Conversion of Retinyl Palmitate to Retinol by Wheat Bran Endogenous Lipase Reduces Vitamin A Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Toasting of Wheat Bran
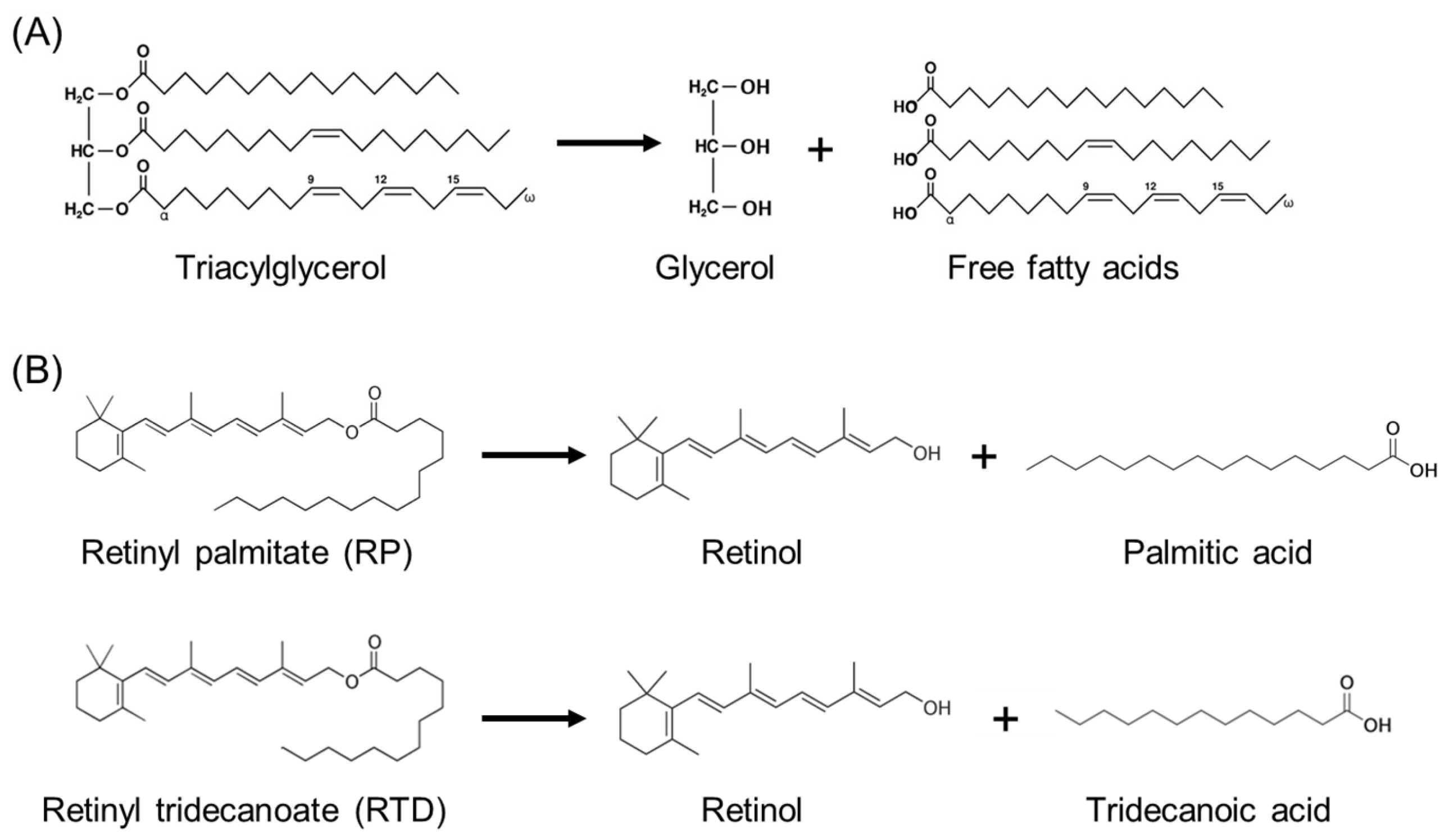
2.3. Synthesis of Retinyl Tridecanoate
2.4. Accelerated Storage Experiments
2.5. Quantification of Retinol (RE) and Retinyl Palmitate (RP)
2.6. Lipid Extraction
2.7. Quantification of Free Fatty Acids and Triacylglycerols
2.8. Primary Oxidation: Peroxide Value
2.9. Determination of the Ratio of Unsaturated over Saturated Fatty Acids
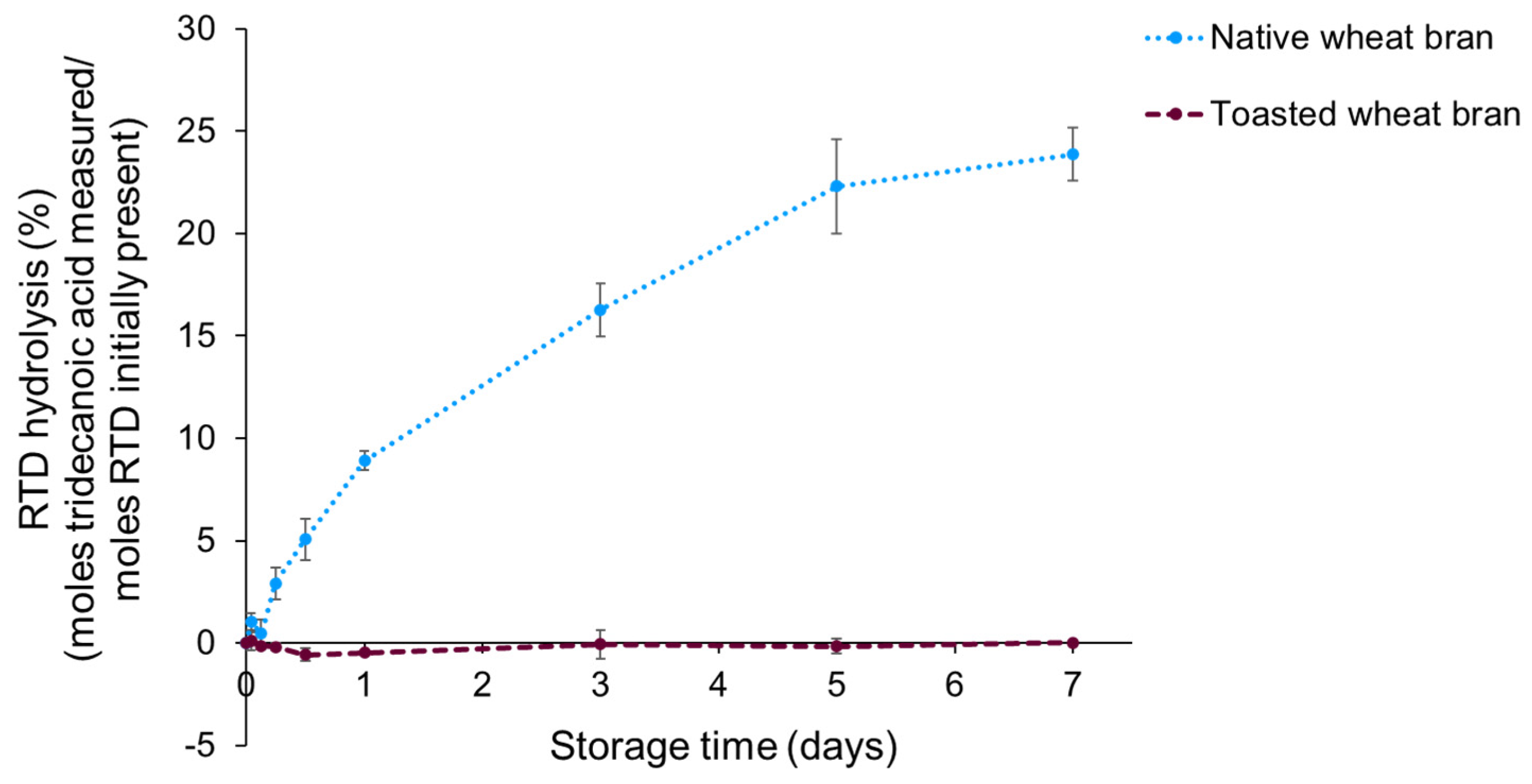
2.10. Quantification of Free Tridecanoic Acid
2.11. Statistical Analysis
3. Results and Discussion
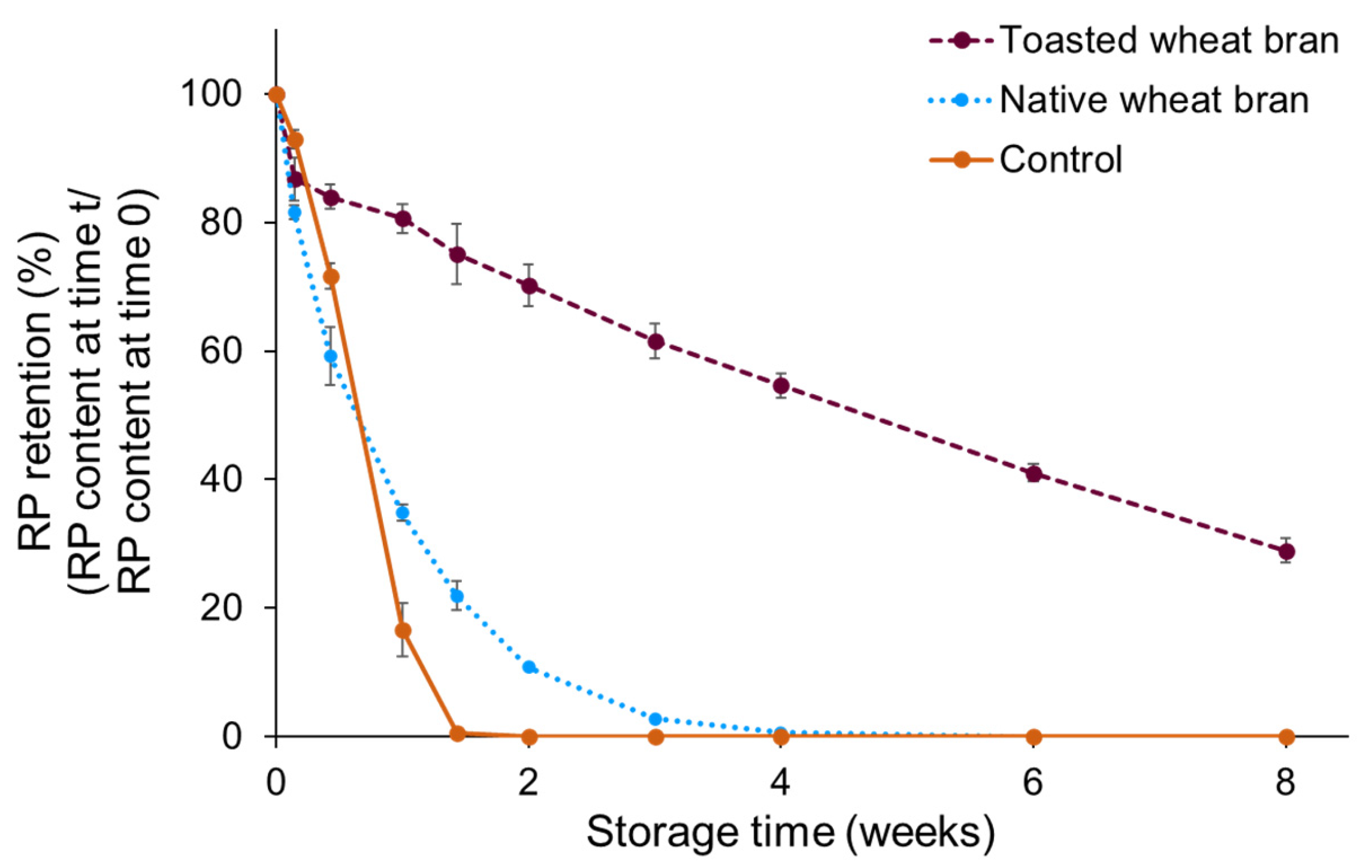
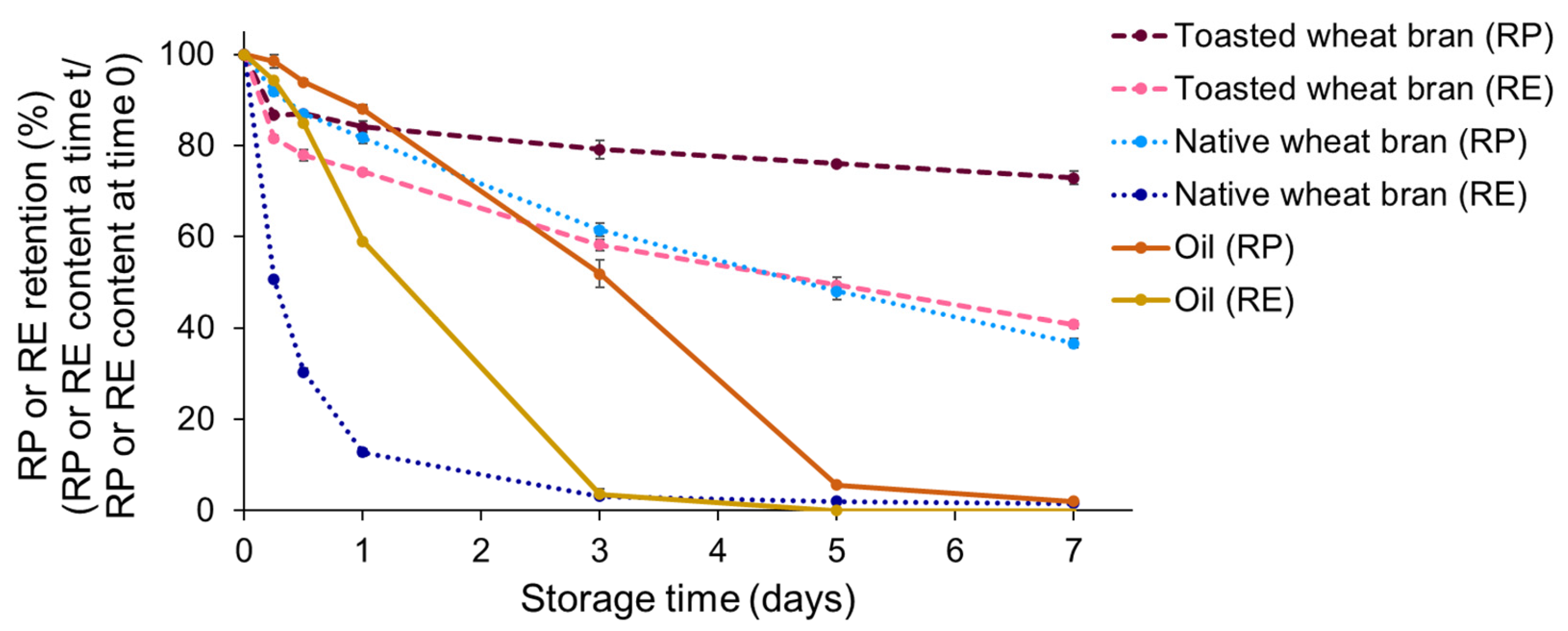
3.1. Vitamin A Degradation during Accelerated Storage
3.2. The Relationship between Free Fatty Acid Production, Lipid Oxidation and Vitamin A Degradation
3.3. Direct Action of Lipase on Retinyl Palmitate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sandjaja; Jusat, I.; Jahari, A.B.; Ifrad; Htet, M.K.; Tilden, R.L.; Soekarjo, D.; Utomo, B.; Moench-Pfanner, R.; Soekirman; et al. Vitamin A-Fortified Cooking Oil Reduces Vitamin A Deficiency in Infants, Young Children and Women: Results from a Programme Evaluation in Indonesia. Public Health Nutr. 2015, 18, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Stevens, G.A.; Bennett, J.E.; Hennocq, Q.; Lu, Y.; De-Regil, L.M.; Rogers, L.; Danaei, G.; Li, G.; White, R.A.; Flaxman, S.R.; et al. Trends and Mortality Effects of Vitamin A Deficiency in Children in 138 Low-Income and Middle-Income Countries between 1991 and 2013: A Pooled Analysis of Population-Based Surveys. Lancet Glob. Health 2015, 3, e528–e536. [Google Scholar] [CrossRef] [PubMed]
