Application and Effect of Pediococcus pentosaceus and Lactiplantibacillus plantarum as Starter Cultures on Bacterial Communities and Volatile Flavor Compounds of Zhayu, a Chinese Traditional Fermented Fish Product
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Pre-Culture of LAB
2.3. Preparation of Zhayu Samples
2.4. Determination of pH and Trichloroacetic Acid (TCA)-Soluble Peptides
2.5. Determination of Microbial Counts
2.6. SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.7. Determination of Thiobarbituric Acid-Reactive Substances (TBARS)
2.8. Texture Profile Analysis (TPA)
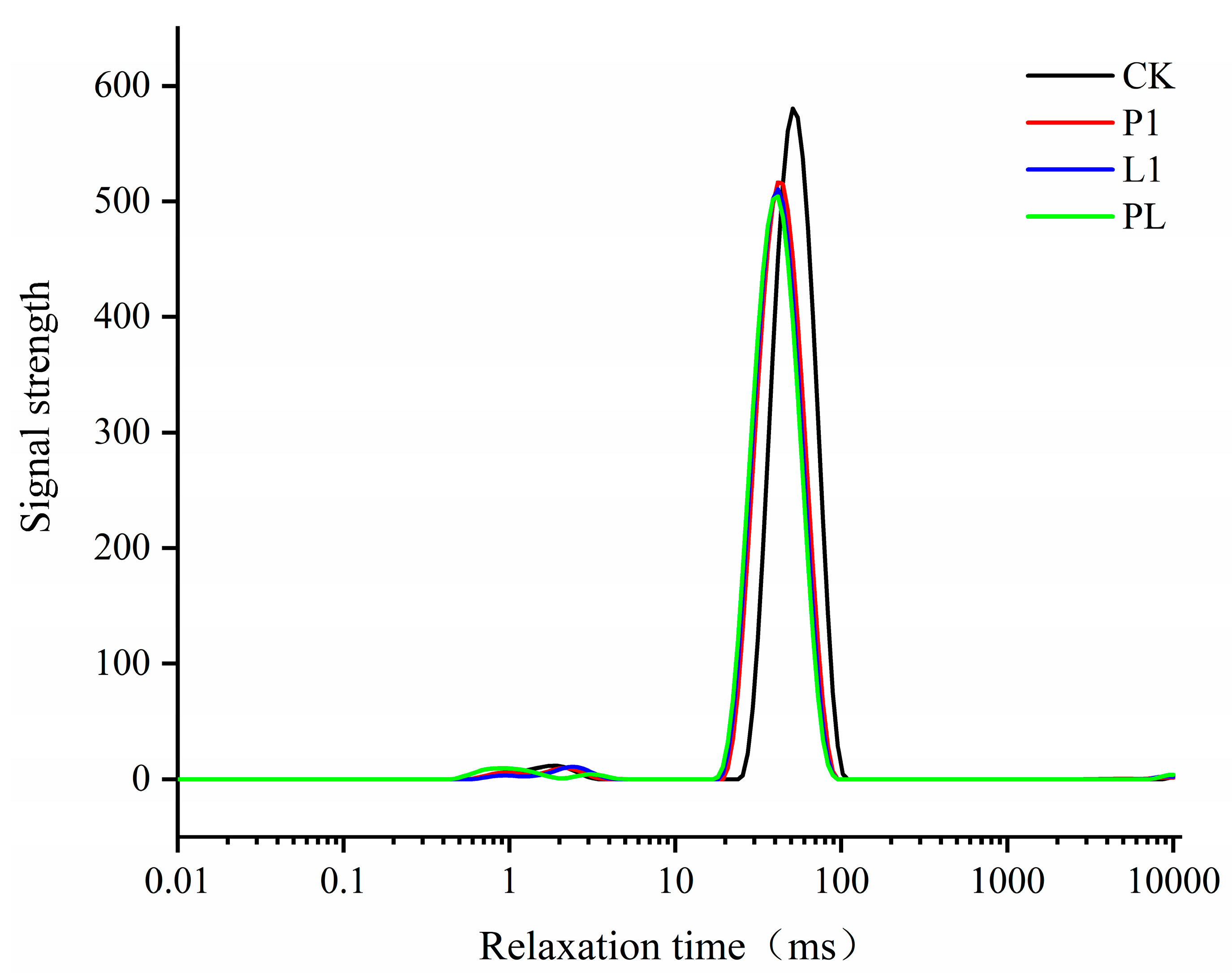
2.9. Low-Field Nuclear Magnetic Resonance (LF-NMR)
2.10. Analysis of Bacterial Diversity
2.11. Determination of Volatile Compounds by HS-SPME-GC-MS Analysis
2.12. Data Analysis
3. Results and Discussion
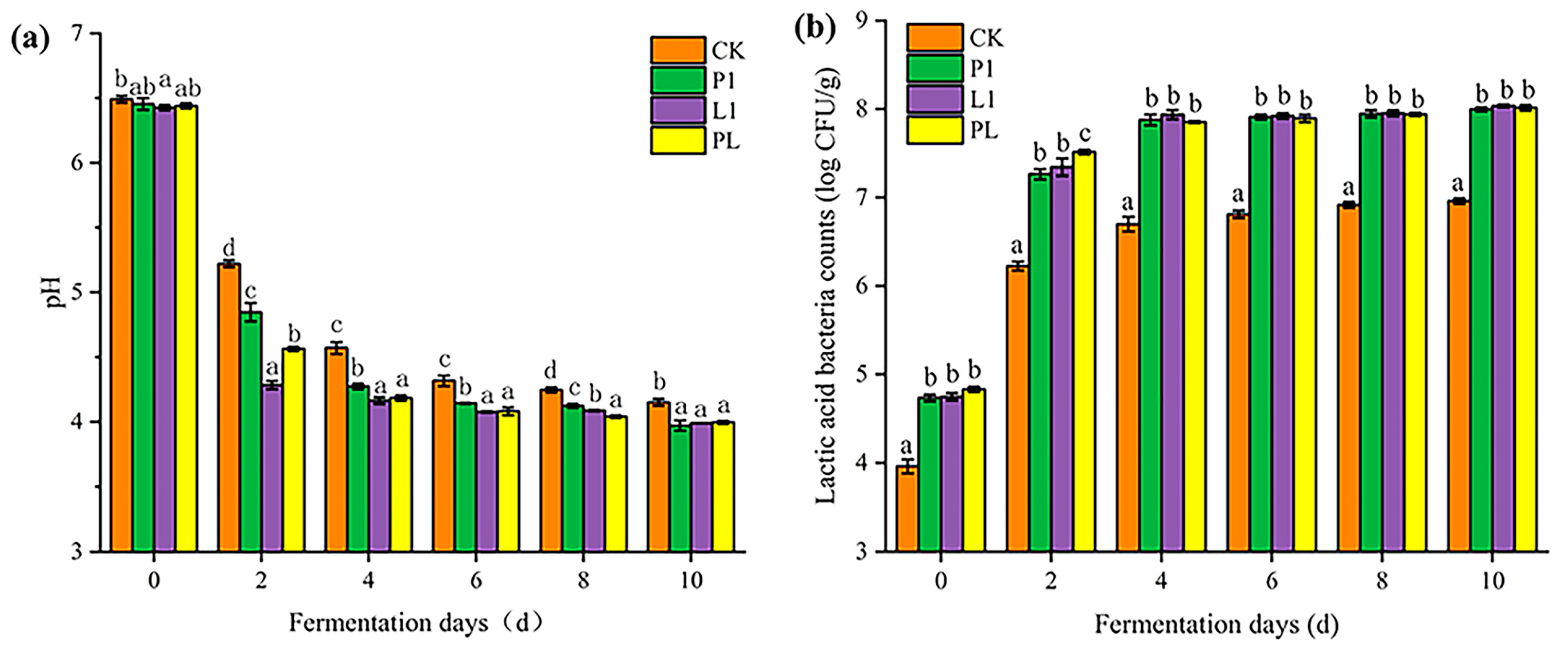
3.1. Growth of LAB Counts and Acid Production
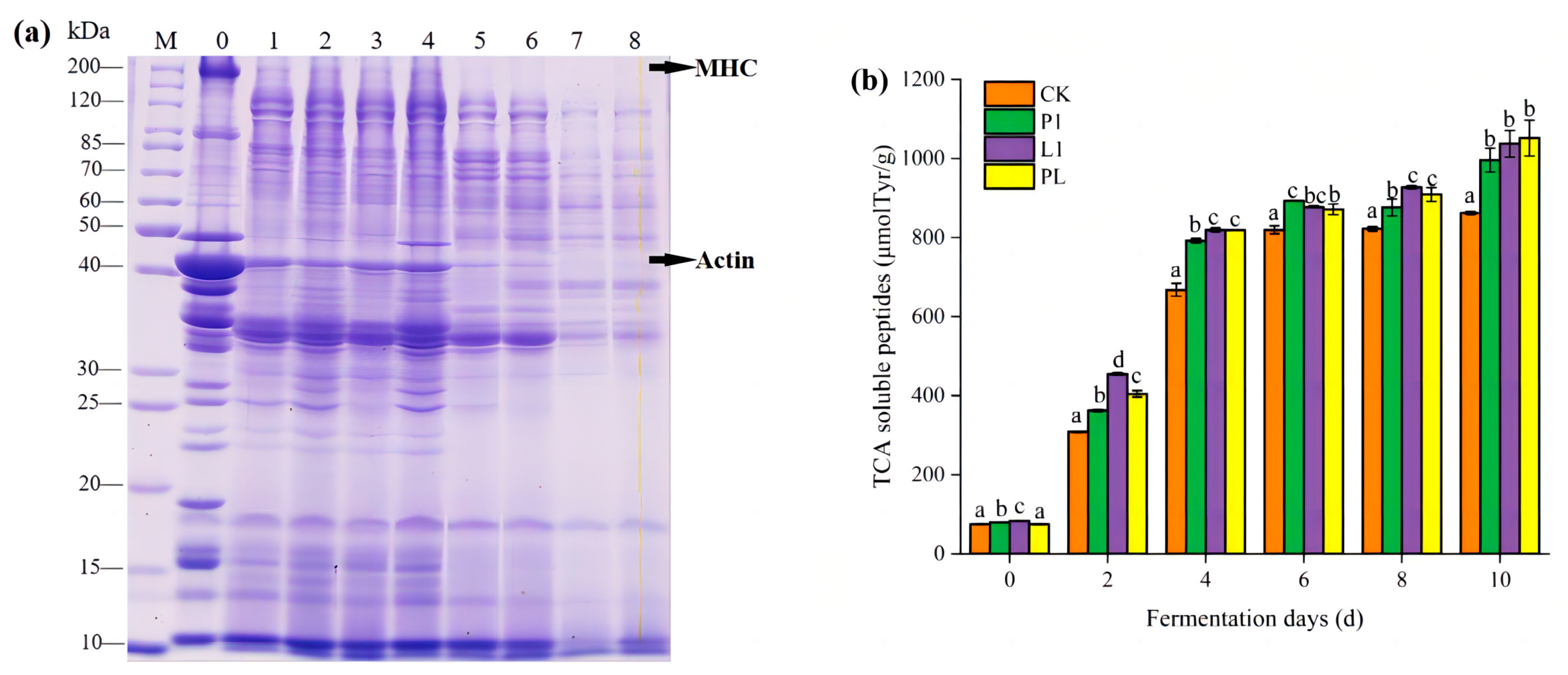
3.2. Indicators Related to Protein Degradation
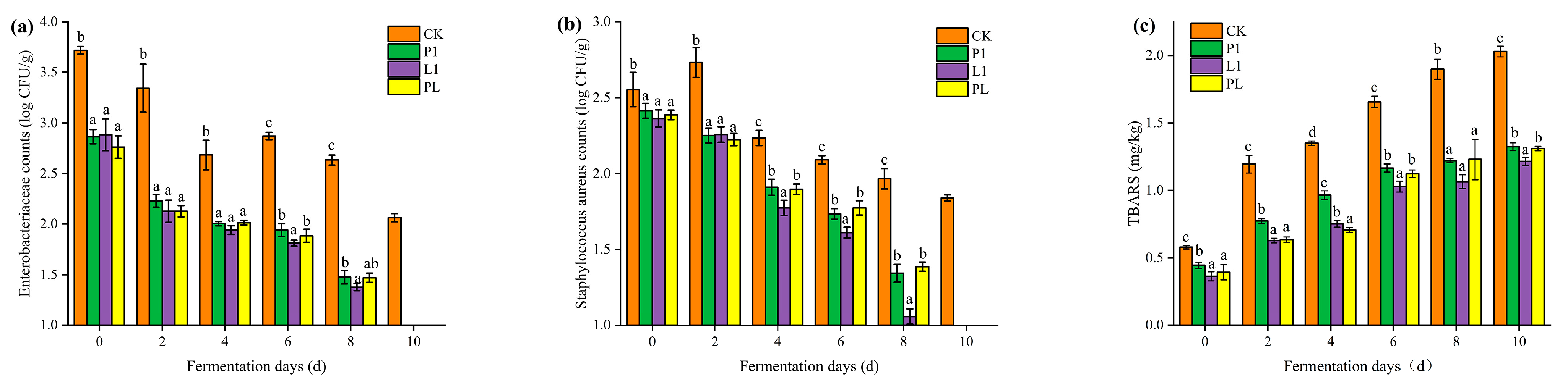
3.3. Improvement of Safety Qualities by LAB Strains
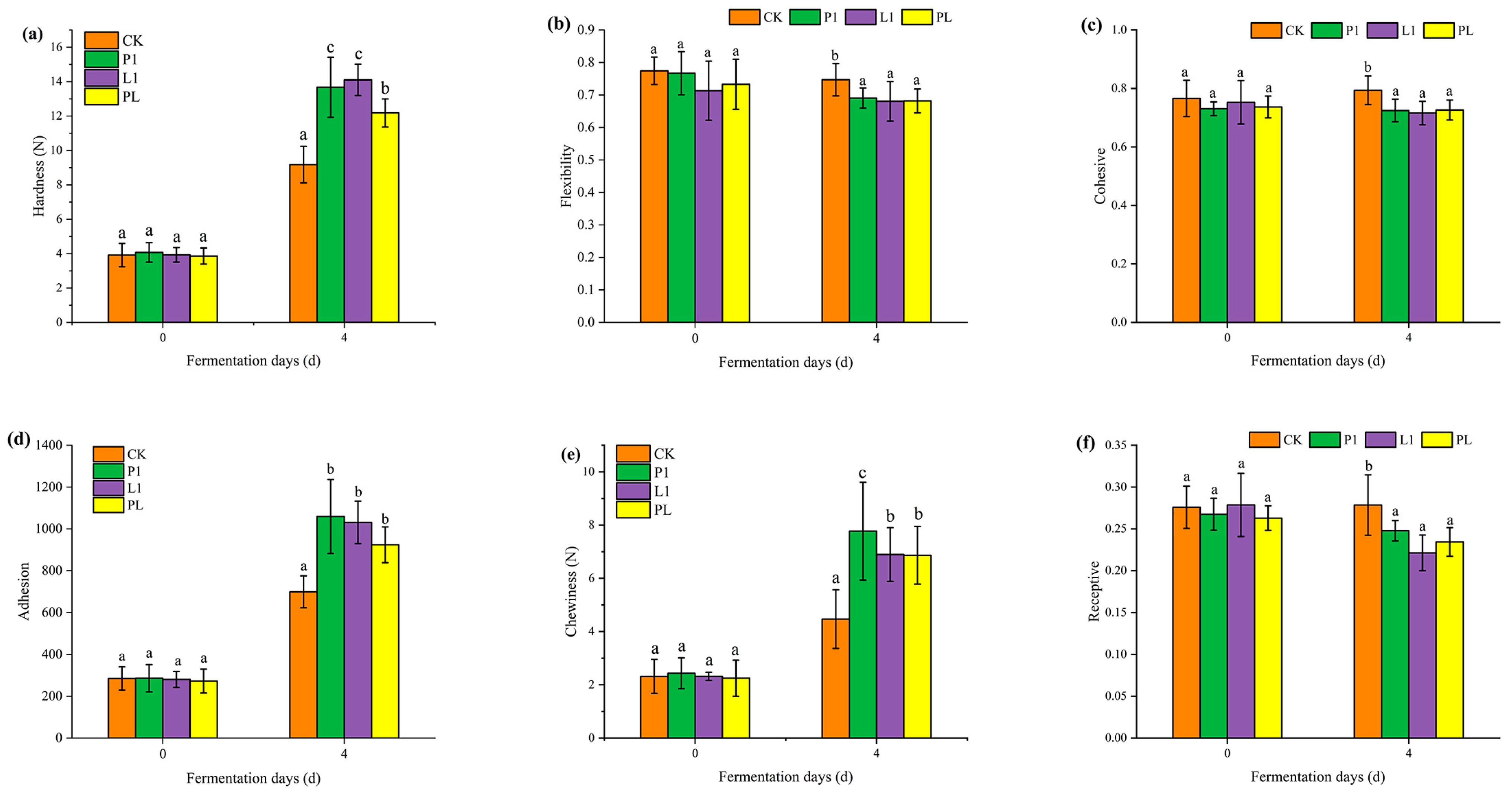
3.4. LAB Strain Influence on the Texture Properties
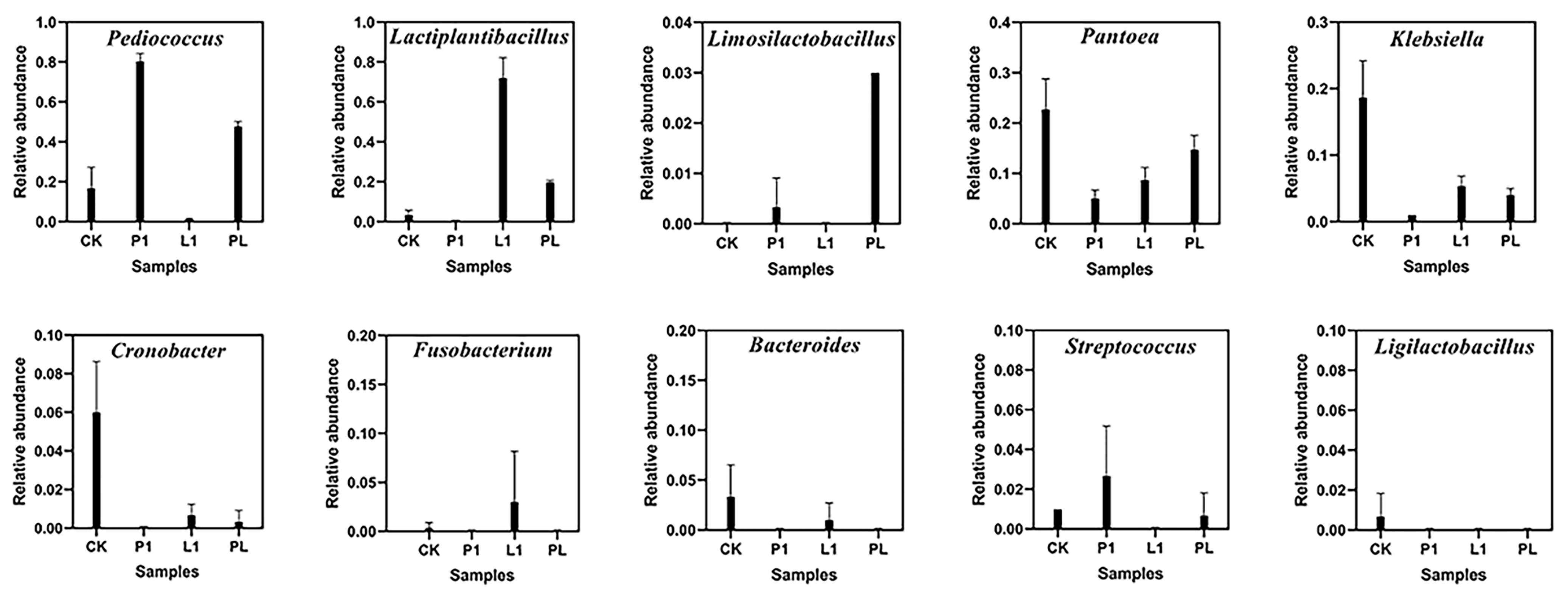
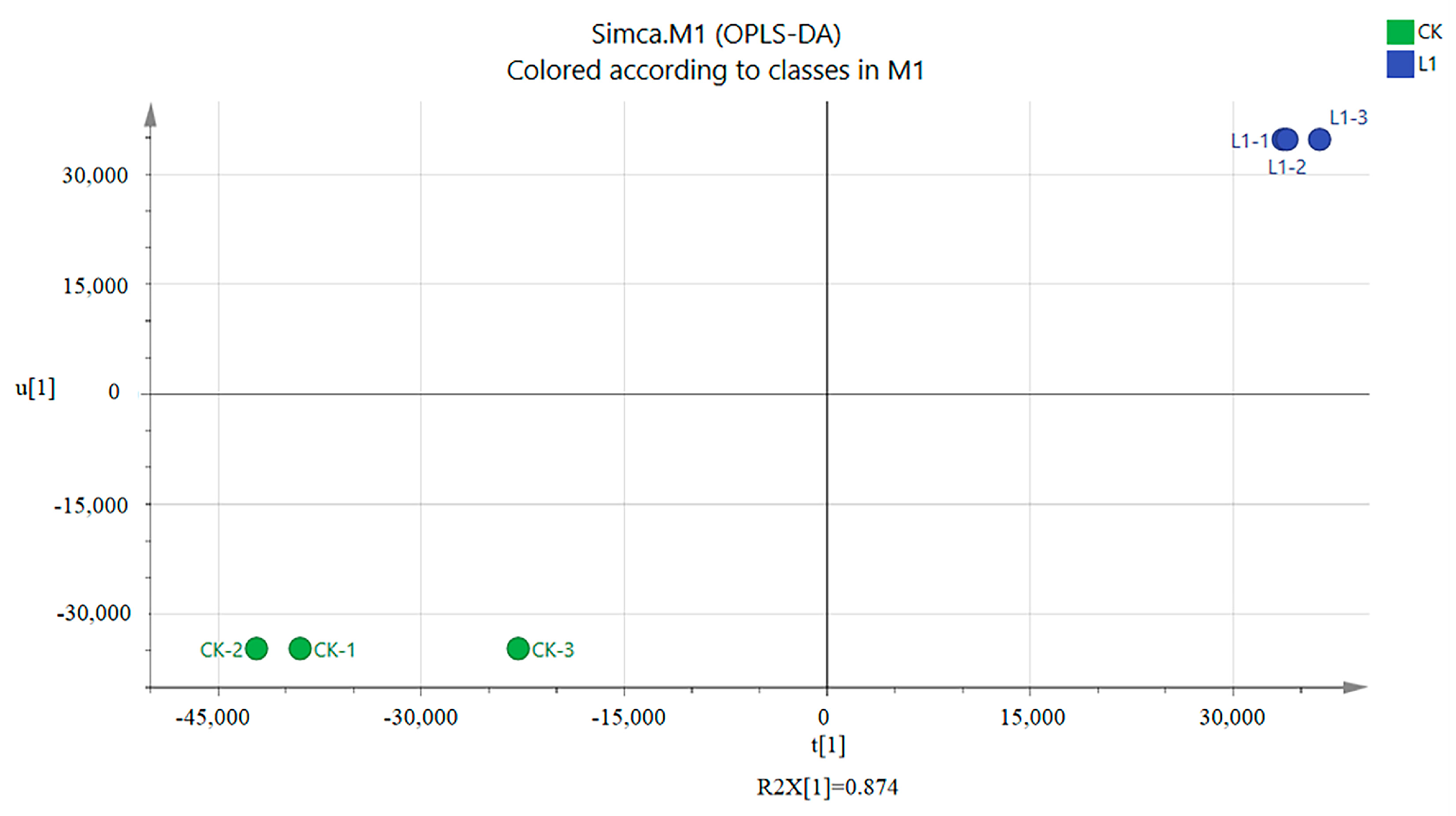
3.5. Abundance and Diversity of Members of the Bacterial Communities
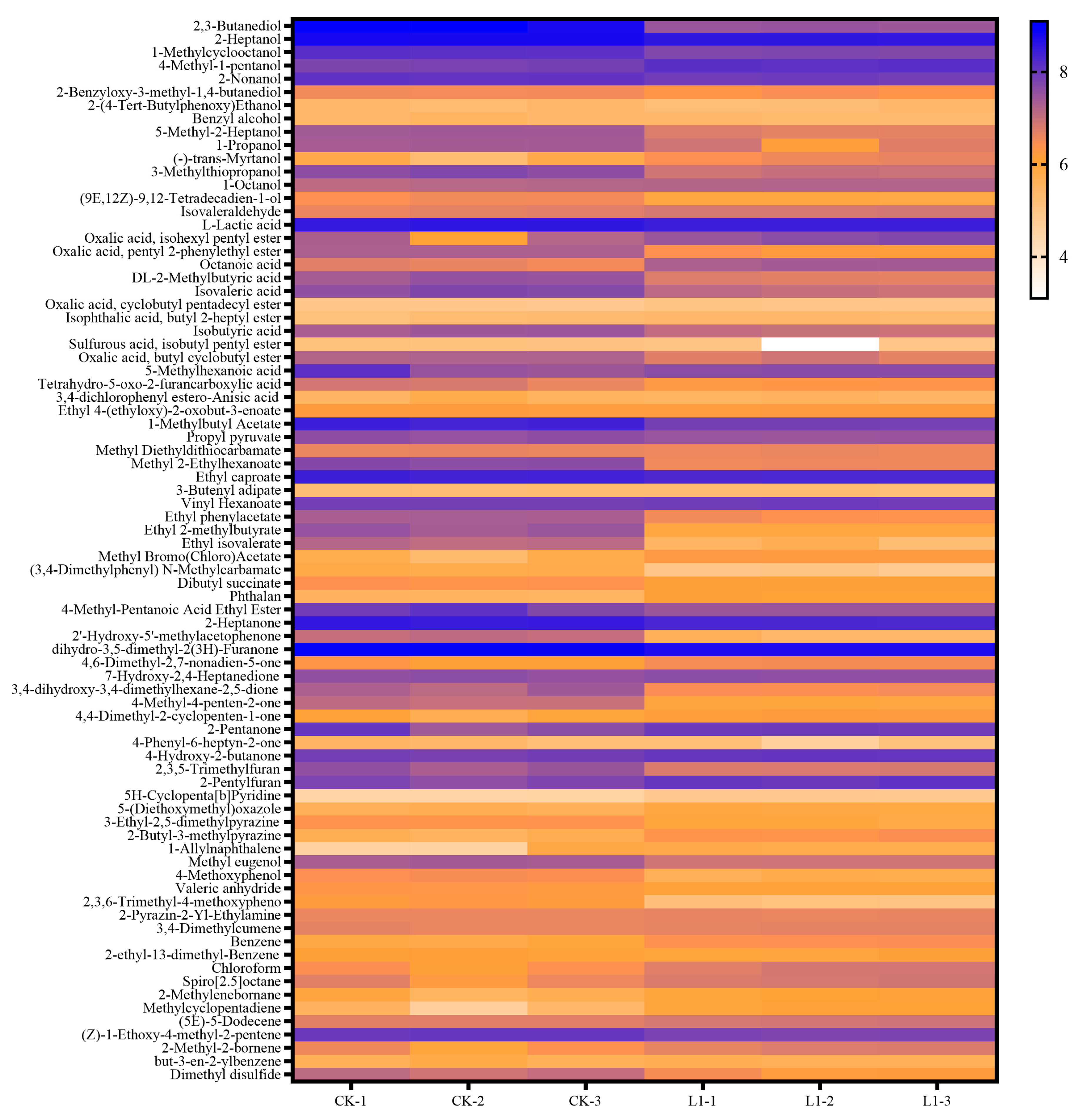
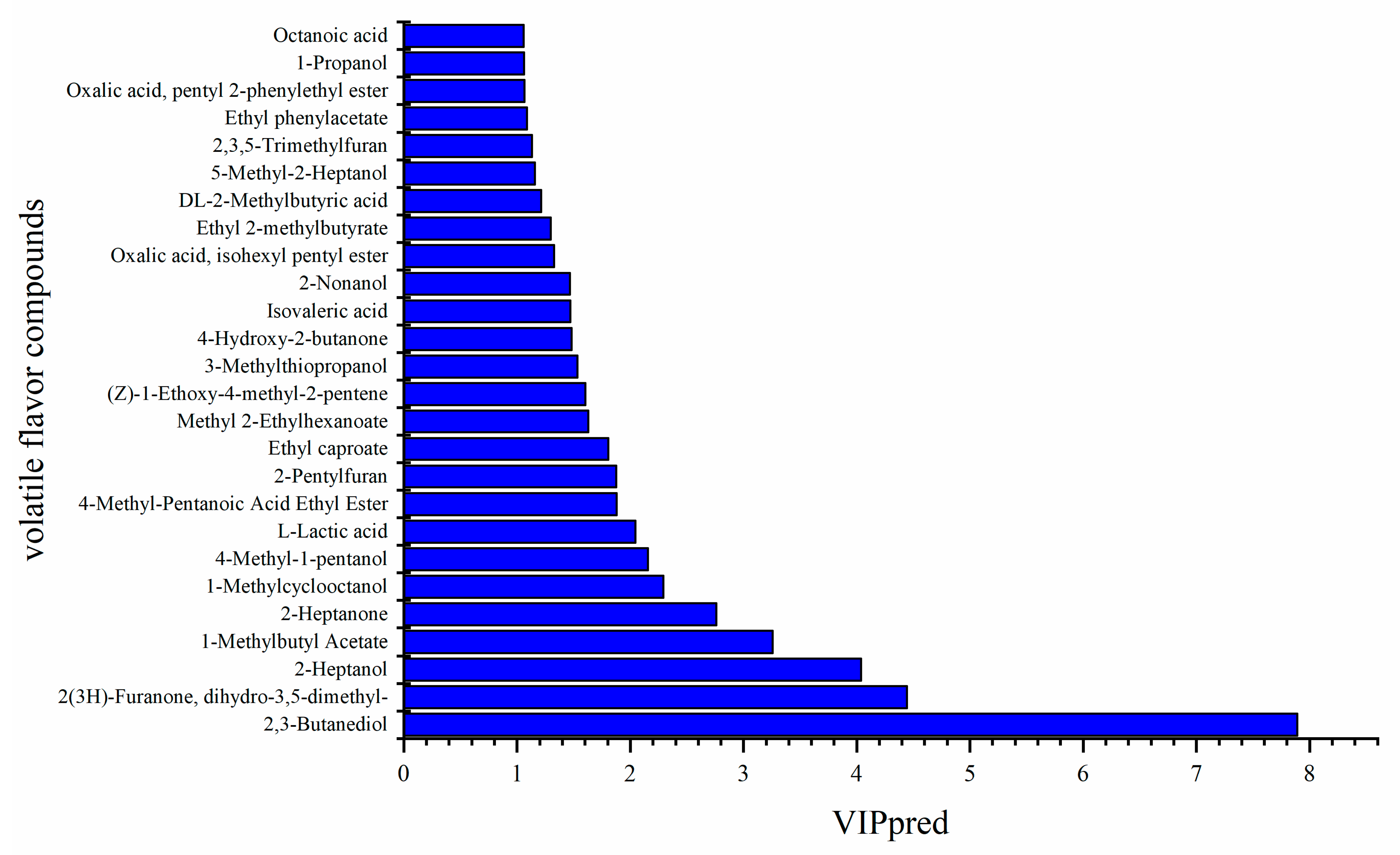
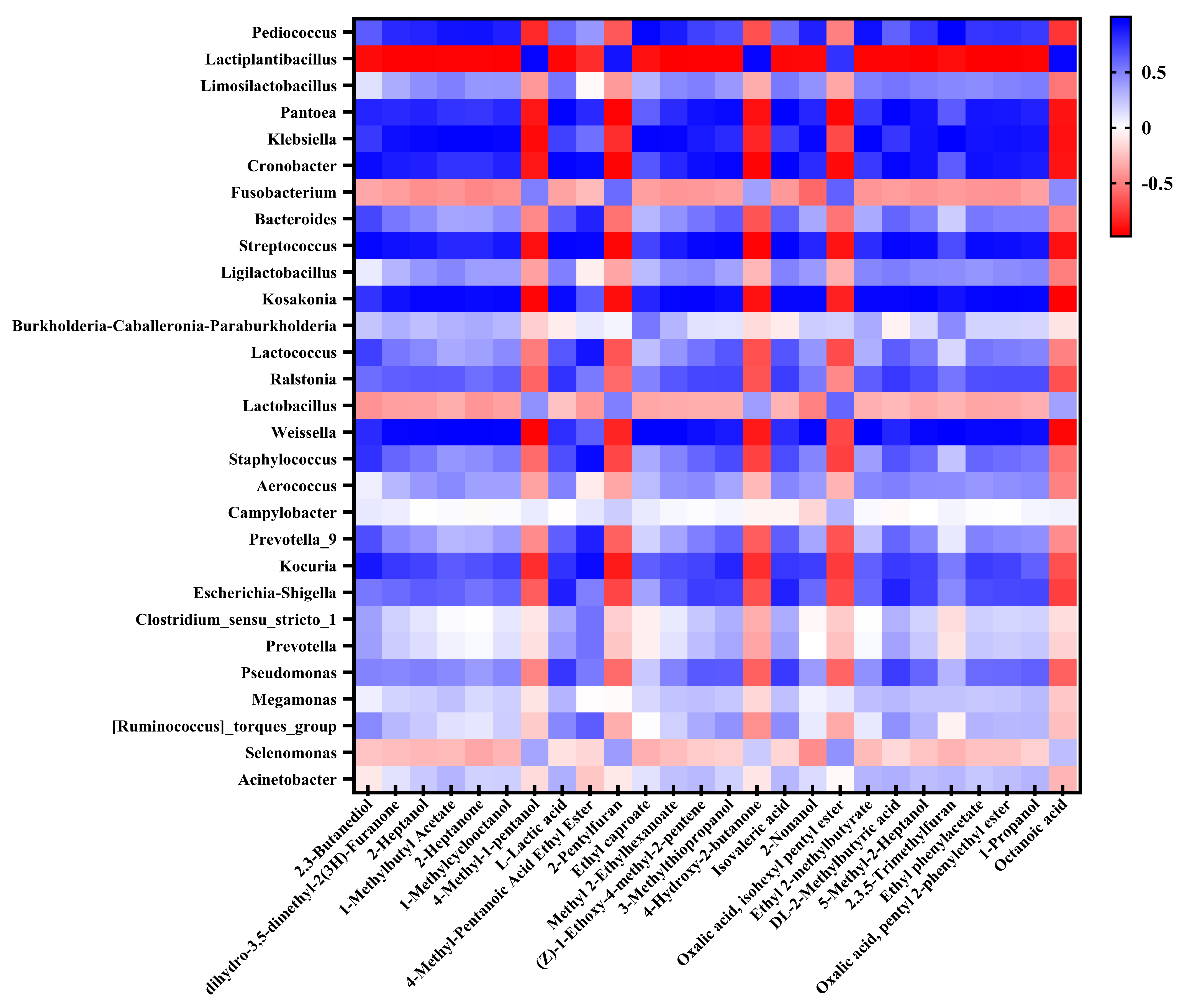
3.6. Analysis of Flavor Compounds and Their Correlation with Bacterial Communities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bao, R.; Liu, S.; Ji, C.; Liang, H.; Yang, S.; Yan, X.; Zhou, Y.; Lin, X.; Zhu, B. Shortening Fermentation Period and Quality Improvement of Fermented Fish, Chouguiyu, by Co-inoculation of Lactococcus lactis M10 and Weissella cibaria M3. Front. Microbiol. 2018, 9, 3003. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wu, R.; Wei, X.; Zhang, Z.; Wang, W.; Liu, A.; Yang, J.; Ji, C.; Liang, H.; Zhang, S.; et al. Moderate fermentation contributes to the formation of typical aroma and good organoleptic properties: A study based on different brands of Chouguiyu. LWT 2021, 152, 112325. