Blackcurrant (Fruits, Pomace, and Leaves) Phenolic Characterization before and after In Vitro Digestion, Free Radical Scavenger Capacity, and Antioxidant Effects on Iron-Mediated Lipid Peroxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Vegetal Materials and Preparation of the Extracts
2.3. Determination of Individual Polyphenolic Compounds
2.4. In Vitro Simulated Gastrointestinal Digestion of Polyphenols
2.5. Antioxidant Activity Assays
2.5.1. Radical Scavenging Assays
2.5.2. In Vitro Induced Lipid Peroxidation
2.6. Statistical Analysis
3. Results
3.1. Effect of Simulated In Vitro Digestion on Bioactive Composition of Blackcurrant
3.2. The Blackcurrant Extracts (Fruits, Pomace and Leaves) Antioxidant Capacity
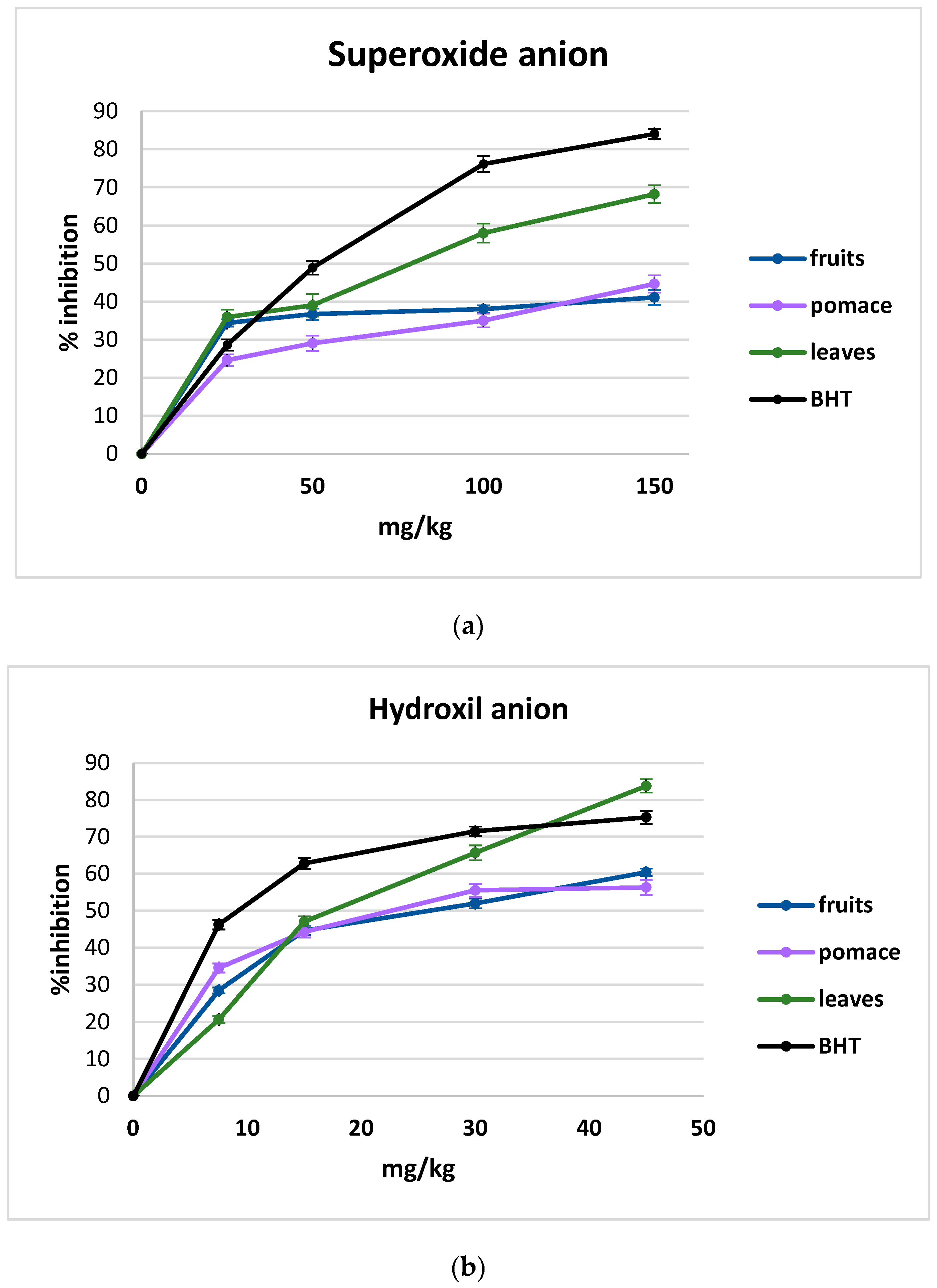
3.3. The Effect of Blackcurrant Extracts (Fruits, Pomace and Leaves) on Inhibition of Free Radicals
3.4. The Effect of Blackcurrant Extracts (Fruits, Pomace, and Leaves) on Inhibition of Lipid Peroxidation
4. Discussion
4.1. Blackcurrant Extracts (Fruits, Pomace, and Leaves): Antioxidant Capacity and Inhibition of Biological Free Radicals
4.2. The Effect of Blackcurrant Extracts on Lipid Peroxidation Inhibition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lavefve, L.; Howard, L.R.; Carbonero, F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2020, 11, 45–65. [Google Scholar] [CrossRef]
- Sánchez-Velázquez, O.A.; Mulero, M.; Cuevas-Rodríguez, E.O.; Mondor, M.; Arcand, Y.; Hernández-Álvarez, A.J. In vitro gastrointestinal digestion impact on stability, bioaccessibility and antioxidant activity of polyphenols from wild and commercial blackberries (Rubus spp.). Food Funct. 2021, 12, 7358–7378. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, A.J.; Somerville, V.X.; Hurst, R.D. The effect of New Zealand blackcurrant on sport performance and related biomarkers: A systematic review and meta-analysis. J. Int. Soc. Sports Nutr. 2020, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Staszowska-Karkut, M.; Chilczuk, B.; Materska, M.; Kontek, R.; Marciniak, B. Phenolic Compounds in Fractionated Blackcurrant Leaf Extracts in Relation to the Biological Activity of the Extracts. Molecules 2023, 28, 7459. [Google Scholar] [CrossRef] [PubMed]
- Teleszko, M.; Wojdyło, A. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Carregosa, D.; Carecho, R.; Figueira, I.; N Santos, C. Low-molecular weight metabolites from polyphenols as effectors for attenuating neuroinflammation. J. Agric. Food Chem. 2019, 68, 1790–1807. [Google Scholar] [CrossRef] [PubMed]