- Wirth, J.P.; Petry, N.; Tanumihardjo, S.A.; Rogers, L.M.; McLean, E.; Greig, A.; Garrett, G.S.; Klemm, R.D.W.; Rohner, F. Vitamin A Supplementation Programs and Country-Level Evidence of Vitamin A Deficiency. Nutrients 2017, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Diosady, L.L.; Vekantesh-Mannar, M.G. Vitamin A Fortification of Cooking Oils. In Handbook of Food Fortification and Health; Preedy, V.R., Srirajaskanthan, R., Patel, V.B., Eds.; Humana Press: London, UK, 2013; pp. 275–292. ISBN 978-1-4614-7076-2. [Google Scholar]
- Maurya, V.K.; Shakya, A.; McClements, D.J. Vitamin A Fortification: Recent Advances in Encapsulation Technologies. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2772–2819. [Google Scholar] [CrossRef] [PubMed]
- Loveday, S.M.; Singh, H. Recent Advances in Technologies for Vitamin A Protection in Foods. Trends Food Sci. Technol. 2008, 19, 657–668. [Google Scholar] [CrossRef]
- Dhakal, S.P.; He, J. Microencapsulation of Vitamins in Food Applications to Prevent Losses in Processing and Storage: A Review. Food Res. Int. 2020, 137, 109326. [Google Scholar] [CrossRef] [PubMed]
- Van Wayenbergh, E.; Struyf, N.; Rezaei, M.N.; Sagalowicz, L.; Bel-Rhlid, R.; Moccand, C.; Courtin, C.M. Cereal Bran Protects Vitamin A from Degradation during Simmering and Storage. Food Chem. 2020, 331, 127292. [Google Scholar] [CrossRef]
- Van Wayenbergh, E.; Langenaeken, N.A.; Struyf, N.; Goos, P.; Foubert, I.; Courtin, C.M. Stabilisation of Vitamin A by Wheat Bran Is Affected by Wheat Bran Antioxidants, Bound Lipids and Endogenous Lipase Activity. Food Res. Int. 2023, 169, 112911. [Google Scholar] [CrossRef]
- Pignitter, M.; Dumhart, B.; Gartner, S.; Jirsa, F.; Steiger, G.; Kraemer, K.; Somoza, V. Vitamin A Is Rapidly Degraded in Retinyl Palmitate-Fortified Soybean Oil Stored under Household Conditions. J. Agric. Food Chem. 2014, 62, 7559–7566. [Google Scholar] [CrossRef]