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, W.; Zhu, Q. Effect of starter cultures on the quality of Suan yu, a Chinese traditional fermented freshwater fish. Int. J. Food Sci.Technol. 2016, 51, 1774–1786. [Google Scholar] [CrossRef]
- Han, J.; Zhang, J.; Lin, X.; Liang, H.; Li, S.; Yu, C.; Zhu, B.; Ji, C. Effect of autochthonous lactic acid bacteria on fermented Yucha quality. LWT 2020, 123, 109060. [Google Scholar] [CrossRef]
- An, Y.; Cai, X.; Cong, L.i.n.; Hu, Y.; Liu, R.; Xiong, S.; Hu, X. Quality Improvement of Zhayu, a Fermented Fish Product in China: Effects of Inoculated Fermentation with Three Kinds of Lactic Acid Bacteria. Foods 2022, 11, 2756. [Google Scholar] [CrossRef]
- Yun, J.; Zhao, F.; Zhang, W.; Yan, H.; Zhao, F.; Ai, D. Monitoring the microbial community succession and diversity of Liangzhou fumigated vinegar during solid-state fermentation with next-generation sequencing. Ann.Microbiol. 2019, 69, 279–289. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, T.; Huang, C.; Hardie, J.; Peng, Z.; Xie, M.; Xiong, T. The microbial communities and flavour compounds of Jiangxi yancai, Sichuan paocai and Dongbei suancai: Three major types of traditional Chinese fermented vegetables. LWT 2020, 121, 108865. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, C.; Bao, R.; Liu, M.; Lv, J.; Yang, Z.; Xu, W.; Liang, H.; Ji, C.; Li, S.; et al. Effects of flavourzyme addition on physicochemical properties, volatile compound components and microbial community succession of Suanzhayu. Int. J. Food Microbiol. 2020, 334, 108839. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Li, C.; Li, L.; Yang, X.; Wu, Y.; Chen, S.; Zhao, Y. Novel insight into physicochemical and flavor formation in naturally fermented tilapia sausage based on microbial metabolic network. Food Res. Int. 2021, 141, 110122. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, X.; Zhang, W. Characterization of the microbial flora from Suan Yu, a Chinese traditional low-salt fermented fish. J. Food Process. Preserv. 2015, 40, 1093–1103. [Google Scholar] [CrossRef]