- Hano, C.; Tungmunnithum, D. Plant polyphenols, more than just simple natural antioxidants: Oxidative stress, aging and age-related diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability and bioaccessibility of food bioactive compounds; overview and assessment by in vitro methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2862–2884. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.O.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Ozkan, G.; Isik, H.; Horoz, O.; Van Camp, J.; Capanoglu, E. Black carrot pomace as a source of polyphenols for enhancing the nutritional value of cake: An in vitro digestion study with a standardized static model. LWT 2017, 77, 475–481. [Google Scholar] [CrossRef]
- Koudoufio, M.; Desjardins, Y.; Feldman, F.; Spahis, S.; Delvin, E.; Levy, E. Insight into polyphenol and gut microbiota crosstalk: Are their metabolites the key to understand protective effects against metabolic disorders? Antioxidants 2020, 9, 982. [Google Scholar] [CrossRef]
- Duque-Soto, C.; Quintriqueo-Cid, A.; Rueda-Robles, A.; Robert, P.; Borrás-Linares, I.; Lozano-Sánchez, J. Evaluation of Different Advanced Approaches to Simulation of Dynamic In Vitro Digestion of Polyphenols from Different Food Matrices—A Systematic Review. Antioxidants 2023, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Li, T.; Diao, X.; Kong, B. Protective effects of black currant (Ribes nigrum L.) extract on hydrogen peroxide-induced damage in lung fibroblast MRC-5 cells in relation to the antioxidant activity. J. Funct. Foods 2014, 11, 142–151. [Google Scholar] [CrossRef]
- Untea, A.E.; Varzaru, I.; Saracila, M.; Panaite, T.D.; Oancea, A.G.; Vlaicu, P.A.; Grosu, I.A. Antioxidant properties of cranberry leaves and walnut meal and their effect on nutritional quality and oxidative stability of broiler breast meat. Antioxidants 2023, 12, 1084. [Google Scholar] [CrossRef]
- Dou, Z.; Chen, C.; Huang, Q.; Fu, X. In vitro digestion of the whole blackberry fruit: Bioaccessibility, bioactive variation of active ingredients and impacts on human gut microbiota. Food Chem. 2022, 370, 131001. [Google Scholar] [CrossRef]
- Untea, A.; Lupu, A.; Saracila, M.; Panaite, T. Comparison of ABTS, DPPH, phosphomolybdenum assays for estimating antioxidant activity and phenolic compounds in five different plant extracts. Bull. UASVM Anim. Sci. Biotechnol. 2018, 75, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Untea, A.E.; Varzaru, I.; Vlaicu, P.A.; Turcu, R.P.; Panaite, T.D. Studies on antioxidant activities of grape pomace using in vitro, ex vivo, and in vivo models. J. Food Meas. Charact. 2023, 17, 121–128. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors affecting their stability and degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- de Ferrars, R.M.; Cassidy, A.; Curtis, P.; Kay, C.D. Phenolic metabolites of anthocyanins following a dietary intervention study in post-menopausal women. Mol. Nutr. Food Res. 2014, 58, 490–502. [Google Scholar] [CrossRef]
