, 73–79. [Google Scholar] [CrossRef]
- Akkerman, M.; Johansen, L.B.; Rauh, V.; Sørensen, J.; Larsen, L.B.; Poulsen, N.A. Relationship between casein micelle size, protein composition and stability of UHT milk. Int. Dairy J. 2021, 112, 104856. [Google Scholar] [CrossRef]
- Uniacke-Lowe, T.; Fox, P.F. Chymosin, pepsins and other aspartyl proteinases: Structures, functions, catalytic mechanism and milk-clotting properties. In Cheese: Chemistry, Physics and Microbiology, 4th ed.; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 69–113. [Google Scholar] [CrossRef]
- Ferrer, M.; Alexander, M.; Corredig, M. Changes in the physico-chemical properties of casein micelles during ultrafiltration combined with diafiltration. LWT Food Sci. Technol. 2014, 59, 173–180. [Google Scholar] [CrossRef]
- Ferrer, M.; Alexander, M.; Corredig, M. Does ultrafiltration have a lasting effect on the physico-chemical properties of the casein micelles? Dairy Sci. Technol. 2011, 91, 151–170. [Google Scholar] [CrossRef]
- Luo, X.; Ramchandran, L.; Vasiljevic, T. Lower ultrafiltration temperature improves membrane performance and emulsifying properties of milk protein concentrates. Dairy Sci. Technol. 2014, 95, 15–31. [Google Scholar] [CrossRef]
- Puri, R.; Singh, U.; O’mahony, J.A. Influence of Processing Temperature on Membrane Performance and Characteristics of Process Streams Generated during Ultrafiltration of Skim Milk. Foods 2020, 9, 1721. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, N.; Ranjan, R.; Kumar, S.; Bhat, Z.F.; Jeong, D.K. Perspective of Membrane Technology in Dairy Industry: A Review. Asian-Australas. J. Anim. Sci. 2013, 26, 1347–1358. [Google Scholar] [CrossRef]
- Maubois, J.L. 50 Years of Membrane Techniques in Dairy Technology. J. Dairy Vet. Sci. 2017, 2, 555576. [Google Scholar] [CrossRef]
- Čítek, J.; Brzáková, M.; Hanusová, L.; Hanuš, O.; Večerek, L.; Samková, E.; Křížová, Z.; Hoštičková, I.; Kávová, T.; Straková, K.; et al. Technological properties of cow’s milk: Correlations with milk composition, effect of interactions of genes and other factors. Czech J. Anim. Sci. 2020, 65, 13–22. [Google Scholar] [CrossRef]
- Balde, A.; Aïder, M. Effect of cryoconcentration, reverse osmosis and vacuum evaporation as concentration step of skim milk prior to drying on the powder properties. Powder Technol. 2017, 319, 463–471. [Google Scholar] [CrossRef]
- Syrios, A.; Faka, M.; Grandison, A.S.; Lewis, M.J. A comparison of reverse osmosis, nanofiltration and ultrafiltration as concentration processes for skim milk prior to drying. Int. J. Dairy Technol. 2011, 64, 467–472. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, W.; Wu, S.; Liu, C.; Li, Y.; Li, H.; Zhang, L. Short communication: Effects of nanofiltration and evaporation on the physiochemical properties of milk protein during processing of milk protein concentrate. J. Dairy Sci. 2015, 98, 100–105. [Google Scholar] [CrossRef]
- Liu, D.Z.; Dunstan, D.E.; Martin, G.J.O. Evaporative concentration of skimmed milk: Effect on casein micelle hydration, composition, and size. Food Chem. 2012, 134, 1446–1452. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jangra, A.; Pramanik, J. Application of Enzymes in Dairy Processing Industry: A Review. Curr. Nutr. Food Sci. 2022, 18, 428–431. [Google Scholar] [CrossRef]
- Law, B.A.; Tamime, A.Y. Technology of Cheesemaking, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Kocabaş, D.S.; Lyne, J.; Ustunol, Z. Hydrolytic enzymes in the dairy industry: Applications, market and future perspectives. Trends Food Sci. Technol. 2022, 119, 467–475. [Google Scholar] [CrossRef]
- Illarionova, E.E.; Kruchinin, A.G.; Turovskaya, S.N.; Bigaeva, A.V. Association of polymorphisms in the biocluster of casein and whey protein genes with technological properties of dairy raw materials. Dairy Ind. 2021, 3, 60–62. [Google Scholar] [CrossRef]
- Salvador, D.; Acosta, Y.; Zamora, A.; Castillo, M. Rennet-Induced Casein Micelle Aggregation Models: A Review. Foods 2022, 11, 1243. [Google Scholar] [CrossRef]
- Troch, T.; Lefébure, É.; Baeten, V.; Colinet, F.; Gengler, N.; Sindic, M. Cow milk coagulation: Process description, variation factors and evaluation methodologies. A review. Biotechnol. Agron. Soc. Environ. 2017, 21, 276–287. [Google Scholar] [CrossRef]
