Challenges of the Application of In Vitro Digestion for Nanomaterials Safety Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. In Vitro Digestion Protocol
2.2.1. Preparation of Reagents and Test Tubes
2.2.2. Digestive Fluids Composition
2.3. Osmolality and pH
2.4. Intestinal Cell Culture and Exposure
2.5. Cell Viability Assay
2.6. Intracellular Reactive Oxygen Species (ROS)
2.7. Statistical Analysis
3. Results and Discussion
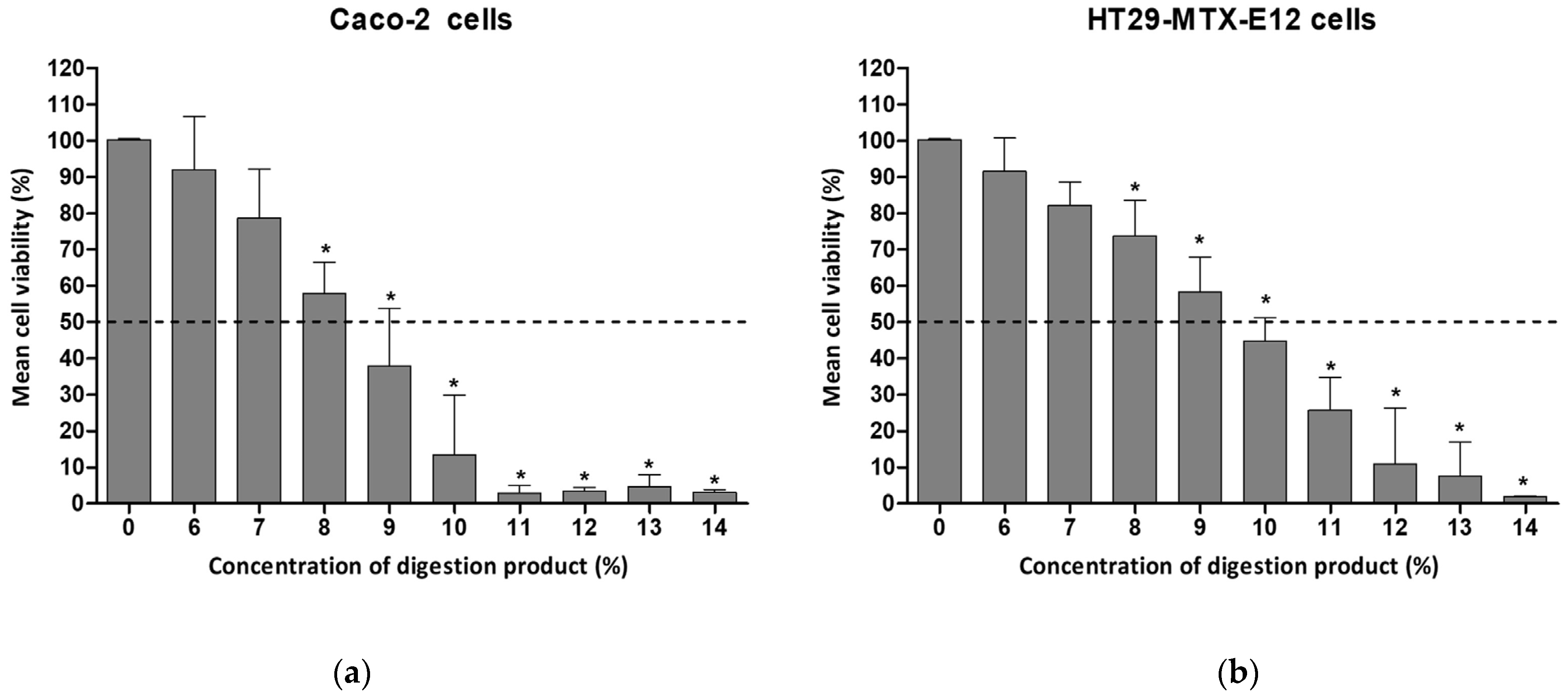
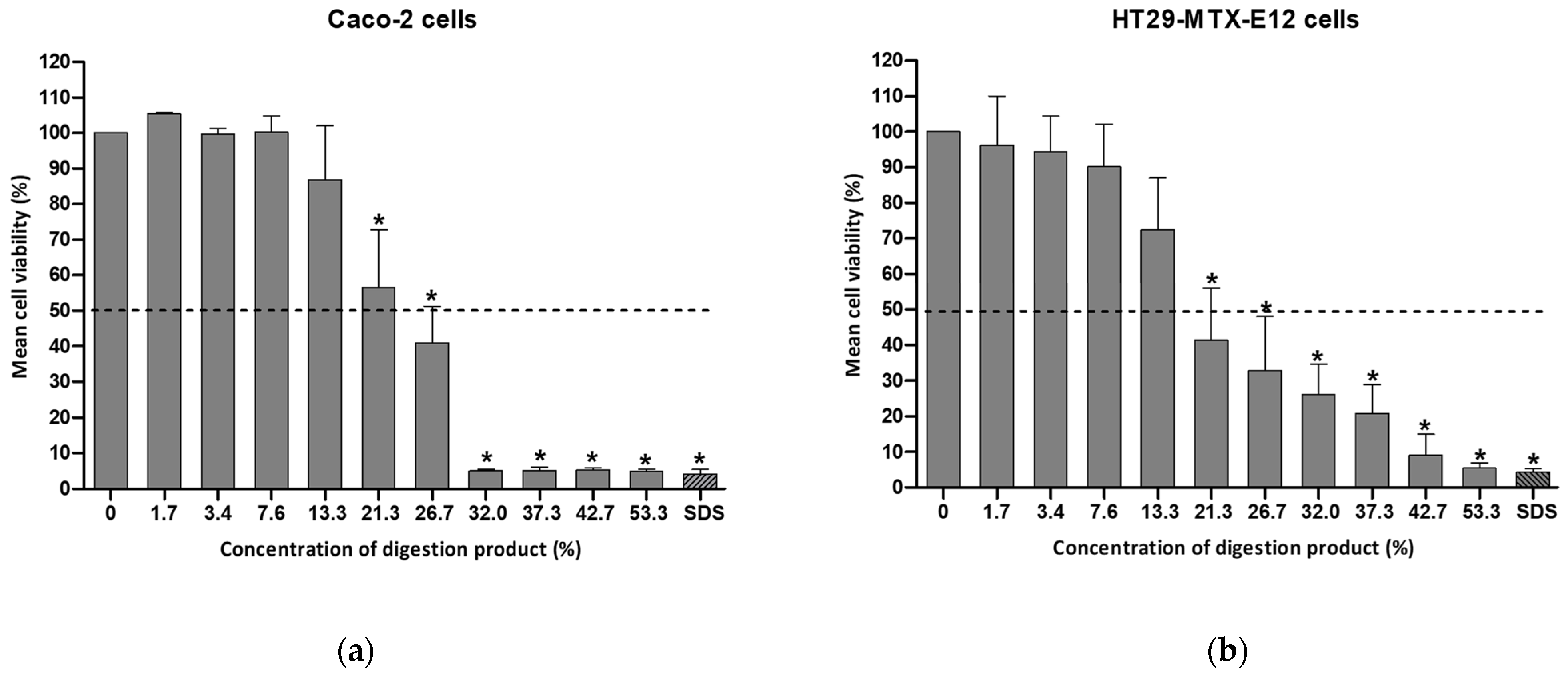
3.1. Cytotoxicity of the Digestion Product on Intestinal Cells
3.2. Osmolality and pH
3.3. The Role of Pefabloc® SC in the Toxicity of the Digestion Product
3.4. Bile Salts’ Effect on Digestion Product Cytotoxicity