- Miyashita, K.; Takagi, T. Study on the Oxidative Rate and Prooxidant Activity of Free Fatty Acids. J. Am. Oil Chem. Soc. 1986, 63, 1380–1384. [Google Scholar] [CrossRef]
- Paradiso, V.M.; Gomes, T.; Nasti, R.; Caponio, F.; Summo, C. Effects of Free Fatty Acids on the Oxidative Processes in Purified Olive Oil. Food Res. Int. 2010, 43, 1389–1394. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, Z.; Emami, S.; Chen, J.; Leite Nobrega de Moura Bell, J.M.; Taha, A.Y. Triacylglycerols Are Preferentially Oxidized over Free Fatty Acids in Heated Soybean Oil. NPJ Sci. Food 2021, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Waraho, T.; McClements, D.J.; Decker, E.A. Impact of Free Fatty Acid Concentration and Structure on Lipid Oxidation in Oil-in-Water Emulsions. Food Chem. 2011, 129, 854–859. [Google Scholar] [CrossRef]
- Gordon, M.H.; Barimalaa, I.S. Co-Oxidation of Fat-Soluble Vitamins by Soybean Lipoxygenase. Food Chem. 1989, 32, 31–37. [Google Scholar] [CrossRef]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.S.; Becker, K. Dietary Roles of Phytate and Phytase in Human Nutrition: A Review. Food Chem. 2010, 120, 945–959. [Google Scholar] [CrossRef]
- Turner, C.; Persson, M.; Mathiasson, L.; Adlercreutz, P.; King, J.W. Lipase-Catalyzed Reactions in Organic and Supercritical Solvents: Application to Fat-Soluble Vitamin Determination in Milk Powder and Infant Formula. Enzyme Microb. Technol. 2001, 29, 111–121. [Google Scholar] [CrossRef]
- Van Wayenbergh, E.; Langenaeken, N.A.; Verheijen, J.; Foubert, I.; Courtin, C.M. Mechanistic Understanding of the Stabilisation of Vitamin A in Oil by Wheat Bran: The Interplay between Vitamin A Degradation, Lipid Oxidation, and Lipase Activity. Food Chem. 2024, 436, 137785. [Google Scholar] [CrossRef] [PubMed]