- Zhao, Z.; Corredig, M. Influence of sodium chloride on the colloidal and rennet coagulation properties of concentrated casein micelle suspensions. J. Dairy Sci. 2016, 99, 6036–6045. [Google Scholar] [CrossRef]
- Bruno Ricardo de Castro Leite, J.; Tribst, A.A.L.; Cristianini, M. The effect of high pressure processing on recombinant chymosin, bovine rennet and porcine pepsin: Influence on the proteolytic and milk-clotting activities and on milk-clotting characteristics. LWT Food Sci. Technol. 2017, 76, 351–360. [Google Scholar] [CrossRef]
- Prestes, A.A.; Helm, C.V.; Esmerino, E.A.; Silva, R.; Prudencio, E.S. Conventional and alternative concentration processes in milk manufacturing: A comparative study on dairy properties. Food Sci. Technol. 2022, 42, 8822. [Google Scholar] [CrossRef]
- Soltani, M.; Saremnezhad, S.; Faraji, A.R.; Hayaloglu, A.A. Perspectives and recent innovations on white cheese produced by conventional methods or ultrafiltration technique. Int. Dairy J. 2022, 125, 105232. [Google Scholar] [CrossRef]
- Petrovska, S.; Jonkus, D.; Zagorska, J.; Ciprovica, I. The influence of k-casein genotype on the coagulation properties of milk collected from the local latvian cow breeds. Agron. Res. 2017, 15, 1411–1418. [Google Scholar]
- Schuck, P.; le Floch-Fouere, C.; Jeantet, R. Changes in Functional Properties of Milk Protein Powders: Effects of Vacuum Concentration and Drying. Dry. Technol. 2013, 31, 1578–1591. [Google Scholar] [CrossRef]
- Freitas, C.D.T.; Silva, M.Z.R.; Oliveira, J.P.B.; Silva, A.F.B.; Ramos, M.V.; de Sousa, J.S. Study of milk coagulation induced by chymosin using atomic force microscopy. Food Biosci. 2019, 29, 81–85. [Google Scholar] [CrossRef]
- O’Sullivan, D.; Nongonierma, A.B.; FitzGerald, R.J. Bitterness in sodium caseinate hydrolysates: Role of enzyme preparation and degree of hydrolysis. J. Sci. Food Agric. 2017, 97, 4652–4655. [Google Scholar] [CrossRef]
- Song, P.; Cheng, L.; Tian, K.; Zhang, M.; Singh, S.; Niu, D.; Prior, B.; Mchunu, N.P.; Wang, Z.X. A novel aminopeptidase with potential debittering properties in casein and soybean protein hydrolysates. Food Sci. Biotechnol. 2020, 29, 1491–1499. [Google Scholar] [CrossRef]
- Li, S.; Ye, A.; Singh, H. The effect of ultrafiltration on the acid gelation properties of protein-standardised skim milk systems. Food Res. Int. 2021, 146, 110432. [Google Scholar] [CrossRef]
- Meyer, P.; Hartinger, M.; Sigler, S.; Kulozik, U. Concentration of Milk and Whey by Membrane Technologies in Alternative Cascade Modes. Food Bioprocess Technol. 2017, 10, 674–686. [Google Scholar] [CrossRef]
- Meyer, P.; Petermeier, J.; Hartinger, M.; Kulozik, U. Concentration of Skim Milk by a Cascade Comprised of Ultrafiltration and Nanofiltration: Investigation of the Nanofiltration of Skim Milk Ultrafiltration Permeate. Food Bioprocess Technol. 2017, 10, 469–478. [Google Scholar] [CrossRef]
- Sandra, S.; Cooper, C.; Alexander, M.; Corredig, M. Coagulation properties of ultrafiltered milk retentates measured using rheology and diffusing wave spectroscopy. Food Res. Int. 2011, 44, 951–956. [Google Scholar] [CrossRef]
- Eshpari, H.; Jimenez-Flores, R.; Tong, P.S.; Corredig, M. Thermal stability of reconstituted milk protein concentrates: Effect of partial calcium depletion during membrane filtration. Food Res. Int. 2017, 102, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Schekotova, A.V.; Zambalova, N.A.; Boiarineva, I.V.; Maradudina, I.P.; Khamagaeva, I.S. The study of the proteolysis of milk proteins obtained by thermal calcium coagulation. J. Pharm. Sci. Res. 2017, 9, 2599–2603. [Google Scholar]
- Anema, S.G. Role of κ-Casein in the Association of Denatured Whey Proteins with Casein Micelles in Heated Reconstituted Skim Milk. J. Agric. Food Chem. 2007, 55, 3635–3642. [Google Scholar] [CrossRef] [PubMed]
- Anema, S.G.; Li, Y. Association of denatured whey proteins with casein micelles in heated reconstituted skim milk and its effect on casein micelle size. J. Dairy Res. 2003, 70, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Tieu, S.; Harte, F. Effect of mild thermal and pH changes on the sol-gel transition in skim milk. J. Dairy Sci. 2022, 105, 7926–7939. [Google Scholar] [CrossRef]

 -CSN3AA;
-CSN3AA;  -CSN3AB;
-CSN3AB;  -CSN3BB). Error bars represent standard errors.
-CSN3BB). Error bars represent standard errors.
 -CSN3AA;
-CSN3AA;  -CSN3AB;
-CSN3AB;  -CSN3BB). Error bars represent standard errors.