- de Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef] [PubMed]
- Celep, E.; Charehsaz, M.; Akyüz, S.; Acar, E.T.; Yesilada, E. Effect of in vitro gastrointestinal digestion on the bioavailability of phenolic components and the antioxidant potentials of some Turkish fruit wines. Food Res. Int. 2015, 78, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Chait, Y.A.; Gunenc, A.; Bendali, F.; Hosseinian, F. Simulated gastrointestinal digestion and in vitro colonic fermentation of carob polyphenols: Bioaccessibility and bioactivity. LWT 2020, 117, 108623. [Google Scholar] [CrossRef]
- Bhat, R.; Liong, M.T.; Abdorreza, M.N.; Karim, A.A. Evaluation of free radical scavenging activity and antioxidant potential of a few popular green leafy vegetables of Malaysia. Int. J. Food Prop. 2013, 16, 1371–1379. [Google Scholar] [CrossRef]
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, É. Comparative evaluation of total antioxidant capacities of plant polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Gupta, P. Plant secondary metabolites for preferential targeting among various stressors of metabolic syndrome. Stud. Nat. Prod. Chem. 2021, 71, 221–261. [Google Scholar] [CrossRef]
- Chen, L.; Xin, X.; Yuan, Q.; Su, D.; Liu, W. Phytochemical properties and antioxidant capacities of various coloredberries. J. Sci. Food Agric. 2014, 94, 180–188. [Google Scholar] [CrossRef]
- Zorzi, M.; Gai, F.; Medana, C.; Aigotti, R.; Morello, S.; Peiretti, P.G. Bioactive compounds and antioxidant capacity of small berries. Foods 2020, 9, 623. [Google Scholar] [CrossRef]
- Ziobroń, M.; Kopeć, A.; Skoczylas, J.; Dziadek, K.; Zawistowski, J. Basic chemical composition and concentration of selected bioactive compounds in leaves of black, red and white currant. Appl. Sci. 2021, 11, 7638. [Google Scholar] [CrossRef]
- Andjelković, M.; Van Camp, J.; De Meulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Wei, Z.; Yin, B.; Man, C.; Jiang, Y. Enhancement of functional characteristics of blueberry juice fermented by Lactobacillus plantarum. LWT 2021, 139, 110590. [Google Scholar] [CrossRef]
