Effect of Microwave Vacuum Freeze-Drying Power on Emulsifying and Structure Properties of Egg White Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Microwave Vacuum Freeze-Dried (100~500 W) EWP Powder
2.3. Preparation of EWP Emulsions
2.4. Determination of Emulsification Activity Index (EAI) and Emulsion Stability Index (ESI)
2.5. Zeta Potential Determination
2.6. Particle Size Determination
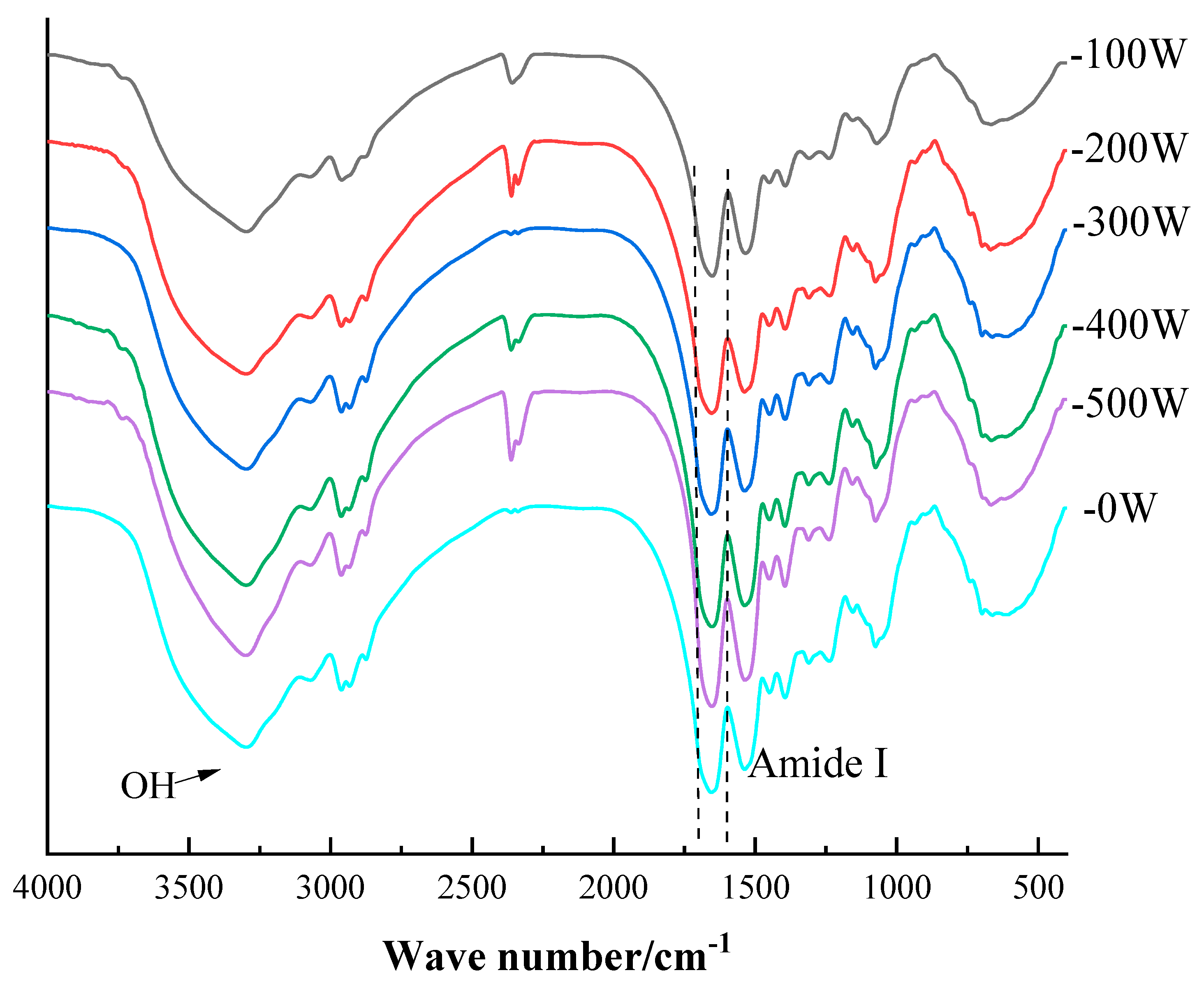
2.7. Fourier Infrared Spectroscopy (FT-IR)
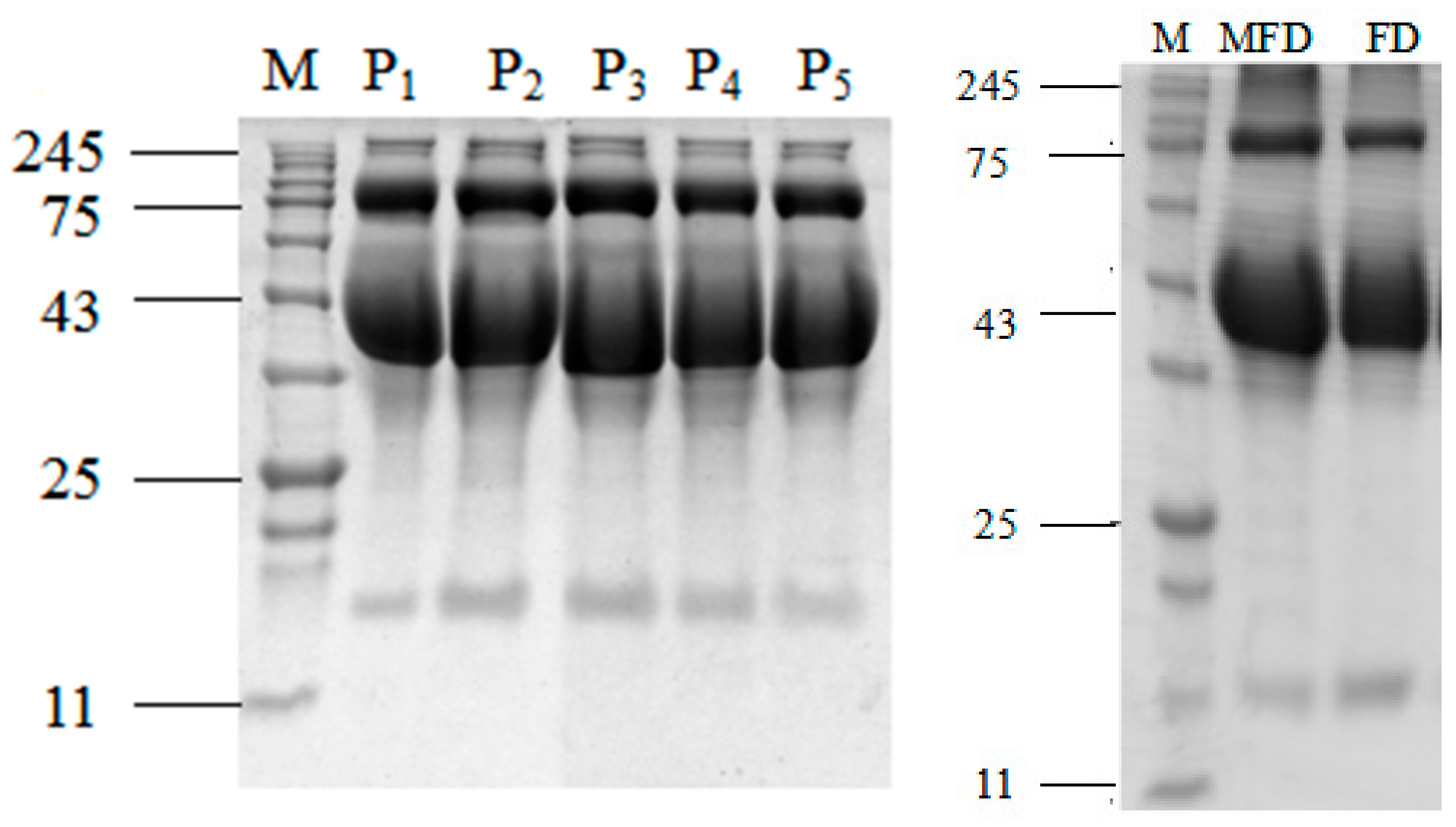
2.8. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
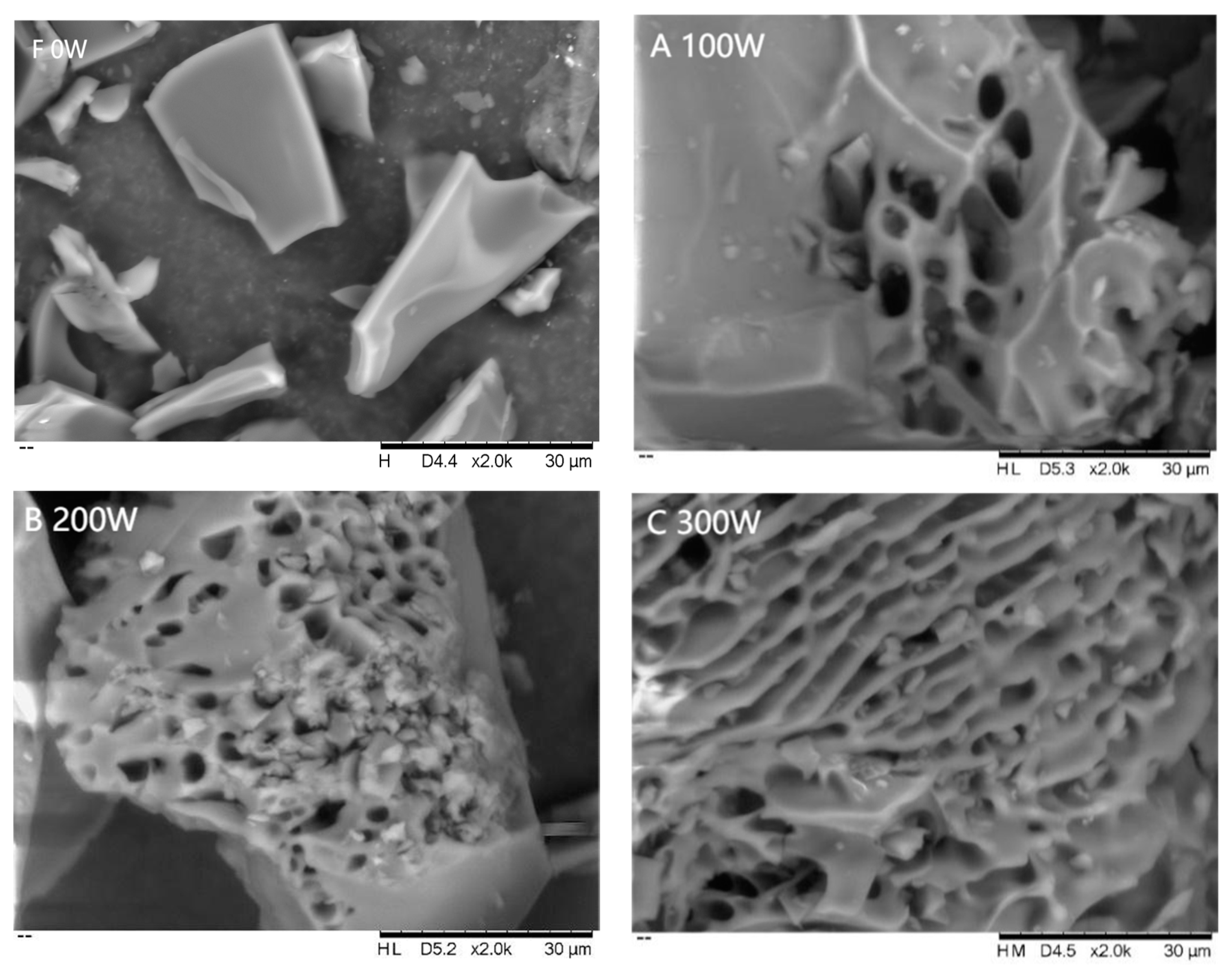
2.9. Scanning Electron Microscopy (SEM) Analysis
2.10. Endogenous Fluorescence Spectroscopy Analysis
2.11. Differential Scanning Calorimetry (DSC) Analysis
2.12. Texture Profile Analysis (TPA) Analysis
2.13. Statistical Analysis
3. Results
3.1. EWP Emulsification Properties Analysis
3.2. Structural Properties of EWP
3.2.1. FT-IR Spectroscopic Analysis
3.2.2. SDS-PAGE Analysis
3.2.3. SEM Analysis
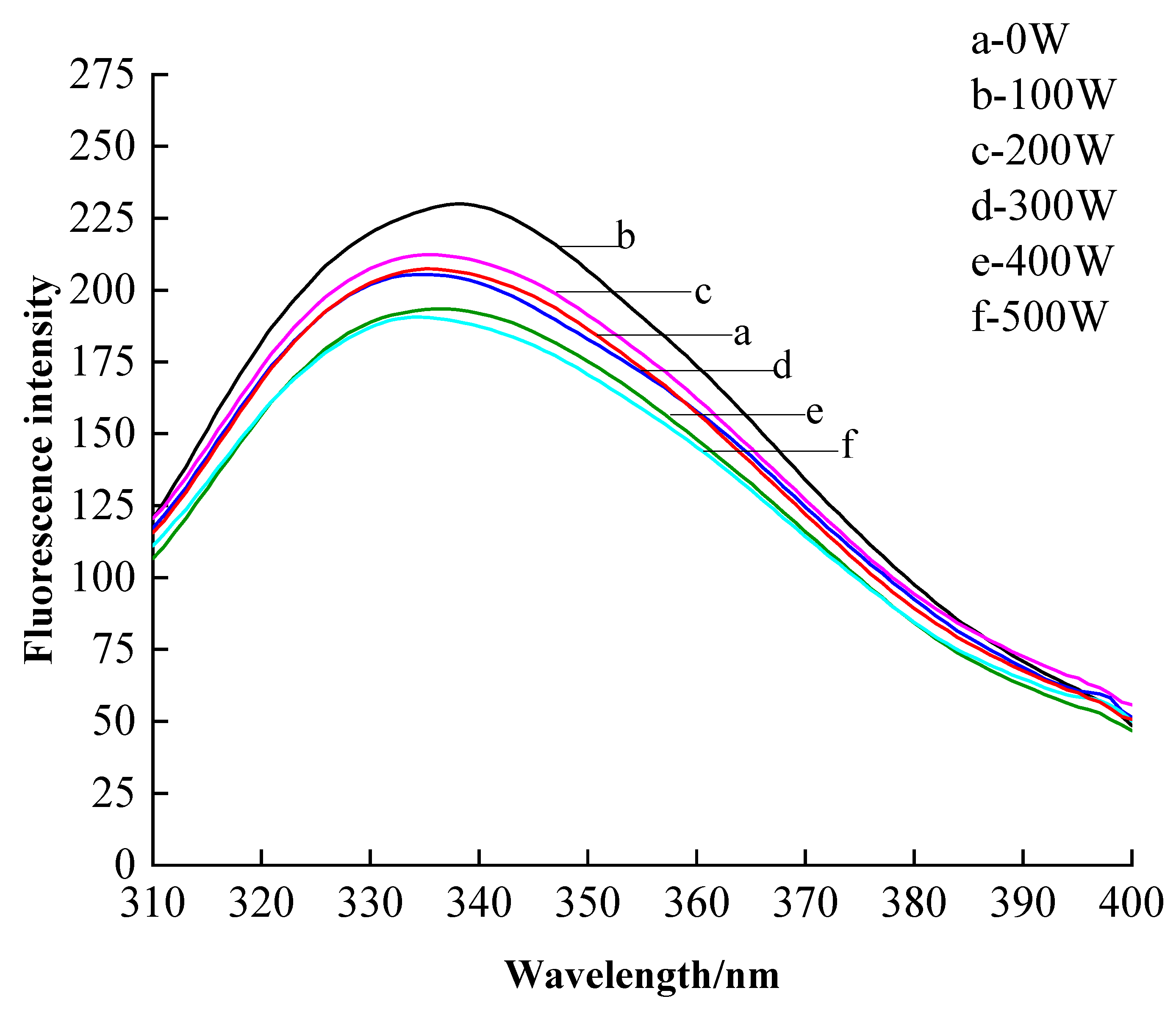
3.2.4. Endogenous Fluorescence Spectroscopy Analysis
3.2.5. DSC Analysis
3.2.6. TPA Analysis
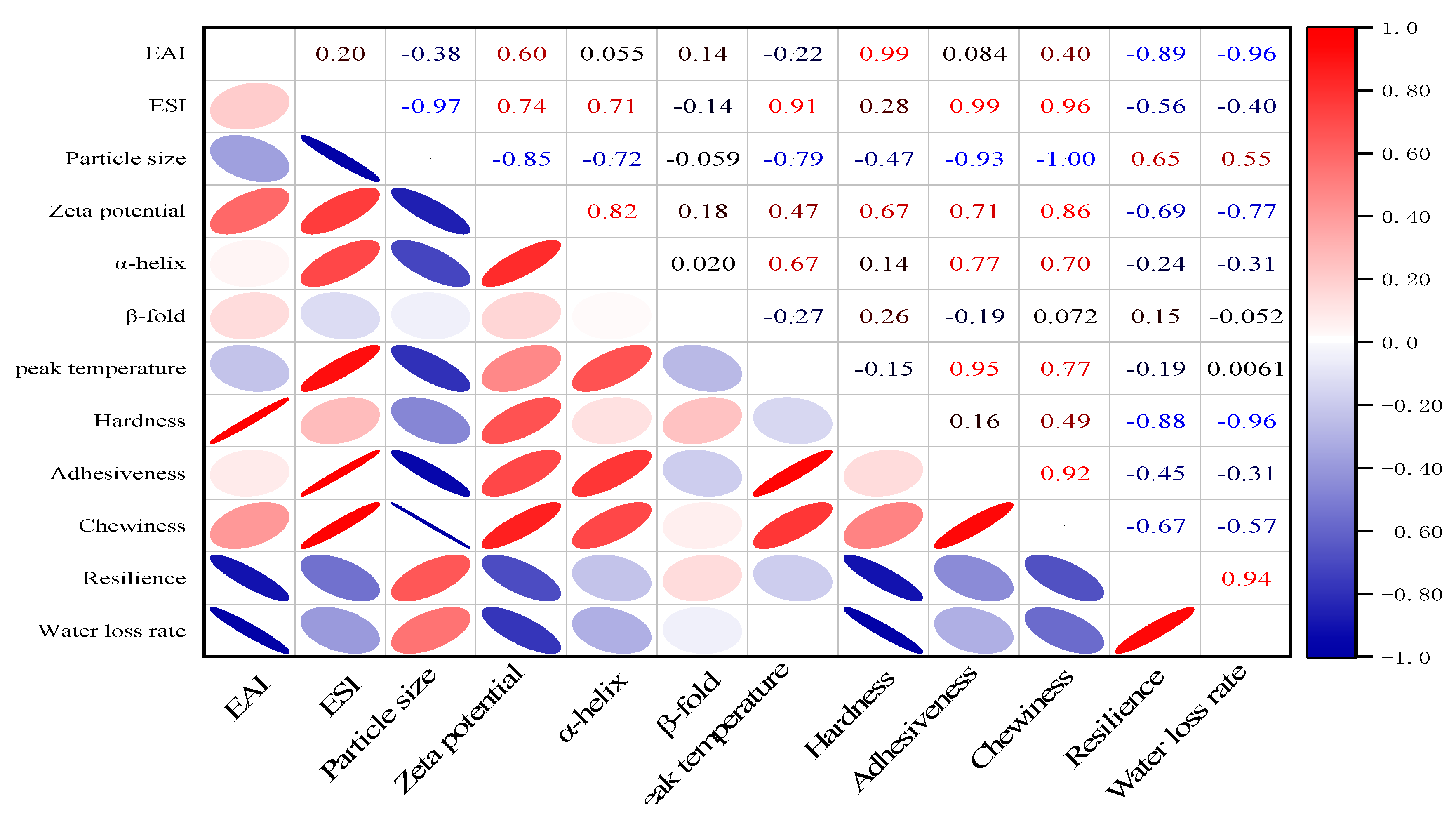
3.2.7. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xue, H.; Liu, H.; Zhang, G.; Tu, Y.; Zhao, Y. Formation mechanism of salted egg yolk mudding during storage: Protein oxidation, gel structure, and conformation. Food Chem. 2023, 413, 135632. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, D.; Li, X.; Xu, B.; Zhang, X.; Liu, J. Effects of inulin on the structure and emulsifying properties of protein components in dough. Food Chem. 2016, 210, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.J.; Zhang, T.B.; Hui, M.; Yin, H.-C.; Qiu, A.-Y.; Liu, X.-Y. Mechanism of microwave-accelerated soy protein isolate–saccharide graft reactions. Food Res. Int. 2011, 44, 2647–2654. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Su, Y.; Gu, L.; Yang, Y.; Chang, C. Composite emulsifying behavior of egg white protein and rhamnolipid: Properties of the constructed high internal phase emulsions. Food Hydrocoll. 2022, 123, 106913. [Google Scholar] [CrossRef]
- Sun, J.; Liu, T.; Zhang, F.; Huang, Y.; Zhang, Y.; Xu, B. Tea polyphenols on emulsifying and antioxidant properties of egg white protein at acidic and neutral pH conditions. LWT 2022, 153, 112537. [Google Scholar] [CrossRef]
- Yu, Y.; Guan, Y.; Liu, J.; Hedi, W.; Yu, Y.; Zhang, T. Molecular structural modification of egg white protein by pH-shifting for improving emulsifying capacity and stability. Food Hydrocoll. 2021, 121, 107071. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Li, X.; Chang, C.; Zhang, M.; Gu, L.; Su, Y.; Yang, Y. Emulsifying properties of glycation or glycation-heat modified egg white protein. Food Res. Int. 2019, 119, 227–235. [Google Scholar] [CrossRef]
- Duan, L.L.; Duan, X.; Ren, G.Y. Evolution of pore structure during microwave freeze-drying of Chinese yam. Int. J. Agric. Biol. Eng. 2018, 11, 208–212. [Google Scholar] [CrossRef]