- Gao, P.; Wang, W.; Jiang, Q.; Xu, Y.; Xia, W. Effect of autochthonous starter cultures on the volatile flavour compounds of Chinese traditional fermented fish (Suan yu). Int. J. Food Sci. Technol. 2016, 51, 1630–1637. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, S.; Lv, J.; Sun, Z.; Xu, W.; Ji, C.; Liang, H.; Li, S.; Yu, C.; Lin, X. Microbial succession and the changes of flavor and aroma in Chouguiyu, a traditional Chinese fermented fish. Food Biosci. 2020, 37, 107725. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Z.; Sun, L.; Dong, L.; Wang, Z.; Du, M. Dynamics of Microbial Communities, Texture and Flavor in Suan zuo yu during Fermentation. Food Chem. 2020, 332, 127364. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chen, H.; Wang, X.; Lin, X.; Ji, C.; Li, S.; Liang, H. Effects of different temperatures on bacterial diversity and volatile flavor compounds during the fermentation of suancai, a traditional fermented vegetable food from northeastern China. LWT 2020, 118, 108773. [Google Scholar] [CrossRef]
- Jusino, M.A.; Banik, M.T.; Palmer, J.M.; Wray, A.K.; Xiao, L.; Pelton, E.; Barber, J.R.; Kawahara, A.Y.; Gratton, C.; Zachariah, P.M.; et al. An improved method for utilizing high-throughput amplicon sequencing to determine the diets of insectivorous animals. Mol. Ecol. Resour. 2018, 19, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Osimani, A.; Ferrocino, I.; Agnolucci, M.; Cocolin, L.; Giovannetti, M.; Cristani, C.; Palla, M.; Milanović, V.; Roncolini, A.; Sabbatini, R.; et al. Unveiling hákarl: A study of the microbiota of the traditional Icelandic fermented fish. Food Microbiol. 2019, 82, 560–572. [Google Scholar] [CrossRef]
- Ming, T.; Han, J.; Li, Y.; Lu, C.; Qiu, D.; Li, Y.; Zhou, J.; Su, X. A metabolomics and proteomics study of the Lactobacillus plantarum in the grass carp fermentation. BMC Microbiol. 2018, 18, 216. [Google Scholar] [CrossRef]