-CSN3BB). Error bars represent standard errors.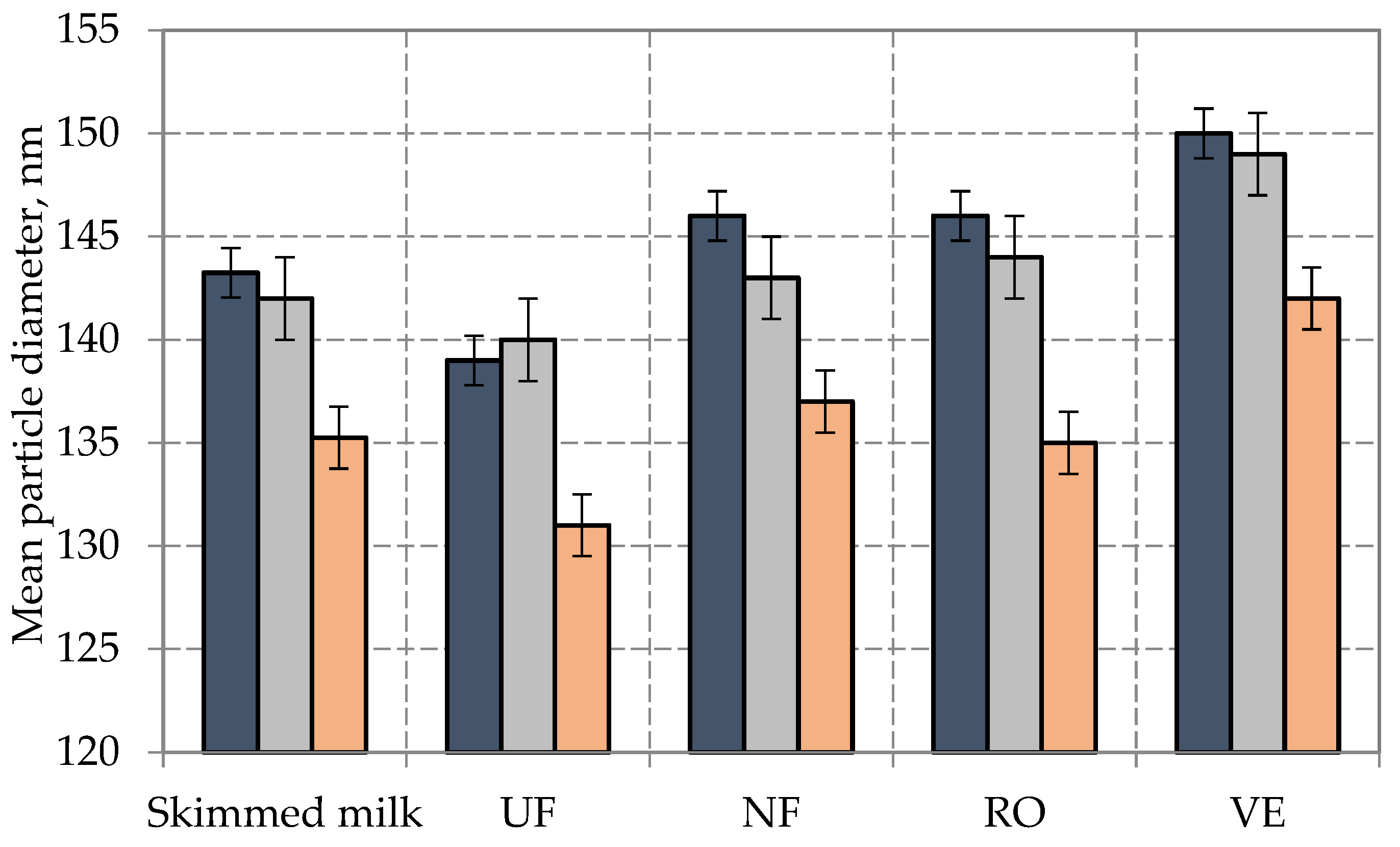
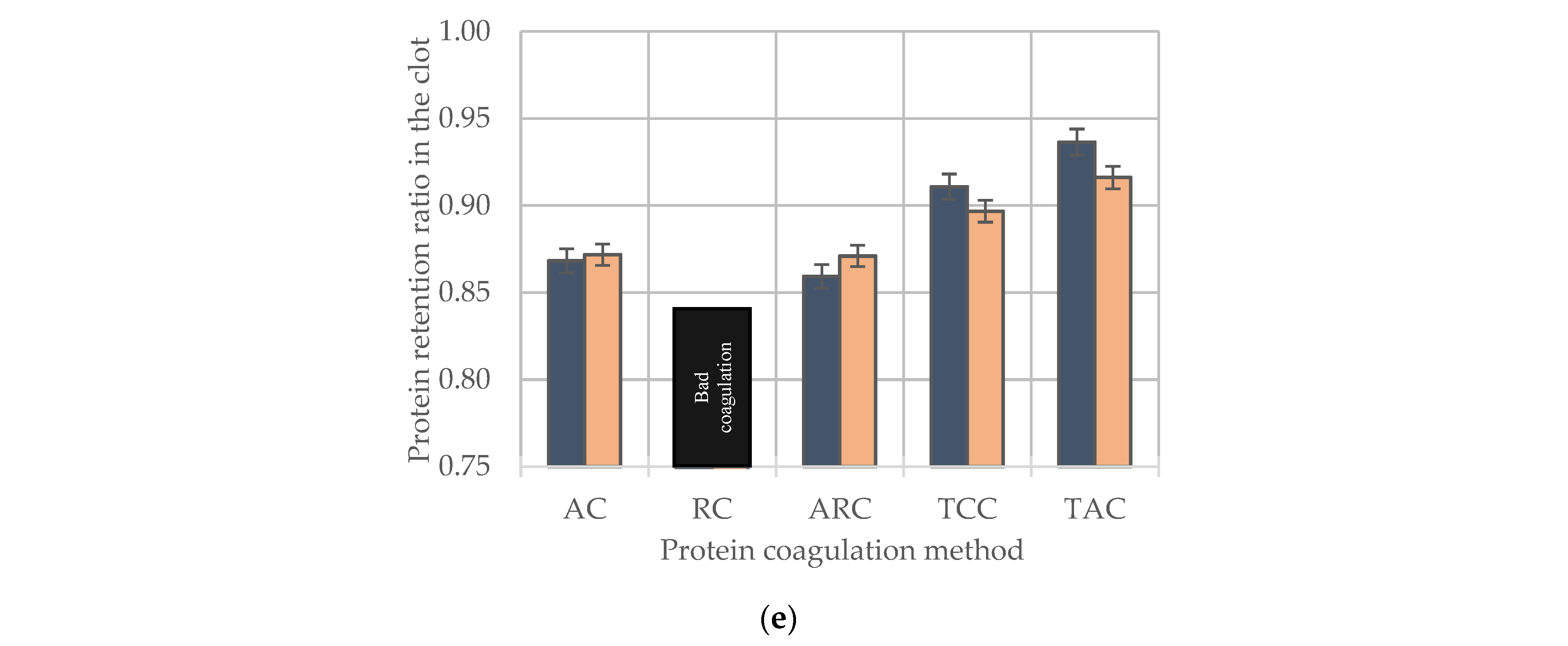
 —ME;
—ME;  —BCP 96/04;
—BCP 96/04;  —BCP 50/50;
—BCP 50/50;  —BCP 05/95).