- Liu, L.; Shi, S.; Zhang, Y.; Li, Y.; Guo, J.; Zhang, M. Effect of microwave freeze drying on moisture migration and gel characteristics of egg white protein. J. Food Process. Preserv. 2022, 46, e16899. [Google Scholar] [CrossRef]
- Le, X.-N.; Hu, S.-C.; Zheng, J.-L.; Cui, E.L.; Zhu, Y.-H.; Zhu, M.-Q. The influence of different drying methods on bioactive components of Eucommia ulmoides Oliver male flower and the comprehensive assessment for industrial application. Ind. Crops Prod. 2022, 177, 114469. [Google Scholar] [CrossRef]
- Sun, X.; Ohanenye, I.C.; Ahmed, T.; Udenigwe, C.C. Microwave treatment increased protein digestibility of pigeon pea (Cajanus cajan) flour: Elucidation of underlying mechanisms. Food Chem. 2020, 329, 127196. [Google Scholar] [CrossRef]
- Liu, L.; Dai, X.; Kang, H.; Xu, Y.; Hao, W. Structural and functional properties of hydrolyzed/glycosylated ovalbumin under spray drying and microwave freeze drying. Food Sci. Hum. Wellness 2020, 9, 80–87. [Google Scholar] [CrossRef]
- Dong, J.; Yi, J.; Li, X.; Duan, X.; Ren, G.Y.; Li, L.; Wang, J.L.; Han, X.Y.; Gao, Y. Effect of microwave vacuum freeze-drying on the flavor of sauerkraut. Food Ferment. Ind. 2023, 2, 12. [Google Scholar] [CrossRef]
- Ren, G.Y.; Zeng, F.L.; Duan, X.; Liu, L.L.; Duan, B.; Wang, M.M.; Liu, Y.H.; Zhu, W.X. The Effect of Glass Transition Temperature on the Procedure of Microwave-Freeze Drying of Mushrooms (Agaricus bisporus). Dry. Technol. 2015, 33, 169–175. [Google Scholar] [CrossRef]