- Zang, J.; Xu, Y.; Xia, W.; Regenstein, J.M.; Yu, D.; Yang, F.; Jiang, Q. Correlations between microbiota succession and flavor formation during fermentation of Chinese low-salt fermented common carp (Cyprinus carpio L.) inoculated with mixed starter cultures. Food Microbiol. 2020, 90, 103487. [Google Scholar] [CrossRef]
- Zeng, X.; Xia, W.; Jiang, Q.; Xu, Y.; Fan, J. Contribution of Mixed Starter Cultures to Flavor Profile of Suanyu—A Traditional Chinese Low−Salt Fermented Whole Fish. J. Food Process. Preserv. 2017, 41, e13131. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Li, L.; Yang, X.; Wu, Y.; Zhao, Y.; Wei, Y. Effect of Bacterial Community and Free Amino Acids on the Content of Biogenic Amines During Fermentation of Yu-lu, a Chinese Fermented Fish Sauce. J. Aquat. Food Prod. Technol. 2018, 27, 496–507. [Google Scholar] [CrossRef]
- GB 5009.237-2016; Determination of pH in food safety national standard. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016. (In Chinese)
- Phetsang, H.; Panpipat, W.; Undeland, I.; Panya, A.; Phonsatta, N.; Chaijan, M. Comparative quality and volatilomic characterisation of unwashed mince, surimi, and pH-shift-processed protein isolates from farm-raised hybrid catfish (Clarias macrocephalus × Clarias gariepinus). Food Chem. 2021, 364, 130365. [Google Scholar] [CrossRef]
- Li, S.; Luo, J.; Zhou, X.; Li, X.; Wang, F.; Liu, Y. Identification of characteristic proteins of wheat varieties used to commercially produce dried noodles by electrophoresis and proteomics analysis. J. Food Compos. Anal. 2020, 96, 103685. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Biomembr. Part C Biol. Oxid. 1978, 52, 302–310. [Google Scholar]
- Qin, N.; Zhang, L.; Zhang, J.; Song, S.; Wang, Z.; Regenstein, J.M.; Luo, Y. Influence of lightly salting and sugaring on the quality and water distribution of grass carp (Ctenopharyngodon idellus) during super-chilled storage. J. Food Eng. 2017, 215, 104–112. [Google Scholar] [CrossRef]