- Rebolleda, S.; Beltrán, S.; Sanz, M.T.; González-Sanjosé, M.L. Supercritical Fluid Extraction of Wheat Bran Oil: Study of Extraction Yield and Oil Quality. Eur. J. Lipid Sci. Technol. 2014, 116, 319–327. [Google Scholar] [CrossRef]
- Sawada, M.M.; Venâncio, L.L.; Toda, T.A.; Rodrigues, C.E.C. Effects of Different Alcoholic Extraction Conditions on Soybean Oil Yield, Fatty Acid Composition and Protein Solubility of Defatted Meal. Food Res. Int. 2014, 62, 662–670. [Google Scholar] [CrossRef]
- Bahtz, J.; Knorr, D.; Tedeschi, C.; Leser, M.E.; Valles-Pamies, B.; Miller, R. Adsorption of Octanoic Acid at the Water/Oil Interface. Colloids Surf. B. 2009, 74, 492–497. [Google Scholar] [CrossRef]
- Jacobs, P.J.; Hemdane, S.; Delcour, J.A.; Courtin, C.M. Dry Heat Treatment Affects Wheat Bran Surface Properties and Hydration Kinetics. Food Chem. 2016, 203, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Bergmeyer, H.U. Methods of Enzymatic Analysis, 2nd ed.; Verlag Chemie G.m.b.H.: Weinheim, Germany, 1974. [Google Scholar]
- Austin-Brown, S.L.; Chapman, K.D. Inhibition of Phospholipase Dα by N-Acylethanolamines. Plant Physiol. 2002, 129, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Van Wayenbergh, E.; Verheijen, J.; Langenaeken, N.A.; Foubert, I.; Courtin, C.M. A Simple Method for Analysis of Vitamin A Palmitate in Fortified Cereal Products Using Direct Solvent Extraction Followed by Reversed-Phase HPLC with UV Detection. Food Chem. 2023, 404, 134584. [Google Scholar] [CrossRef] [PubMed]
- Ryckebosch, E.; Muylaert, K.; Foubert, I. Optimization of an Analytical Procedure for Extraction of Lipids from Microalgae. J. Am. Oil Chem. Soc. 2012, 89, 189–198. [Google Scholar] [CrossRef]