- Fan, L.; Ding, S.; Liu, Y.; Ai, L. Dehydration of crude protein from Ginkgo biloba L. by microwave freeze drying. Int. J. Biol. Macromol. 2012, 50, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Vanga, S.K.; Wang, J.; Raghavan, V. Effect of ultrasound and microwave processing on the structure, in-vitro digestibility and trypsin inhibitor activity of soymilk proteins. LWT 2020, 131, 109708. [Google Scholar] [CrossRef]
- Cao, H.; Zhu, H.; Wang, Q.; Fan, D.; Huang, J.; Zhao, J.; Jiao, X.; Yan, B.; Zhou, W.; Zhang, H. Intervention on activity and structure of cathepsin L during surimi gel degradation under microwave irradiation. Food Hydrocoll. 2020, 103, 105705. [Google Scholar] [CrossRef]
- Katidi, A.; Xypolitaki, K.; Vlassopoulos, A.; Kapsokefalou, M. Nutritional Quality of Plant-Based Meat and Dairy Imitation Products and Comparison with Animal-Based Counterparts. Nutrients 2023, 15, 401. [Google Scholar] [CrossRef]
- Xu, D.; Liu, L.; Ren, G.Y.; Zhu, W.X. Microwave Freeze Drying of Type I Collagen from Bovine Bone. Dry. Technol. 2013, 31, 1701–1706. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, M.; Yang, X.; Jiang, Y. Improvement on functional properties of wheat gluten by enzymatic hydrolysis and ultrafiltration. J. Cereal Sci. 2006, 44, 93–100. [Google Scholar] [CrossRef]
- Xue, X.; He, H.; Liu, C.; Han, Y.; He, J.; Zhou, J.; Guo, X.; Wang, J.; Cheng, J. Effects of zein modifying polar amino acids as surface stabilizers on the emulsification stability of milk cream diacylglycerol. LWT 2022, 165, 113676. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, T.; Chu, Y.; Cao, Y.; Cheng, S.; Na, R.; Wang, Y. Comparison of the interactions of flupyrimin and nitenpyram with serum albumins via multiple analysis methods. Chemosphere 2022, 289, 133139. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, X.; Zhang, M.; Zhang, M.; Cheng, W.; Xu, B. Effect of Catechin on Yolk Immunoglobulin Structure and Properties: A Polyphenol–Protein Interaction Approach. Foods 2023, 12, 462. [Google Scholar] [CrossRef]
- Han, T.; Li, F.G.; Li, C. Preparation and properties of emulsion gels based on glucomannan and gelatin. J. Food Saf. Qual. Test. 2022, 13, 7524–7529. [Google Scholar]
- Yalcin, E.; Sakiyan, O.; Sumnu, G.; Celik, S.; Koksel, H. Functional properties of microwave-treated wheat gluten. Eur. Food Res. Technol. 2008, 227, 1411–1417. [Google Scholar] [CrossRef]
- Yan, B.; Jiao, X.; Zhu, H.; Wang, Q.; Huang, J.; Zhao, J.; Cao, H.; Zhou, W.; Zhang, W.; Ye, W.; et al. Chemical interactions involved in microwave heat-induced surimi gel fortified with fish oil and its formation mechanism. Food Hydrocoll. 2020, 105, 105779. [Google Scholar] [CrossRef]
- Schmitt, C.; Bovay, C.; VuiIliomenet, A.M.; Rouvet, M.; Bovetto, L.; Barbar, R.; Sanchez, C. Multiscale characterization of individualized β-lactoglobulin microgels formed upon heat treatment under narrow pH range conditions. Langmuir 2009, 25, 7899–7909. [Google Scholar] [CrossRef]
- Matheis, G. Phosphorylation of food proteins with phosphorus oxychloridea improvement of functional and nutritional properties: A review. Food Chem. 1991, 39, 13–26. [Google Scholar] [CrossRef]