- Nie, X.; Lin, S.; Zhang, Q. Proteolytic Characterisation in Grass Carp Sausage Inoculated with Lactobacillus plantarum and Pediococcus pentosaceus. Food Chem. 2014, 145, 840–844. [Google Scholar] [CrossRef]
- Zeng, X.; Xia, W.; Jiang, Q.; Yang, F. Effect of autochthonous starter cultures on microbiological and physico-chemical characteristics of Suan yu, a traditional Chinese low salt fermented fish. Food Control. 2013, 33, 344–351. [Google Scholar] [CrossRef]
- Wang, W.; Xia, W.; Gao, P.; Xu, Y.; Jiang, Q. Proteolysis during fermentation of Suanyu as a traditional fermented fish product of China. Int. J. Food Prop. 2017, 20 (Suppl. S1), S166–S176. [Google Scholar] [CrossRef]
- Kopermsub, P.; Yunchalard, S. Identification of lactic acid bacteria associated with the production of plaa-som, a traditional fermented fish product of Thailand. Int. J. Food Microbiol. 2010, 138, 200–204. [Google Scholar] [CrossRef]
- Xu, Y.; Xia, W.; Yang, F.; Nie, X. Physical and chemical changes of silver carp sausages during fermentation with Pediococcus pentosaceus. Food Chem. 2010, 122, 633–637. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Ren, H.; Zhan, Y. Comparison of bacterial diversity profiles and microbial safety assessment of salami, Chinese dry-cured sausage and Chinese smoked-cured sausage by high-throughput sequencing. LWT 2018, 90, 108–115. [Google Scholar] [CrossRef]
- Sikorski, Z.E.; Kolakowska, A.; Burt, J.R. Post harvest biochemical and microbial changes seafood. In Resources, Nutritional Composition and Preservation; Sikorski, Z.E., Ed.; CRC Press: Boca Raton, FL, USA, 1990; pp. 55–75. [Google Scholar]
- Zang, j.; Xu, Y.; Xia, W.; Yu, D.; Gao, P.; Jiang, Q.; Yang, F. Dynamics and diversity of microbial community succession during fermentation of Suan yu, a Chinese traditional fermented fish, determined by high throughput sequencing. Food Res. Int. 2018, 111, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, L.; Regenstein, J.M.; Gao, P.; Zang, J.; Xia, W.; Jiang, Q. The contribution of autochthonous microflora on free fatty acids release and