- Melis, S.; Foubert, I.; Delcour, J.A. Normal-Phase HPLC-ELSD to Compare Lipid Profiles of Different Wheat Flours. Foods 2021, 10, 428. [Google Scholar] [CrossRef] [PubMed]
- Gheysen, L.; Dejonghe, C.; Bernaerts, T.; Van Loey, A.; De Cooman, L.; Foubert, I. Measuring Primary Lipid Oxidation in Food Products Enriched with Colored Microalgae. Food Anal. Methods 2019, 12, 2150–2160. [Google Scholar] [CrossRef]
- Gheysen, L.; Bernaerts, T.; Bruneel, C.; Goiris, K.; Van Durme, J.; Van Loey, A.; De Cooman, L.; Foubert, I. Impact of Processing on N-3 LC-PUFA in Model Systems Enriched with Microalgae. Food Chem. 2018, 268, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Van Meulebroek, L.; De Paepe, E.; Vercruysse, V.; Pomian, B.; Bos, S.; Lapauw, B.; Vanhaecke, L. Holistic Lipidomics of the Human Gut Phenotype Using Validated Ultra-High-Performance Liquid Chromatography Coupled to Hybrid Orbitrap Mass Spectrometry. Anal. Chem. 2017, 89, 12502–12510. [Google Scholar] [CrossRef]
- Hemery, Y.M.; Fontan, L.; Moench-Pfanner, R.; Laillou, A.; Berger, J.; Renaud, C.; Avallone, S. Influence of Light Exposure and Oxidative Status on the Stability of Vitamins A and D3 during the Storage of Fortified Soybean Oil. Food Chem. 2015, 184, 90–98. [Google Scholar] [CrossRef]
- Rohfritsch, Z.; Canelli, G.; Pollien, P.; Bel-Rhlid, R. Wheat and Rice Bran as Natural Additives for the Protection of Fish Oil from Oxidation. ACS Food Sci. Technol. 2021, 1, 1160–1168. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Nam, K.; Ahn, D.U. Analytical Methods for Lipid Oxidation and Antioxidant Capacity in Food Systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Van Wayenbergh, E.; Coddens, L.; Langenaeken, N.A.; Foubert, I.; Courtin, C.M. Stabilisation of Vitamin A by Cereal Bran: Importance of the Balance between Antioxidants, pro-Oxidants and Oxidation Sensitive Components. J. Agric. Food Chem. 2023, 71, 15296–15304. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Mcclements, D.J.; Decker, E.A. Minor Components in Food Oils: A Critical Review of Their Roles on Lipid Oxidation Chemistry in Bulk Oils and Emulsions. Crit. Rev. Food Sci. Nutr. 2011, 51, 901–916. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, V.M.; Pasqualone, A.; Summo, C.; Caponio, F. An “Omics” Approach for Lipid Oxidation in Foods: The Case of Free Fatty Acids in Bulk Purified Olive Oil. Eur. J. Lipid Sci. Technol. 2018, 120, 1800102. [Google Scholar] [CrossRef]
- Roppongi, T.; Mizuno, N.; Miyagawa, Y.; Kobayashi, T.; Nakagawa, K.; Adachi, S. Solubility and Mass Transfer Coefficient of Oxygen through Gas– and Water–Lipid Interfaces. J. Food Sci. 2021, 86, 867–873. [Google Scholar] [CrossRef]
- Balduyck, L.; Bijttebier, S.; Bruneel, C.; Jacobs, G.; Voorspoels, S.; Van Durme, J.; Muylaert, K.; Foubert, I. Lipolysis in T-Isochrysis Lutea during Wet Storage at Different Temperatures. Algal Res. 2016, 18, 281–287. [Google Scholar] [CrossRef]
- Rose, D.J.; Pike, O.A. A Simple Method to Measure Lipase Activity in Wheat and Wheat Bran as an Estimation of Storage Quality. JAOCS J. Am. Oil Chem. Soc. 2006, 83, 415–419. [Google Scholar] [CrossRef]
- Eyley, S.; Thielemans, W. Surface modification of cellulose nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef]
- Kim, H.; Hu, Y.; Jeong, D.; Jun, B.H.; Cho, E.; Jung, S. Synthesis, characterization, and retinol stabilization of fatty amide-β-cyclodextrin conjugates. Molecules 2016, 21, 963. [Google Scholar] [CrossRef]