—BCP 05/95).
 —ME;
—ME;  —BCP 96/04;
—BCP 96/04;  —BCP 50/50;
—BCP 50/50;  —BCP 05/95).
—BCP 05/95).


 -CSN3 AA;
-CSN3 AA;  -CSN3 BB. Error bars represent standard errors.
-CSN3 BB. Error bars represent standard errors.
 -CSN3 AA;
-CSN3 AA;  -CSN3 BB. Error bars represent standard errors.
-CSN3 BB. Error bars represent standard errors.
| Genotype | Number of Cows, Head | Age of Cows, Calves | Frequency Occurrence Genotypes, % | Frequency of Alleles, % | |
|---|---|---|---|---|---|
| A | B | ||||
| AA | 225 | 2.3 ± 0.1 | 63.7 | 77.5 | 22.5 |
| AB | 97 | 2.6 ± 0.1 | 27.5 | ||
| BB | 31 | 2.1 ± 0.2 | 8.8 | ||
| Genotype | Milk Yield (kg) | Fat Content (%) | Fat Yield (kg) | Protein Contnet (%) | Protein Yield (kg) |
|---|---|---|---|---|---|
| AA | 10,359 ± 443 | 3.69 ± 0.15 | 382.3 ± 15.5 | 3.22 ± 0.07 | 333.6 ± 7.3 |
| AB | 10,297 ± 405 | 3.71 ± 0.12 | 382.0 ± 12.4 | 3.17 ± 0.13 | 326.4 ± 13.4 |
| BB | 9983 ± 381 | 3.88 ± 0.21 | 387.3 ± 21.0 | 3.30 ± 0.09 | 329.4 ± 9.0 |
| Name of Parameter | Skim Milk | UF-Retentat | NF-Retentat | RO-Retentat | VE-Concentrat | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CSN3AA | CSN3AB | CSN3BB | CSN3AA | CSN3AB | CSN3BB | CSN3AA | CSN3AB | CSN3BB | CSN3AA | CSN3AB | CSN3BB | CSN3AA | CSN3AB | CSN3BB | |
| Fat, % | 0.07 ± 0.01 a | 0.06 ± 0.01 a | 0.10 ± 0.01 b | 0.14 ± 0.02 b | 0.12 ± 0.01 b | 0.20 ± 0.03 c | 0.14 ± 0.02 b | 0.12 ± 0.02 b | 0.19 ± 0.03 c | 0.14 ± 0.01 b | 0.12 ± 0.02 b | 0.19 ± 0.03 c | 0.15 ± 0.02 bc | 0.12 ± 0.02 b | 0.20 ± 0.02 c |
| Protein, % | 3.30 ± 0.12 a | 3.24 ± 0.09 a | 3.39 ± 0.10 a | 6.64 ± 0.12 b | 6.62 ± 0.10 b | 6.61 ± 0.09 b | 6.63 ± 0.10 b | 6.64 ± 0.12 b | 6.61 ± 0.10 b | 6.62 ± 0.11 b | 6.64 ± 0.14 b | 6.61 ± 0.09 b | 6.60 ± 0.09 b | 6.63 ± 0.13 b | 6.64 ± 0.11 b |
| Casein, % | 2.58 ± 0.09 a | 2.53 ± 0.14 a | 2.71 ± 0.14 a | 5.58 ± 0.09 b | 5.53 ± 0.09 b | 5.63 ± 0.11 b | 5.16 ± 0.10 c | 5.19 ± 0.14 c | 5.29 ± 0.12 c | 5.16 ± 0.14 c | 5.20 ± 0.13 c | 5.29 ± 0.10 c | 5.16 ± 0.09 c | 5.18 ± 0.14 c | 5.32 ± 0.10 c |
| αS1-CN | 1.13 ± 0.03 a | 1.11 ± 0.03 a | 1.20 ± 0.04 a | 2.39 ± 0.07 b | 2.41 ± 0.05 b | 2.50 ± 0.08 b | 2.28 ± 0.04 c | 2.26 ± 0.06 c | 2.32 ± 0.03 c | 2.29 ± 0.04 c | 2.26 ± 0.04 c | 2.35 ± 0.03 bc | 2.24 ± 0.04 c | 2.25 ± 0.02 c | 2.33 ± 0.06 c |
| αS2-CN | 0.25 ± 0.02 a | 0.26 ± 0.02 a | 0.23 ± 0.01 a | 0.55 ± 0.03 b | 0.53 ± 0.02 b | 0.47 ± 0.04 c | 0.48 ± 0.02 c | 0.53 ± 0.04 bc | 0.46 ± 0.03 c | 0.49 ± 0.02 c | 0.53 ± 0.01 b | 0.45 ± 0.03 c | 0.51 ± 0.03 bc | 0.54 ± 0.02 b | 0.46 ± 0.01 c |
| β-CN | 0.97 ± 0.03 a | 0.92 ± 0.02 a | 0.98 ± 0.02 a | 2.12 ± 0.02 b | 2.05 ± 0.03 b | 2.03 ± 0.03 b | 1.92 ± 0.05 c | 1.90 ± 0.02 c | 1.93 ± 0.03 c | 1.96 ± 0.05 c | 1.91 ± 0.04 c | 1.90 ± 0.02 c | 1.96 ± 0.05 c | 1.90 ± 0.03 c | 1.93 ± 0.02 c |