- Tian, Y.; Puganen, A.; Alakomi, H.L.; Uusitupa, A.; Saarela, M.; Yang, B. Antioxidative and antibacterial activities of aqueous ethanol extracts of berries, leaves, and branches of berry plants. Food Res. Int. 2018, 106, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, L.; Wang, D.; Huo, Y.; Ji, B. Anthocyanin-rich extracts from blackberry, wild blueberry, strawberry, and chokeberry: Antioxidant activity and inhibitory effect on oleic acid-induced hepatic steatosis in vitro. J. Sci. Food Agric. 2016, 96, 2494–2503. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Ma, G.; Li, N.; Deng, Q.; Yin, Y.; Huang, R. Investigation of in vitro and in vivo antioxidant activities of flavonoids rich extract from the berries of Rhodomyrtus tomentosa (Ait.) Hassk. Food Chem. 2015, 173, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Kong, B.; Liu, Q.; Diao, X.; Xia, X. Antioxidant activity of black currant (Ribes nigrum L.) extract and its inhibitory effect on lipid and protein oxidation of pork patties during chilled storage. Meat Sci. 2012, 91, 533–539. [Google Scholar] [CrossRef]
- Varzaru, I.; Untea, A.E.; Saracila, M. In vitro antioxidant properties of berry leaves and their inhibitory effect on lipid peroxidation of thigh meat from broiler chickens. Eur. J. Lipid Sci. Technol. 2020, 122, 1900384. [Google Scholar] [CrossRef]


| Fruits mg/g | Leaves mg/g | Pomace mg/g | SEM | p-Value | |
|---|---|---|---|---|---|
| Phenolic acids | 0.628 b | 11.39 a | 0.612 b | 0.443 | 0.0001 |
| Hydroxybenzoic acids | 0.185 b | 3.319 a | 0.489 b | 0.183 | 0.0001 |
| Gallic acid | 0.048 c | 0.506 a | 0.113 b | 0.004 | 0.0001 |
| Vanillic acid | 0.034 b | 1.166 a | 0.035 b | 0.016 | 0.0001 |
| Syringic acid | 0.030 b | 0.290 a | 0.000 b | 0.010 | 0.0001 |
| Hydroxybenzoic acid | 0.050 b | 1.153 a | 0.300 b | 0.176 | 0.010 |
| Ellagic acid | 0.023 b | 0.189 a | 0.041 b | 0.010 | 0.0001 |
| Protocatechuic acid | 0.000 b | 0.014 a | 0.000 b | 0.000 | 0.0001 |
| Hydroxycinnamic acids | 0.443 b | 8.074 a | 0.123 b | 0.261 | 0.0001 |
| Chlorogenic acid | 0.316 b | 2.293 a | 0.000 c | 0.038 | 0.0001 |
| Caffeic acid | 0.021 b | 0.176 a | 0.023 b | 0.017 | 0.001 |
| Metoxicinnamic acid | 0.013 b | 1.078 a | 0.039 b | 0.022 | 0.0001 |
| Ferulic acid | 0.087 b | 3.588 a | 0.050 b | 0.211 | 0.0001 |
| Coumaric acid | 0.006 b | 0.925 a | 0.011 b | 0.016 | 0.0001 |
| Cinnamic acid | nd 1 | 0.014 a | nd 1 | 0.000 | 0.0001 |
| Flavonoids | 0.183 c | 2.809 a | 0.411 b | 0.026 | 0.0001 |
| Flavanols | 0.18 c | 2.62 a | 0.40 b | 0.028 | 0.0001 |
| Epigallocatechin | nd 1 | 1.369 | nd 1 | - | - |
| Catechin | 0.031 b | 0.088 b | 0.253 a | 0.018 | 0.0001 |
| Epicatechin | 0.146 b | 1.163 a | 0.147 b | 0.053 | 0.0001 |
| Flavonols | 0.006 b | 0.189 a | 0.012 b | 0.011 | 0.0001 |
| Rutin | 0.006 b | 0.184 a | 0.010 b | 0.010 | 0.0001 |
| Quercetin | nd 1 | 0.005 a | 0.002 b | 0.001 | 0.0001 |
| Stilbene | nd 1 | 0.006 | nd 1 | - | - |
| Resveratrol | nd 1 | 0.006 | nd 1 | - | - |
| Phenolic Compounds in Fruit Samples | Digestive Phases | |||||
|---|---|---|---|---|---|---|