- Li, X.; Liu, T.; Song, L.; Zhang, H.; Li, L.; Gao, X. Influence of high-molecular-weight glutenin subunit composition at Glu-A1 and Glu-D1 loci on secondary and micro structures of gluten in wheat (Triticum aestivum L.). Food Chem. 2016, 213, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Singh, H. Aspects of milk-protein-stabilised emulsions. Food Hydrocoll. 2011, 25, 1938–1944. [Google Scholar] [CrossRef]
- Teng, Y.T.; Freire, P.; Zamora, A.; Castillo, M. Tryptophan front-face fluorescence and functional properties of whey: A preliminary study. LWT 2022, 163, 113589. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Jiang, L.; Qi, B.; Zhou, L. Relationship between Secondary Structure and Surface Hydrophobicity of Soybean Protein Isolate Subjected to Heat Treatment. J. Chem. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Bai, X.; Shi, S.; Kong, B.; Chen, Q.; Liu, Q.; Li, Z.; Wu, K.; Xia, X. Analysis of the influencing mechanism of the freeze-thawing cycles on in vitro chicken meat digestion based on protein structural changes. Food Chem. 2022, 399, 134020. [Google Scholar] [CrossRef] [PubMed]
- Gunel, Z.; Torun, M.; Sahin-Nadeem, H. Sugar, D-pinitol, Volatile Composition, and Antioxidant Activity of Carob Powder Roasted By Microwave, Hot Air, and Combined Microwave/hot Air. J. Food Process. Preserv. 2020, 44, e14371. [Google Scholar] [CrossRef]
- Jiang, Z.; Yu, H.; Chen, W.; Gantumur, M.-A.; Bilawala, A.; Hou, J.; Wang, H. Comparisons of characteristics, kinetics and biological activities of glycosylated α-lactalbumin produced by microwave and conventional heating. LWT 2021, 151, 112111. [Google Scholar] [CrossRef]