| κ-CN | 0.23 ± 0.02 a | 0.24 ± 0.01 a | 0.30 ± 0.02 b | 0.52 ± 0.01 c | 0.54 ± 0.02 c | 0.61 ± 0.02 d | 0.48 ± 0.01 e | 0.50 ± 0.01 c | 0.58 ± 0.02 d | 0.46 ± 0.02 e | 0.50 ± 0.02 c | 0.57 ± 0.03 cd | 0.45 ± 0.01 e | 0.49 ± 0.01 c | 0.60 ± 0.03 d |
| Whey protein, % | 0.72 ± 0.07 a | 0.71 ± 0.05 a | 0.68 ± 0.05 a | 1.06 ± 0.03 b | 1.09 ± 0.04 b | 0.98 ± 0.04 b | 1.47 ± 0.03 c | 1.45 ± 0.06 c | 1.32 ± 0.04 d | 1.46 ± 0.03 c | 1.44 ± 0.05 c | 1.32 ± 0.05 d | 1.44 ± 0.07 c | 1.45 ± 0.04 c | 1.32 ± 0.03 d |
| β-LG | 0.40 ± 0.03 a | 0.38 ± 0.03 a | 0.40 ± 0.04 a | 0.57 ± 0.03 b | 0.56 ± 0.02 b | 0.55 ± 0.03 b | 0.79 ± 0.04 c | 0.76 ± 0.05 c | 0.75 ± 0.03 c | 0.81 ± 0.05 c | 0.78 ± 0.04 c | 0.79 ± 0.04 c | 0.82 ± 0.04 c | 0.79 ± 0.02 c | 0.76 ± 0.03 c |
| α-LA | 0.17 ± 0.03 a | 0.18 ± 0.03 a | 0.14 ± 0.03 a | 0.22 ± 0.02 a b | 0.24 ± 0.04 ab | 0.18 ± 0.03 a | 0.32 ± 0.03 c | 0.34 ± 0.02 c | 0.26 ± 0.01 b | 0.33 ± 0.02 c | 0.38 ± 0.03 c | 0.27 ± 0.01 b | 0.33 ± 0.02 c | 0.36 ± 0.01 c | 0.29 ± 0.02 bc |
| BSA | 0.03 ± 0.002 a | 0.03 ± 0.002 a | 0.04 ± 0.003 b | 0.06 ± 0.002 c | 0.06 ± 0.002 c | 0.08 ± 0.002 d | 0.06 ± 0.001 c | 0.06 ± 0.002 c | 0.08 ± 0.002 d | 0.06 ± 0.002 c | 0.05 ± 0.001 e | 0.07 ± 0.003 f | 0.05 ± 0.001 e | 0.06 ± 0.003 c | 0.08 ± 0.003 d |
| Lactose, % | 5.01 ± 0.12 a | 4.96 ± 0.09 a | 5.10 ± 0.14 a | 5.26 ± 0.16 a | 5.31 ± 0.14 a | 5.27 ± 0.17 b | 9.66 ± 0.18 b | 9.84 ± 0.21 b | 9.65 ± 0.15 b | 10.15 ± 0.19 b | 10.18 ± 0.20 b | 9.95 ± 0.16 b | 10.01 ± 0.21 b | 10.17 ± 0.21 b | 10.00 ± 0.19 b |
| Ash, % | 0.76 ± 0.03 a | 0.72 ± 0.01 a | 0.79 ± 0.03 a | 0.88 ± 0.03 a | 0.85 ± 0.04 a | 0.96 ± 0.04 a | 1.21 ± 0.02 b | 1.14 ± 0.04 b | 1.23 ± 0.02 b | 1.44 ± 0.03 c | 1.39 ± 0.03 c | 1.46 ± 0.04 c | 1.44 ± 0.02 c | 1.40 ± 0.04 c | 1.55 ± 0.04 c |
| Calcium, mg/100 g | 119.37 ± 3.15 a | 122.19 ± 3.76 a | 131.49 ± 3.48 a | 186.39 ± 4.11 b | 191.53 ± 4.23 b | 203.47 ± 4.15 c | 201.18 ± 4.15 c | 205.36 ± 3.95 c | 219.79 ± 3.87 d | 233.83 ± 3.78 e | 239.35 ± 4.02 e | 251.57 ± 4.13 f | 235.71 ± 3.56 e | 248.03 ± 3.92 e | 252.48 ± 4.15 f |
| Magnesium, mg/100 g | 13.71 ± 0.22 a | 13.87 ± 0.24 a | 14.64 ± 0.27 a | 16.28 ± 0.33 b | 16.68 ± 0.18 b | 17.32 ± 0.18 b | 23.56 ± 0.24 c | 24.04 ± 0.26 c | 24.98 ± 0.19 c | 26.23 ± 0.29 d | 27.16 ± 0.27 d | 27.36 ± 0.31 d | 26.45 ± 0.32 d | 27.49 ± 0.24 d | 27.91 ± 0.43 d |
| Sodium, mg/100 g | 24.87 ± 0.33 a | 24.35 ± 0.18 a | 24.14 ± 0.21 a | 25.96 ± 0.27 a | 25.52 ± 0.24 a | 24.91 ± 0.20 a | 34.80 ± 0.31 b | 34.07 ± 0.34 b | 33.77 ± 0.41 b | 48.15 ± 0.21 c | 47.15 ± 0.42 c | 46.74 ± 0.26 c | 48.84 ± 0.31 c | 48.53 ± 0.39 c | 46.68 ± 0.27 c |
| Potassium, mg/100 g | 164.08 ± 2.15 a | 162.03 ± 1.76 a | 158.45 ± 1.82 a | 126.57 ± 3.11 b | 124.13 ± 1.97 b | 121.96 ± 2.13 b | 203.51 ± 3.26 c | 201.04 ± 3.17 c | 196.95 ± 2.66 c | 317.65 ± 3.51 d | 319.18 ± 3.42 d | 298.38 ± 3.45 e | 322.33 ± 4.15 d | 325.31 ± 4.25 d | 304.57 ± 4.03 e |