flavor development in low-salt fermented fish. Food Chem. 2018, 256, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, L.; Chen, H.; Zhang, W.; He, L.; Zeng, X. Effects of autochthonous starter cultures on bacterial communities and metabolites during fermentation of Yu jiangsuan, a Chinese traditional fermented condiment. LWT 2022, 168, 113874. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Chen, C.; Xie, T.; Li, P. Effect of Lactobacillus plantarum and Staphylococcus xylosus on flavour development and bacterial communities in Chinese dry fermented sausages. Food Res. Int. 2020, 135, 109247. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Li, C.; Li, S.; Liang, H.; Ji, C.; Zhu, B.; Lin, X. Effects of temperature on microbial succession and quality of sour meat during fermentation. LWT 2019, 114, 108391. [Google Scholar] [CrossRef]
- Ji, X.; Zhu, J.; Gao, Z.; Ren, L.; Huang, H. Research progress on the production of 2,3-butanediol by microbial fermentation. Mod. Chem. 2006, 8, 23–27. [Google Scholar]
- Maione, A.; La Pietra, A.; de Alteriis, E.; Mileo, A.; De Falco, M.; Guida, M.; Galdiero, E. Effect of Myrtenol and Its Synergistic Interactions with Antimicrobial Drugs in the Inhibition of Single and Mixed Biofilms of Candida auris and Klebsiella pneumoniae. Microorganisms 2022, 10, 1773. [Google Scholar] [CrossRef]
- Yang, S.; Duan, J.; Lv, S.; Xu, L.; Li, H. Revealing the Changes in Compounds When Producing Strong-Flavor Daqu by Statistical and Instrumental Analysis. Fermentation 2022, 8, 720. [Google Scholar] [CrossRef]
- Beare-Rogers, J.L.; Dieffenbacher, A.; Holm, J.V. Lexicon of lipid nutrition (IUPAC Technical Report). Pure Appl. Chem. 2013, 73, 685–744. [Google Scholar] [CrossRef]
- Menezes, A.G.T.; Batista, N.N.; Ramos, C.L.; e Silva, A.R.D.A.; Efraim, P.; Pinheiro, A.C.M.; Schwan, R.F. Investigation of chocolate produced from four different Brazilian varieties of cocoa (Theobroma cacao L.) inoculated with Saccharomyces cerevisiae. Food Res. Int. 2016, 81, 83–90. [Google Scholar] [CrossRef]