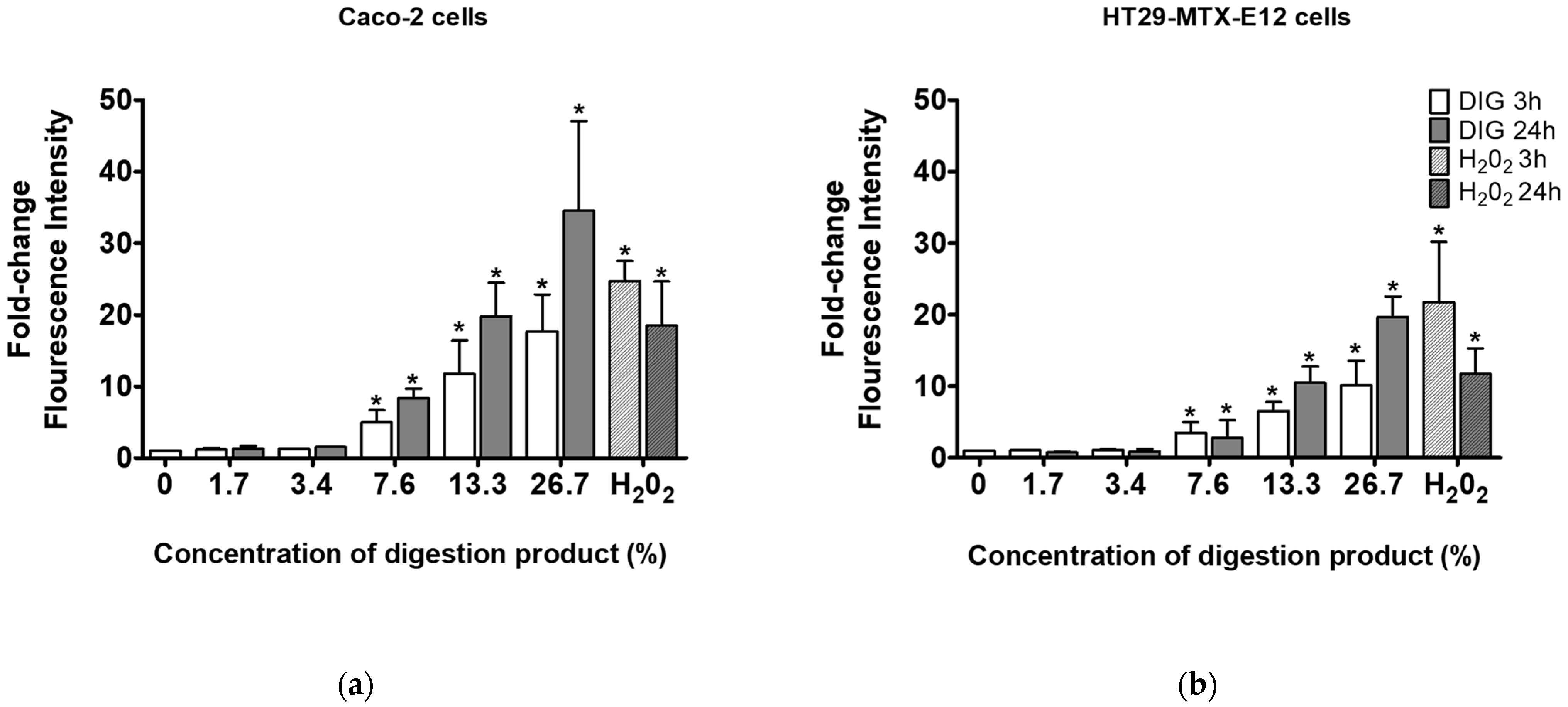
3.5. Modified Digestion Product Induces Reactive Oxygen Species
4. Conclusions
- In toxicology testing, include a digestion product control, corresponding to each concentration assayed when evaluating digested NM;
- Conduct preliminary experiments to assess the sensitivity of the selected cell model and culture system to the digestion product;
- When feasible, start the digestion process with the highest attainable concentration of the test material, enabling maximum dilution levels of both the sample and the digestion product before testing;
- Consider reducing the bile salts concentration in the digestion product, particularly when the experiment aims to replicate a fasting-diet state.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EFSA Panel on Food Additives and Flavourings (FAF); Younes, M.; Aquilina, G.; Castle, L.; Engel, K.H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; et al. Scientific opinion on the safety assessment of titanium dioxide (E171) as a food additive. EFSA J. 2021, 19, e6585. [Google Scholar] [CrossRef]
- EFSA Scientific Panel on Food Additives and Flavourings (FAF); Castle, L.; Degen, G.; Engel, K.H.; Frutos Fernández, M.J.; Remy, U.G.; Gürtler, R.; Husoy, T.; Manco, M.; Mennes, W.; et al. In Proceedings of the 41st FAF Panel Meeting, 14–15 November 2023. Available online: https://www.efsa.europa.eu/sites/default/files/2023-12/141123-m.pdf (accessed on 16 May 2024).