| Phosphates, mg/100 g | 118.42 ± 4.12 a | 110.35 ± 3.19 a | 104.00 ± 3.03 a | 154.43 ± 3.75 b | 143.90 ± 3.82 b | 135.62 ± 3.24 ab | 191.83 ± 2.99 c | 178.76 ± 3.18 c | 168.47 ± 3.42 bc | 222.03 ± 3.87 d | 208.94 ± 4.02 d | 195.65 ± 3.94 c | 233.91 ± 3.75 e | 222.54 ± 4.00 d | 201.46 ± 4.15 c |
| Citrates, mg/100 g | 173.01 ± 3.02 a | 151.21 ± 3.41 b | 122.18 ± 2.95 c | 208.98 ± 3.45 d | 194.27 ± 3.12 a | 147.84 ± 3.05 c | 257.38 ± 3.28 e | 220.77 ± 3.21 d | 179.51 ± 3.01 a | 337.90 ± 3.12 f | 301.61 ± 3.41 g | 232.53 ± 3.30 d | 341.13 ± 3.64 f | 306.15 ± 3.7 g | 235.12 ± 3.32 d |
| Total solids, % | 9.41 ± 0.17 a | 9.21 ± 0.18 a | 9.57 ± 0.14 a | 13.97 ± 0.16 b | 13.91 ± 0.15 b | 14.14 ± 0.19 b | 17.81 ± 0.14 c | 17.98 ± 0.22 c | 17.79 ± 0.19 c | 18.47 ± 0.20 c | 18.45 ± 0.20 c | 18.36 ± 0.17 c | 18.76 ± 0.21 c | 18.45 ± 0.20 c | 19.10 ± 0.20 c |
| Titratable acidity, °T | 16.5 ± 0.5 a | 16.4 ± 0.4 a | 17.0 ± 0.5 a | 27.0 ± 0.3 a | 27.5 ± 0.4 a | 25.6 ± 0.3 a | 23.5 ± 0.3 a | 23.4 ± 0.5 a | 25.0 ± 0.2 a | 39.1 ± 0.5 a | 38.5 ± 0.6 a | 36.8 ± 0.6 a | 35.2 ± 0.4 a | 34.9 ± 0.5 a | 36.1 ± 0.5 a |
| pH | 6.79 ± 0.04 a | 6.77 ± 0.04 a | 6.75 ± 0.05 a | 6.81 ± 0.02 b | 6.79 ± 0.05 b | 6.75 ± 0.03 c | 6.71 ± 0.03 c | 6.73 ± 0.04 c | 6.69 ± 0.02 c | 6.50 ± 0.04 d | 6.52 ± 0.05 d | 6.54 ± 0.03 e | 6.49 ± 0.03 e | 6.51 ± 0.02 e | 6.42 ± 0.04 e |
| Name of Parameter | Enzyme Preparations | |||||||
|---|---|---|---|---|---|---|---|---|
| ME | BCP 96/04 | BCP 50/50 | BCP 05/95 | ME | BCP 96/04 | BCP 50/50 | BCP 05/95 | |
| CSN3AA | CSN3BB | |||||||
| Clot yield, % | 15.2 ± 0.3 a | 14.6 ± 0.2 b | 15.8 ± 0.3 c | 16.0 ± 0.3 c | 16.1 ± 0.2 c | 15.4 ± 0.1 a | 16.6 ± 0.4 c | 17.2 ± 0.4 d |
| Total solids, % | 22.50 ± 0.21 a | 23.04 ± 0.34 b | 21.56 ± 0.17 c | 21.93 ± 0.14 c | 23.10 ± 0.27 b | 23.50 ± 0.25 b | 22.48 ± 0.19 a | 22.15 ± 0.17 a |
| Protein, % | 16.19 ± 0.13 a | 17.71 ± 0.15 b | 16.16 ± 0.10 a | 15.84 ± 0.08 a | 16.85 ± 0.16 c | 18.07 ± 0.14 b | 16.45 ± 0.11 a | 16.07 ± 0.09 a |
| Protein retention factor | 0.746 ± 0.006 a | 0.784 ± 0.011 b | 0.774 ± 0.009 b | 0.768 ± 0.009 b | 0.800 ± 0.006 c | 0.821 ± 0.012 c | 0.806 ± 0.001 c | 0.815 ± 0.001 c |
| UF-CSN3AA | UF-CSN3BB | |||||||
| Clot yield, % | 27.3 ± 0.5 a | 26.5 ± 0.6 a | 27.4 ± 0.5 a | 28.7 ± 0.7 b | 28.3 ± 0.7 b | 27.6 ± 0.5 ab | 28.2 ± 0.7 ab | 29.6 ± 0.8 b |
| Total solids, % | 24.61 ± 0.25 a | 24.74 ± 0.24 a | 24.31 ± 0.21 a | 24.25 ± 0.26 a | 27.71 ± 0.31 b | 28.03 ± 0.27 b | 26.89 ± 0.24 b | 26.52 ± 0.19 b |
| Protein, % | 21.32 ± 0.16 a | 21.80 ± 0.18 a | 21.15 ± 0.16 a | 19.01 ± 0.14 b | 20.43 ± 0.17 c | 21.85 ± 0.20 a | 20.61 ± 0.15 c | 20.12 ± 0.14 c |