| Oral | Gastric | Intestinal | SEM | p-Value | RI 1 | |
| mg/g | mg/g | mg/g | % | |||
| Phenolic acids | 0.220 b | 0.262 b | 0.489 a | 0.026 | 0.0001 | 72.81 |
| Hydroxybenzoic acids | 0.064 c | 0.080 b | 0.140 a | 0.001 | 0.0001 | 73.94 |
| Gallic acid | 0.018 c | 0.021 b | 0.045 a | 0.000 | 0.0001 | 93.43 |
| Vanillic acid | 0.014 b | 0.016 b | 0.026 a | 0.000 | 0.0001 | 76.50 |
| Syringic acid | 0.011 b | 0.013 b | 0.021 a | 0.000 | 0.0001 | 68.85 |
| Hydroxybenzoic acid | 0.014 c | 0.018 b | 0.033 a | 0.000 | 0.0001 | 66.68 |
| Ellagic acid | 0.008 c | 0.011 b | 0.014 a | 0.000 | 0.0001 | 64.26 |
| Protocatechuic acid | nd 2 | nd 2 | nd 2 | nd 2 | ||
| Hydroxycinnamic acids | 0.156 c | 0.182 b | 0.348 a | 0.027 | 0.0001 | 71.68 |
| Chlorogenic acid | 0.103 c | 0.134 b | 0.255 a | 0.001 | 0.0001 | 80.80 |
| Caffeic acid | 0.005 b | 0.006 b | 0.012 a | 0.000 | 0.0001 | 57.75 |
| Metoxicinnamic acid | 0.002 c | 0.007 b | 0.011 a | 0.000 | 0.0001 | 89.86 |
| Ferulic acid | 0.042 b | 0.033 c | 0.067 a | 0.001 | 0.0001 | 76.68 |
| Coumaric acid | 0.003 a | 0.002 b | 0.003 a | 0.000 | 0.0001 | 53.29 |
| Cinnamic acid | nd 2 | nd 2 | nd 2 | nd 2 | ||
| Flavonoids | 0.183 a | 0.079 c | 0.095 b | 0.014 | 0.0001 | 91.04 |
| Flavanols | 0.08 b | 0.09 b | 0.17 a | 0.001 | 0.0001 | 89.88 |
| Epigallocatechin | nd 2 | nd 2 | nd 2 | nd 2 | ||
| Catechin | 0.013 b | 0.012 b | 0.026 a | 0.001 | 0.0001 | 82.60 |
| Epicatechin | 0.064 c | 0.080 b | 0.142 a | 0.002 | 0.0001 | 97.15 |
| Flavonols | 0.001 c | 0.003 b | 0.005 a | 0.000 | 0.0001 | 93.36 |
| Rutin | 0.001 c | 0.003 b | 0.005 a | 0.000 | 0.0001 | 93.36 |
| Quercetin | nd 2 | nd 2 | nd 2 | nd 2 | ||
| Stilbene | ||||||
| Resveratrol | nd 2 | nd 2 | nd 2 | nd 2 | ||
| Phenolic Compounds in Pomace Samples | Digestive Phases | |||||
|---|---|---|---|---|---|---|
| Oral | Gastric | Intestinal | SEM | p-Value | RI 1 | |
| mg/g | mg/g | mg/g | % | |||
| Phenolic acids | 0.237 b | 0.231 b | 0.383 a | 0.024 | 0.0001 | 56.87 |
| Hydroxybenzoic acids | 0.188 b | 0.193 b | 0.314 a | 0.009 | 0.0001 | 61.08 |
| Gallic acid | 0.036 b | 0.040 b | 0.123 a | 0.001 | 0.0001 | 109.09 |
| Vanillic acid | 0.015 b | 0.014 b | 0.017 a | 0.001 | 0.0023 | 48.47 |
| Syringic acid | nd 2 | nd 2 | nd 2 | 0.000 | 0.0001 | nd 2 |
| Hydroxybenzoic acid | 0.106 c | 0.131 b | 0.160 a | 0.007 | 0.0001 | 53.22 |
| Ellagic acid | 0.031 a | 0.008 c | 0.014 b | 0.000 | 0.0001 | 33.54 |
| Protocatechuic acid | nd 2 | nd 2 | nd 2 | nd 2 | ||
| Hydroxycinnamic acids | 0.049 b | 0.037 c | 0.069 a | 0.027 | 0.0001 | 52.65 |
| Chlorogenic acid | nd 2 | nd 2 | nd 2 | nd 2 | ||
| Caffeic acid | 0.011 a | 0.009 b | 0.012 a | 0.000 | 0.0010 | 51.77 |