- EFSA Scientific Committee; More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hernández-Jerez, A.; Bennekou, S.H.; Koutsoumanis, K.; Lambré, C.; et al. Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles. EFSA J. 2021, 19, e6769. [Google Scholar] [CrossRef]
- EFSA Scientific Committee; More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hernández-Jerez, A.; Bennekou, S.H.; Koutsoumanis, K.; Lambré, C.; et al. Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: Human and animal health. EFSA J. 2021, 19, e06768. [Google Scholar] [CrossRef]
- Bolan, S.; Sharma, S.; Mukherjee, S.; Zhou, P.; Mandal, J.; Srivastava, P.; Hou, D.; Edussuriya, R.; Vithanage, M.; Truong, V.K.; et al. The distribution, fate, and environmental impacts of food additive nanomaterials in soil and aquatic ecosystems. Sci. Total Environ. 2024, 916, 170013. [Google Scholar] [CrossRef]
- Louro, H. Relevance of physicochemical characterization of nanomaterials for understanding nano-cellular interactions. In Cellular and Molecular Toxicology of Nanoparticles, 1st ed.; Saquib, Q., Faisal, M., Al-Khedhairy, A., Alatar, A., Eds.; Springer: Cham, Switzerland, 2018; Volume 1048, pp. 123–142. ISBN 978-3-319-72041-8. [Google Scholar] [CrossRef]
- Louro, H.; Saruga, A.; Santos, J.; Pinhão, M.; Silva, M.J. Biological impact of metal nanomaterials in relation to their physicochemical characteristics. Toxicol. Vitr. 2019, 56, 172–183, Erratum in Toxicol. Vitr. 2019, 59, 323. [Google Scholar] [CrossRef]
- Vieira, A.; Vital, N.; Rolo, D.; Roque, R.; Gonçalves, L.M.; Bettencourt, A.; Silva, M.J.; Louro, H. Investigation of the genotoxicity of digested titanium dioxide nanomaterials in human intestinal cells. Food. Chem. Toxicol. 2022, 161, 112841. [Google Scholar] [CrossRef]
- Bettencourt, A.; Gonçalves, L.M.; Gramacho, A.C.; Vieira, A.; Rolo, D.; Martins, C.; Assunção, R.; Alvito, P.; Silva, M.J.; Louro, H. Analysis of the characteristics and cytotoxicity of titanium dioxide nanomaterials following simulated in vitro digestion. Nanomaterials 2020, 10, 1516. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food. Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Assunção, R.; Martins, C.; Dupont, D.; Alvito, P. Patulin and ochratoxin A co-occurrence and their bioaccessibility in processed cereal-based foods: A contribution for Portuguese children risk assessment. Food Chem. Toxicol. 2016, 96, 205–214. [Google Scholar] [CrossRef]
- Vital, N.; Ventura, C.; Kranendonk, M.; Silva, M.J.; Louro, H. Toxicological assessment of cellulose nanomaterials: Oral exposure. Nanomaterials 2022, 12, 3375. [Google Scholar] [CrossRef]
- Kondrashina, A.; Arranz, E.; Cilla, A.; Faria, M.A.; Santos-Hernández, M.; Miralles, B.; Hashemi, N.; Rasmussen, M.K.; Young, J.F.; Barberá, R.; et al. Coupling in vitro food digestion with in vitro epithelial absorption; recommendations for biocompatibility. Crit. Rev. Food Sci. Nutr. 2023, 1–19. [Google Scholar] [CrossRef]