- Marikhin, V.A.; Myasnikova, L.P.; Radovanova, E.I.; Volchek, B.Z.; Medvedeva, D.A. Fourier transform infrared spectroscopic study of the kinetics of a first-order phase transition in tridecanoic acid CH3(CH2)11COOH. Phys. Solid State 2017, 59, 331–337. [Google Scholar] [CrossRef]


| Sample | Wheat Bran (%) | Soy Oil (%) | RP (%) | RE (%) | RTD (%) |
|---|---|---|---|---|---|
| 1 | 0 | 99.20 | 0.80 | 0 | 0 |
| 2 | 80.00 (native) | 19.84 | 0.16 | 0 | 0 |
| 3 | 80.00 (toasted) | 19.84 | 0.16 | 0 | 0 |
| 4 | 0 | 99.56 | 0 | 0.44 | 0 |
| 5 | 80.00 (native) | 19.91 | 0 | 0.09 | 0 |
| 6 | 80.00 (toasted) | 19.91 | 0 | 0.09 | 0 |
| 7 | 80.00 (native) | 19.85 | 0 | 0 | 0.15 |
| 8 | 80.00 (toasted) | 19.85 | 0 | 0 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Wayenbergh, E.; Blockx, J.; Langenaeken, N.A.; Foubert, I.; Courtin, C.M. Conversion of Retinyl Palmitate to Retinol by Wheat Bran Endogenous Lipase Reduces Vitamin A Stability. Foods 2024, 13, 80. https://doi.org/10.3390/foods13010080
Van Wayenbergh E, Blockx J, Langenaeken NA, Foubert I, Courtin CM. Conversion of Retinyl Palmitate to Retinol by Wheat Bran Endogenous Lipase Reduces Vitamin A Stability. Foods. 2024; 13(1):80. https://doi.org/10.3390/foods13010080
Chicago/Turabian StyleVan Wayenbergh, Eline, Jonas Blockx, Niels A. Langenaeken, Imogen Foubert, and Christophe M. Courtin. 2024. "Conversion of Retinyl Palmitate to Retinol by Wheat Bran Endogenous Lipase Reduces Vitamin A Stability" Foods 13, no. 1: 80. https://doi.org/10.3390/foods13010080
APA StyleVan Wayenbergh, E., Blockx, J., Langenaeken, N. A., Foubert, I., & Courtin, C. M. (2024). Conversion of Retinyl Palmitate to Retinol by Wheat Bran Endogenous Lipase Reduces Vitamin A Stability. Foods, 13(1), 80. https://doi.org/10.3390/foods13010080