- Viesser, J.A.; de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Vandenberghe, L.P.D.S.; Azevedo, V.; Brenig, B.; Rogez, H.; Góes-Neto, A.; Soccol, C.R. Exploring the contribution of fructophilic lactic acid bacteria to cocoa beans fermentation: Isolation, selection and evaluation. Food Res. Int. 2020, 136, 109478. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, Y.; Wu, Y.; Li, C.; Li, L.; Zhao, Y.; Hu, X.; Wei, Y.; Huang, H. Comparison of the microbial community and flavor compounds in fermented mandarin fish (Siniperca chuatsi): Three typical types of Chinese fermented mandarin fish products. Food Res. Int. 2021, 144, 110365. [Google Scholar] [CrossRef]
- Sidira, M.; Kandylis, P.; Kanellaki, M.; Kourkoutas, Y. Effect of curing salts and probiotic cultures on the evolution of flavor compounds in dry-fermented sausages during ripening. Food Chem. 2016, 201, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, P.; Xia, W.; Jiang, Q.; Liu, S.; Xu, Y. Characterization of key aroma compounds in low-salt fermented sour fish by gas chromatography-mass spectrometry, odor activity values, aroma recombination and omission experiments. Food Chem. 2022, 397, 133773. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Wu, Y.; Li, C.; Li, L.; Yang, X.; Chen, S.; Zhao, Y.; Cen, J.; Yang, S.; et al. Investigation of fermentation-induced changes in the volatile compounds of Trachinotus ovatus (meixiangyu) based on molecular sensory and interpretable machine-learning techniques: Comparison of different fermentation stages. Food Res. Int. 2021, 150, 110739. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.-R.; Liu, R.-S.; He, L.; Li, H.-M.; Tang, Y.-L.; Liang, X.-H.; Chen, T.; Tang, Y.-J. Aroma improvement by repeated freeze-thaw treatment during Tuber melanosporum fermentation. Sci. Rep. 2015, 5, 17120. [Google Scholar] [CrossRef]
- Müller, R.; Rappert, S. Pyrazines: Occurrence, formation and biodegradation. Appl. Microbiol. Biotechnol. 2010, 85, 1315–1320. [Google Scholar] [CrossRef]