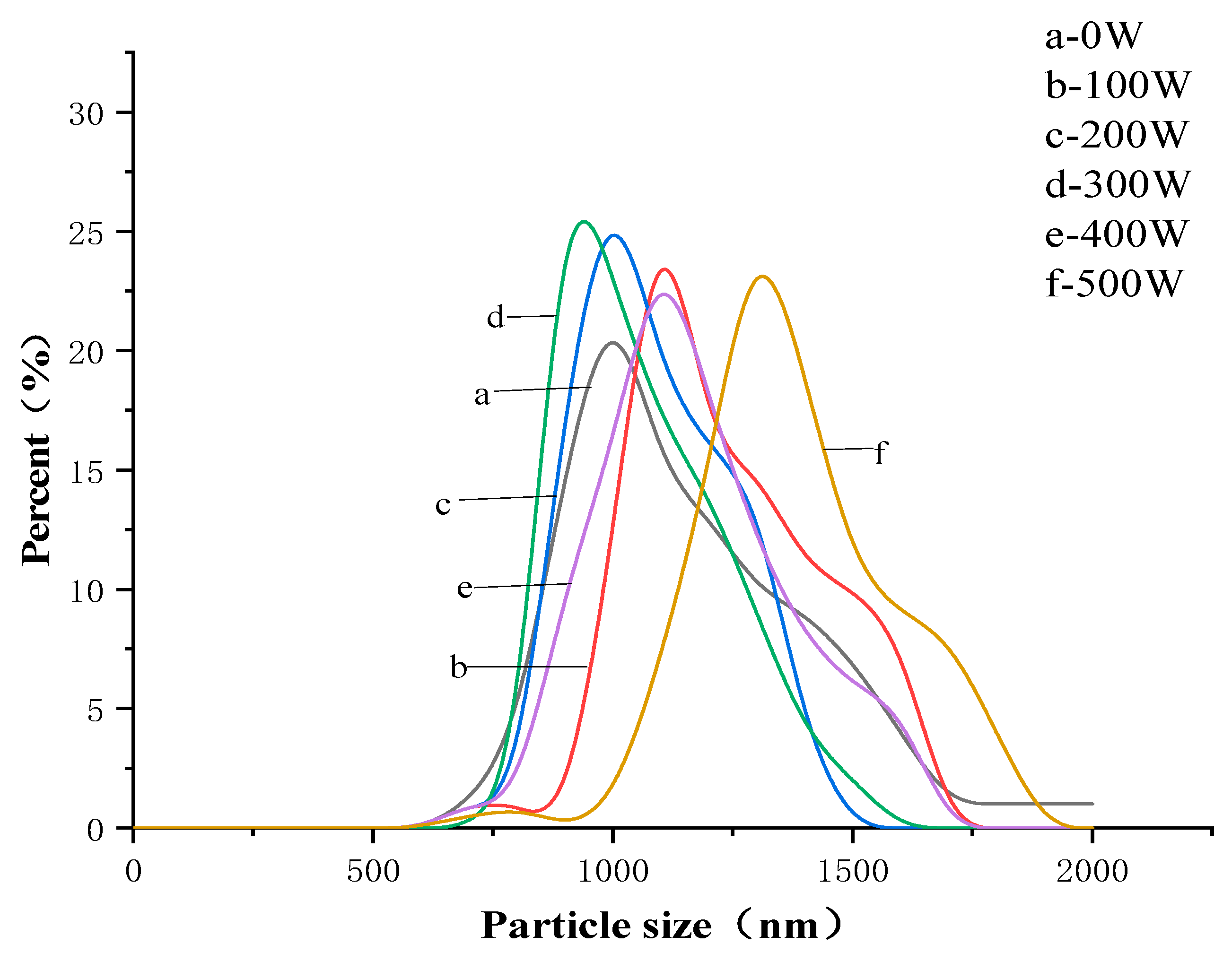




| Microwave Power/W | EAI/m2·g−1 | ESI/min | Particle Size/nm | Absolute Value of the Zeta Potential/mV |
|---|---|---|---|---|
| 0 | 35.27 ± 0.29 e | 26.51 ± 0.84 c | 1507.23 ± 14.88 d | 35.57 ± 0.89 d |
| 100 | 33.40 ± 0.31 f | 26.92 ± 1.14 d | 1619.01 ± 16.52 c | 34.53 ± 1.33 e |
| 200 | 51.19 ± 0.59 c | 29.91 ± 1.13 b | 1494.11 ± 38.65 e | 37.93 ± 0.99 c |
| 300 | 61.35 ± 0.58 a | 33.53 ± 0.62 a | 1203.66 ± 13.66 f | 41.35 ± 1.61 a |
| 400 | 58.88 ± 0.86 b | 23.17 ± 0.95 e | 1668.33 ± 28.82 b | 39.37 ± 1.38 b |
| 500 | 48.17 ± 0.25 d | 20.09 ± 0.88 f | 1923.67 ± 16.49 a | 29.57 ± 0.33 f |
| Microwave Power/W | Secondary Structure Content/% | |||
|---|---|---|---|---|
| α-helix | β-fold | β-rotation angle | Irregularconvolution | |
| 0 | 37.19 ± 0.02 a | 32.71 ± 0.02 f | 15.67 ± 0.04 c | 14.27 ± 0.01 f |
| 100 | 28.28 ± 0.01 d | 40.81 ± 0.04 c | 11.89 ± 0.04 d | 18.10 ± 0.03 c |
| 200 | 29.90 ± 0.04 b | 33.29 ± 0.03 e | 19.44 ± 0.02 b | 17.34 ± 0.02 e |
| 300 | 28.79 ± 0.03 c | 41.58 ± 0.01 b | 25.83 ± 0.03 a | 18.21 ± 0.07 b |
| 400 | 26.75 ± 0.01 e | 42.27 ± 0.05 a | 11.47 ± 0.04 f | 17.51 ± 0.03 d |
| 500 | 16.21 ± 0.02 f | 38.71 ± 0.02 d | 11.52 ± 0.05 e | 19.25 ± 0.01 a |
| Microwave Power/w | Hardness/g | Adhesiveness | Chewiness | Resilience | Water Loss Rate/% |
|---|---|---|---|---|---|
| 0 | 307.28 ± 2.47 c | 0.689 ± 0.02 e | 201.271 ± 2.87 b | 0.076 ± 0.002 b | 34.21 ± 0.07 b |