| Metoxicinnamic acid | 0.018 b | 0.021 b | 0.032 a | 0.001 | 0.0001 | 82.84 |
| Ferulic acid | 0.015 b | 0.005 c | 0.022 a | 0.001 | 0.0001 | 43.14 |
| Coumaric acid | 0.004 a | 0.002 b | 0.004 a | 0.000 | 0.0001 | 32.86 |
| Cinnamic acid | nd 2 | nd 2 | nd 2 | nd 2 | ||
| Flavonoids | 0.318 b | 0.314 b | 0.364 a | 0.014 | 0.0010 | 80.75 |
| Flavanols | 0.312 b | 0.309 b | 0.356 a | 0.014 | 0.0010 | 83.68 |
| Epigallocatechin | nd 2 | nd 2 | nd 2 | nd 2 | ||
| Catechin | 0.192 b | 0.197 b | 0.216 a | 0.008 | 0.0001 | 85.31 |
| Epicatechin | 0.119 b | 0.113 b | 0.140 a | 0.008 | 0.0001 | 82.04 |
| Flavonols | 0.006 b | 0.005 b | 0.009 a | 0.000 | 0.0001 | 77.82 |
| Rutin | 0.005 b | 0.004 b | 0.007 a | 0.000 | 0.0001 | 72.81 |
| Quercetin | 0.001 b | 0.001 b | 0.002 a | 0.000 | 0.0001 | 82.84 |
| Stilbene | ||||||
| Resveratrol | nd 2 | nd 2 | nd 2 | nd 2 | ||
| Phenolic Compounds in Leaf Samples | Digestive Phases | |||||
|---|---|---|---|---|---|---|
| Oral | Gastric | Intestinal | SEM | p-Value | RI 1 | |
| mg/g | mg/g | mg/g | % | |||
| Phenolic acids | 1.372 c | 3.666 b | 6.255 a | 0.132 | 0.0001 | 62.96 |
| Hydroxybenzoic acids | 0.297 c | 0.906 b | 1.955 a | 0.028 | 0.0001 | 64.19 |
| Gallic acid | 0.047 c | 0.149 b | 0.497 a | 0.005 | 0.0001 | 98.14 |
| Vanillic acid | 0.130 c | 0.460 b | 0.905 a | 0.010 | 0.0001 | 74.52 |
| Syringic acid | 0.037 c | 0.100 b | 0.155 a | 0.009 | 0.0001 | 59.52 |
| Hydroxybenzoic acid | 0.060 c | 0.152 b | 0.293 a | 0.008 | 0.0001 | 46.36 |
| Ellagic acid | 0.020 c | 0.043 b | 0.099 a | 0.005 | 0.0001 | 62.46 |
| Protocatechuic acid | 0.001 b | 0.002 b | 0.007 a | 0.000 | 0.0001 | 44.15 |
| Hydroxycinnamic acids | 1.075 c | 2.761 b | 4.300 a | 0.106 | 0.0001 | 61.73 |
| Chlorogenic acid | 0.222 c | 0.587 b | 0.984 a | 0.025 | 0.0001 | 45.18 |
| Caffeic acid | 0.017 c | 0.050 b | 0.087 a | 0.005 | 0.0001 | 68.48 |
| Metoxicinnamic acid | 0.189 c | 0.449 b | 0.714 a | 0.017 | 0.0001 | 62.43 |
| Ferulic acid | 0.536 c | 1.346 b | 2.092 a | 0.075 | 0.0001 | 70.78 |
| Coumaric acid | 0.110 c | 0.326 b | 0.412 a | 0.014 | 0.0001 | 47.05 |
| Cinnamic acid | 0.002 b | 0.003 b | 0.011 a | 0.000 | 0.0001 | 76.46 |
| Flavonoids | 0.434 c | 0.989 b | 1.580 a | 0.042 | 0.0001 | 54.87 |
| Flavanols | 0.417 c | 0.951 b | 1.500 a | 0.042 | 0.0001 | 56.78 |
| Epigallocatechin | 0.296 c | 0.658 b | 0.923 a | 0.025 | 0.0001 | 59.27 |
| Catechin | 0.015 b | 0.026 b | 0.054 a | 0.003 | 0.0001 | 59.00 |
| Epicatechin | 0.106 c | 0.267 b | 0.523 a | 0.018 | 0.0001 | 52.08 |
| Flavonols | 0.017 c | 0.038 b | 0.080 a | 0.003 | 0.0001 | 52.95 |
| Rutin | 0.015 c | 0.037 b | 0.077 a | 0.003 | 0.0001 | 50.09 |