- Jeong, D.-W.; Heo, S.; Lee, B.; Lee, H.; Jeong, K.; Her, J.-Y.; Lee, K.-G.; Lee, J.-H. Effects of the predominant bacteria from meju and doenjang on the production of volatile compounds during soybean fermentation. Int. J. Food Microbiol. 2017, 262, 8–13. [Google Scholar] [CrossRef]


| Observed_Species | Shannon | Simpson | Chao1 | ACE | PD_Whole_Tree | OTU | Goods_Coverage | |
| CK | 1065 | 4.347 | 0.878 | 1278.517 | 1312.548 | 509.538 | 2690 | 0.994 |
| P1 | 657 | 1.859 | 0.375 | 786.27 | 858.857 | 232.568 | 1725 | 0.996 |
| L1 | 628 | 1.959 | 0.456 | 780.442 | 888.153 | 236.48 | 1667 | 0.995 |
| PL | 181 | 2.953 | 0.784 | 196.266 | 204.191 | 39.671 | 313 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Liu, Y.; Li, X.; Wang, F.; Huang, Y.; Ma, X. Application and Effect of Pediococcus pentosaceus and Lactiplantibacillus plantarum as Starter Cultures on Bacterial Communities and Volatile Flavor Compounds of Zhayu, a Chinese Traditional Fermented Fish Product. Foods 2023, 12, 1768. https://doi.org/10.3390/foods12091768
Xu D, Liu Y, Li X, Wang F, Huang Y, Ma X. Application and Effect of Pediococcus pentosaceus and Lactiplantibacillus plantarum as Starter Cultures on Bacterial Communities and Volatile Flavor Compounds of Zhayu, a Chinese Traditional Fermented Fish Product. Foods. 2023; 12(9):1768. https://doi.org/10.3390/foods12091768
Chicago/Turabian StyleXu, Dongmei, Yongle Liu, Xianghong Li, Faxiang Wang, Yiqun Huang, and Xiayin Ma. 2023. "Application and Effect of Pediococcus pentosaceus and Lactiplantibacillus plantarum as Starter Cultures on Bacterial Communities and Volatile Flavor Compounds of Zhayu, a Chinese Traditional Fermented Fish Product" Foods 12, no. 9: 1768. https://doi.org/10.3390/foods12091768
APA StyleXu, D., Liu, Y., Li, X., Wang, F., Huang, Y., & Ma, X. (2023). Application and Effect of Pediococcus pentosaceus and Lactiplantibacillus plantarum as Starter Cultures on Bacterial Communities and Volatile Flavor Compounds of Zhayu, a Chinese Traditional Fermented Fish Product. Foods, 12(9), 1768. https://doi.org/10.3390/foods12091768