| 100 | 226.57 ± 4.44 f | 0.733 ± 0.03 bc | 157.334 ± 2.15 d | 0.077 ± 0.001 a | 36.43 ± 0.08 a |
| 200 | 299.77 ± 5.01 d | 0.751 ± 0.02 b | 176.342 ± 5.44 c | 0.067 ± 0.003 e | 31.07 ± 0.10 d |
| 300 | 369.62 ± 4.19 a | 0.764 ± 0.03 a | 220.737 ± 3.31 a | 0.064 ± 0.002 f | 29.14 ± 0.09 f |
| 400 | 353.55 ± 3.92 b | 0.7 ± 0.02 d | 152.583 ± 4.13 e | 0.069 ± 0.001 d | 29.67 ± 0.11 e |
| 500 | 289.46 ± 5.34 e | 0.675 ± 0.01 f | 114.847 ± 2.51 f | 0.072 ± 0.002 c | 33.92 ± 0.07 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, K.; Liu, L.; Pan, X.; Chen, S.; Zhang, X.; Cheng, W.; Xu, B. Effect of Microwave Vacuum Freeze-Drying Power on Emulsifying and Structure Properties of Egg White Protein. Foods 2023, 12, 1792. https://doi.org/10.3390/foods12091792
Su K, Liu L, Pan X, Chen S, Zhang X, Cheng W, Xu B. Effect of Microwave Vacuum Freeze-Drying Power on Emulsifying and Structure Properties of Egg White Protein. Foods. 2023; 12(9):1792. https://doi.org/10.3390/foods12091792
Chicago/Turabian StyleSu, Kenan, Lili Liu, Xingyu Pan, Shuxing Chen, Xiaodan Zhang, Weiwei Cheng, and Baocheng Xu. 2023. "Effect of Microwave Vacuum Freeze-Drying Power on Emulsifying and Structure Properties of Egg White Protein" Foods 12, no. 9: 1792. https://doi.org/10.3390/foods12091792
APA StyleSu, K., Liu, L., Pan, X., Chen, S., Zhang, X., Cheng, W., & Xu, B. (2023). Effect of Microwave Vacuum Freeze-Drying Power on Emulsifying and Structure Properties of Egg White Protein. Foods, 12(9), 1792. https://doi.org/10.3390/foods12091792