| Protein retention factor | 0.882 ± 0.001 a | 0.875 ± 0.006 a | 0.878 ± 0.002 a | 0.827 ± 0.006 b | 0.876 ± 0.016 a | 0.914 ± 0.011 c | 0.881 ± 0.015 a | 0.902 ± 0.017 ac |
| NF-CSN3AA | NF-CSN3BB | |||||||
| Clot yield, % | 29.6 ± 0.6 a | 29.0 ± 0.7 a | 29.4 ± 0.5 a | 30.9 ± 0.9 ab | 30.9 ± 0.7 ab | 30.4 ± 0.6 ab | 30.2 ± 0.6 ab | 31.6 ± 0.8 b |
| Total solids, % | 25.34 ± 0.24 a | 25.52 ± 0.26 a | 24.76 ± 0.21 a | 23.49 ± 0.19 b | 24.03 ± 0.23 b | 25.41 ± 0.23 a | 25.06 ± 0.27 a | 24.96 ± 0.21 a |
| Protein, % | 20.05 ± 0.16 a | 20.31 ± 0.18 a | 19.87 ± 0.16 a | 18.61 ± 0.14 b | 17.97 ± 0.17 b | 20.00 ± 0.19 a | 18.69 ± 0.15 b | 18.59 ± 0.17 b |
| Protein retention factor | 0.899 ± 0.008 a | 0.892 ± 0.012 a | 0.885 ± 0.005 a | 0.871 ± 0.015 a | 0.841 ± 0.018 b | 0.921 ± 0.012 c | 0.855 ± 0.010 b | 0.890 ± 0.016 a |
| RO-CSN3AA | RO-CSN3BB | |||||||
| Clot yield, % | 29.4 ± 0.6 a | 28.7 ± 0.5 a | 29.1 ± 0.7 a | 30.9 ± 0.9 b | 30.9 ± 0.7 b | 29.2 ± 0.4 a | 29.9 ± 0.6 a | 31.4 ± 0.9 b |
| Total solids, % | 28.30 ± 0.27 a | 28.66 ± 0.24 a | 27.96 ± 0.25 b | 27.68 ± 0.19 b | 27.89 ± 0.24 b | 28.73 ± 0.29 a | 27.96 ± 0.18 b | 27.81 ± 0.21 b |
| Protein, % | 20.04 ± 0.18 a | 21.11 ± 0.16 b | 19.86 ± 0.14 a | 18.48 ± 0.18 c | 18.50 ± 0.19 c | 21.07 ± 0.21 b | 19.12 ± 0.15 c | 18.81 ± 0.17 c |
| Protein retention factor | 0.893 ± 0.008 a | 0.918 ± 0.04 a | 0.876 ± 0.010 a | 0.865 ± 0.017 a | 0.866 ± 0.016 a | 0.932 ± 0.008 b | 0.866 ± 0.011 a | 0.895 ± 0.020 a |
| Name of Parameter | Protein Coagulation Method | |||||||
|---|---|---|---|---|---|---|---|---|
| AC | ARC | TCC | TAC | AC | ARC | TCC | TAC | |
| CSN3AA | CSN3BB | |||||||
| Coagulation duration, min | 600 ± 5 aA | 480 ± 5 bA | 5.0 ± 0.1 | 10 ± 1 | 600 ± 5 aA | 480 ± 5 bA | 5.0 ± 0.1 | 10 ± 1 |
| Clot yield, % | 13.9 ± 0.4 a | 14.8 ± 0.5 a | 15.9 ± 0.7 b | 20.7 ± 0.5 c | 16.2 ± 0.3 b | 15.8 ± 0.4 b | 16.3 ± 0.4 b | 16.6 ± 0.5 b |
| Total solids, % | 26.60 ± 0.21 a | 25.11 ± 0.19 b | 27.53 ± 0.24 c | 23.60 ± 0.22 b | 24.55 ± 0.23 b | 25.84 ± 0.19 b | 27.52 ± 0.25 c | 28.31 ± 0.27 c |
| Protein, % | 20.26 ± 0.16 | 18.93 ± 0.14 | 19.97 ± 0.17 | 15.31 ± 0.18 | 17.37 ± 0.17 | 19.31 ± 0.15 | 19.44 ± 0.19 | 19.80 ± 0.23 |
| UF-CSN3AA | UF-CSN3BB | |||||||
| Coagulation duration, min | 690 ± 5 aB | 520 ± 5 bB | 5.0 ± 0.1 | 10 ± 1 | 670 ± 5 cB | 505 ± 5 bAB | 5.0 ± 0.1 | 10 ± 1 |
| Clot yield, % | 29.7 ± 0.8 a | 27.9 ± 0.7 a | 37.3 ± 1.1 b | 25.2 ± 0.6 a | 33.4 ± 0.9 a | 28.0 ± 0.6 a | 41.5 ± 1.0 c | 24.9 ± 0.6 a |
| Total solids, % | 25.62 ± 0.24 a | 25.13 ± 0.22 a | 23.64 ± 0.19 a | 31.44 ± 0.29 b | 23.81 ± 0.17 a | 25.78 ± 0.21 a | 18.38 ± 0.15 c | 29.90 ± 0.27 b |
| Protein, % | 20.24 ± 0.14 | 20.95 ± 0.16 | 16.97 ± 0.13 | 25.55 ± 0.21 | 18.16 ± 0.14 | 21.32 ± 0.18 | 14.86 ± 0.11 | 25.40 ± 0.20 |