| Quercetin | 0.001 b | 0.001 b | 0.003 a | 0.000 | 0.0001 | 55.81 |
| Stilbene | 0.001 b | 0.002 b | 0.004 a | 0.000 | 0.0001 | 74.37 |
| Resveratrol | 0.001 b | 0.002 b | 0.004 a | 0.000 | 0.0001 | 74.37 |
| Fruits | Leaves | Pomace | SEM | p-Value | |
|---|---|---|---|---|---|
| Phosphomolybdate assay (mM equiv ascorbic acid) | 92.03 c | 472.1 a | 299.3 b | 7.529 | 0.0001 |
| DPPH 1 (mM equiv Trolox) | 235.6 b | 1041.6 a | 72.70 c | 7.191 | 0.0001 |
| TEAC 2 (mM equiv Trolox) | 86.33 b | 360.6 a | 34.17 c | 1.160 | 0.0001 |
| Iron chelation capacity (mM equiv EDTA) | 0.380 c | 2.450 a | 0.414 b | 0.020 | 0.0001 |
| Samples | MDA 1, (mg/kg) |
|---|---|
| Fresh meat (unperoxidized) | 0.568 d |
| Fresh meat (in vitro peroxidized with Fe3+/AA system) | 1.150 a |
| Fresh meat (in vitro peroxidized with Fe3+/AA system, inhibited with BHT) | 0.648 cd |
| Fresh meat (in vitro peroxidized with Fe3+/AA system, inhibited with blackcurrant leaf extract) | 0.714 c |
| Fresh meat (in vitro peroxidized with Fe3+/AA system, inhibited with blackcurrant fruit extract) | 0.880 b |
| Fresh meat (in vitro peroxidized with Fe3+/AA system, inhibited with blackcurrant pomace extract) | 0.951 b |
| SEM | 0.025 |
| p-value | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Untea, A.E.; Oancea, A.-G.; Vlaicu, P.A.; Varzaru, I.; Saracila, M. Blackcurrant (Fruits, Pomace, and Leaves) Phenolic Characterization before and after In Vitro Digestion, Free Radical Scavenger Capacity, and Antioxidant Effects on Iron-Mediated Lipid Peroxidation. Foods 2024, 13, 1514. https://doi.org/10.3390/foods13101514
Untea AE, Oancea A-G, Vlaicu PA, Varzaru I, Saracila M. Blackcurrant (Fruits, Pomace, and Leaves) Phenolic Characterization before and after In Vitro Digestion, Free Radical Scavenger Capacity, and Antioxidant Effects on Iron-Mediated Lipid Peroxidation. Foods. 2024; 13(10):1514. https://doi.org/10.3390/foods13101514
Chicago/Turabian StyleUntea, Arabela Elena, Alexandra-Gabriela Oancea, Petru Alexandru Vlaicu, Iulia Varzaru, and Mihaela Saracila. 2024. "Blackcurrant (Fruits, Pomace, and Leaves) Phenolic Characterization before and after In Vitro Digestion, Free Radical Scavenger Capacity, and Antioxidant Effects on Iron-Mediated Lipid Peroxidation" Foods 13, no. 10: 1514. https://doi.org/10.3390/foods13101514
APA StyleUntea, A. E., Oancea, A.-G., Vlaicu, P. A., Varzaru, I., & Saracila, M. (2024). Blackcurrant (Fruits, Pomace, and Leaves) Phenolic Characterization before and after In Vitro Digestion, Free Radical Scavenger Capacity, and Antioxidant Effects on Iron-Mediated Lipid Peroxidation. Foods, 13(10), 1514. https://doi.org/10.3390/foods13101514