| NF-CSN3AA | NF-CSN3BB | |||||||
| Coagulation duration, min | 700 ± 5 aB | 540 ± 5 bC | 5.0 ± 0.1 | 10 ± 1 | 690 ± 5 aC | 525 ± 5 bB | 5.0 ± 0.1 | 10 ± 1 |
| Clot yield, % | 31.1 ± 0.9 a | 29.6 ± 0.7 a | 38.3 ± 1.0 b | 24.9 ± 0.5 c | 31.3 ± 0.9 a | 29.7 ± 0.7 a | 41.3 ± 1.1 b | 26.5 ± 0.8 c |
| Total solids, % | 22.68 ± 0.19 a | 23.16 ± 0.24 a | 25.46 ± 0.23 a | 35.43 ± 0.27 b | 23.24 ± 0.21 a | 23.61 ± 0.23 a | 23.25 ± 0.19 a | 36.03 ± 0.32 b |
| Protein, % | 18.44 ± 0.14 | 19.23 ± 0.19 | 16.42 ± 0.18 | 25.91 ± 0.24 | 18.57 ± 0.16 | 19.52 ± 0.15 | 15.03 ± 0.15 | 23.93 ± 0.27 |
| RO-CSN3AA | RO-CSN3BB | |||||||
| Coagulation duration, min | 690 ± 5 aB | 540 ± 5 bC | 5.0 ± 0.1 | 10 ± 1 | 670 ± 5 cB | 525 ± 5 bB | 5.0 ± 0.1 | 10 ± 1 |
| Clot yield, % | 30.5 ± 0.7 a | 29.1 ± 0.5 a | 38.0 ± 1.1 b | 24.8 ± 0.4 c | 30.7 ± 0.9 a | 29.2 ± 0.6 a | 40.9 ± 1.1 b | 26.3 ± 0.8 c |
| Total solids, % | 26.17 ± 0.24 a | 27.26 ± 0.27 a | 25.71 ± 0.23 a | 35.55 ± 0.31 b | 26.06 ± 0.24 a | 27.59 ± 0.28 a | 23.43 ± 0.19 c | 35.74 ± 0.30 b |
| Protein, % | 19.31 ± 0.19 | 20.11 ± 0.22 | 16.59 ± 0.18 | 26.03 ± 0.26 | 19.24 ± 0.20 | 20.27 ± 0.22 | 15.28 ± 0.14 | 24.15 ± 0.25 |
| VE-CSN3AA | VE-CSN3BB | |||||||
| Coagulation duration, min | 650 ± 5 aC | 550 ± 5 bC | 5.0 ± 0.1 | 10 ± 1 | 645 ± 5 aB | 550 ± 5 bC | 5.0 ± 0.1 | 10 ± 1 |
| Clot yield, % | 42.7 ± 1.2 a | 41.7 ± 1.0 a | 29.7 ± 0.7 b | 27.0 ± 0.5 b | 43.1 ± 1.0 a | 41.9 ± 0.9 a | 31.1 ± 0.7 b | 27.1 ± 0.5 b |
| Total solids, % | 21.30 ± 0.20 a | 20.80 ± 0.18 a | 31.03 ± 0.27 b | 31.18 ± 0.30 b | 21.21 ± 0.19 a | 20.95 ± 0.18 a | 28.74 ± 0.24 c | 31.43 ± 0.28 b |
| Protein, % | 13.42 ± 0.17 | 13.60 ± 0.14 | 20.24 ± 0.22 | 22.89 ± 0.24 | 13.35 ± 0.12 | 13.72 ± 0.13 | 19.03 ± 0.18 | 22.31 ± 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruchinin, A.G.; Illarionova, E.E.; Galstyan, A.G.; Turovskaya, S.N.; Bigaeva, A.V.; Bolshakova, E.I.; Strizhko, M.N. Effect of CSN3 Gene Polymorphism on the Formation of Milk Gels Induced by Physical, Chemical, and Biotechnological Factors. Foods 2023, 12, 1767. https://doi.org/10.3390/foods12091767
Kruchinin AG, Illarionova EE, Galstyan AG, Turovskaya SN, Bigaeva AV, Bolshakova EI, Strizhko MN. Effect of CSN3 Gene Polymorphism on the Formation of Milk Gels Induced by Physical, Chemical, and Biotechnological Factors. Foods. 2023; 12(9):1767. https://doi.org/10.3390/foods12091767
Chicago/Turabian StyleKruchinin, Aleksandr G., Elena E. Illarionova, Aram G. Galstyan, Svetlana N. Turovskaya, Alana V. Bigaeva, Ekaterina I. Bolshakova, and Mariya N. Strizhko. 2023. "Effect of CSN3 Gene Polymorphism on the Formation of Milk Gels Induced by Physical, Chemical, and Biotechnological Factors" Foods 12, no. 9: 1767. https://doi.org/10.3390/foods12091767