- Mulet-Cabero, A.I.; Egger, L.; Portmann, R.; Ménard, O.; Marze, S.; Minekus, M.; Le Feunteun, S.; Sarkar, A.; Grundy, M.M.; Carrière, F.; et al. A standardised semi-dynamic in vitro digestion method suitable for food—An international consensus. Food Funct. 2020, 11, 1702–1720. [Google Scholar] [CrossRef]
- Madalena, D.; Fernandes, J.M.; Avelar, Z.; Gonçalves, R.F.S.; Ramos, Ó.L.; Vicente, A.A.; Pinheiro, A.C. Emerging challenges in assessing bio-based nanosystems’ behaviour under in vitro digestion focused on food applications—A critical view and future perspectives. Food Res. Int. 2022, 157, 111417. [Google Scholar] [CrossRef]
- Gonçalves, R.F.S.; Martins, J.T.; Abrunhosa, L.; Baixinho, J.; Matias, A.A.; Vicente, A.A.; Pinheiro, A.C. Lipid-based nanostructures as a strategy to enhance curcumin bioaccessibility: Behavior under digestion and cytotoxicity assessment. Food Res. Int. 2021, 143, 110278. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Gonçalves, R.F.S.; Madalena, D.A.; Vicente, A.A. Towards the understanding of the behavior of bio-based nanostructures during in vitro digestion. Curr. Opin. Food Sci. 2017, 15, 79–86. [Google Scholar] [CrossRef]
- Li, C.; Yu, W.; Wu, P.; Chen, X.D. Current in vitro digestion systems for understanding food digestion in human upper gastrointestinal tract. Trends Food Sci. Technol. 2020, 96, 114–126. [Google Scholar] [CrossRef]
- Jensen, A.K.; Kembouche, Y.; Christiansen, E.; Jacobsen, N.R.; Wallin, H.; Guiot, C.; Spalla, O.; Witschger, O. Towards a Method for Detecting the Potential Genotoxicity of Nanomaterials: Final Protocol for Producing Suitable Manufactured Nanomaterials Exposure Media Report; The Generic NANOGENOTOX Dispersion Protocol Standard Operation Procedure (SOP) and Background Documentation; NANOGENOTOX: Copenhagen, Denmark, 2011. [Google Scholar]
- Gramacho, A. Assessment of the Cellular Effects of Ingested Titanium Dioxide Nanomaterials in Intestinal. Master’s Thesis, Universidade Nova de Lisboa, Lisboa, Portugal, 2019. [Google Scholar]
- Gil-Sánchez, I.; Monge, M.; Miralles, B.; Armentia, G.; Cueva, C.; Crespo, J.; López-de-Luzuriaga, J.M.; Olmos, M.E.; Bartolomé, B.; Llano, D.G.; et al. Some new findings on the potential use of biocompatible silver nanoparticles in winemaking. Innov. Food Sci. Emerg. Technol. 2018, 51, 64–72. [Google Scholar] [CrossRef]
- Xavier, M.; Rodrigues, P.M.; Neto, M.D.; Guedes, M.I.; Calero, V.; Pastrana, L.; Gonçalves, C. From mouth to gut: Microfluidic in vitro simulation of human gastro-intestinal digestion and intestinal permeability. Analyst 2023, 148, 3193–3203. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Wang, Y.; Kapronezai, K.; Lorente, L.R.; Zhang, R.; Pyrgiotakis, G.; Konduru, N.V.; Ericsson, M.; White, J.C.; De La Torre-Roche, R.; et al. An integrated methodology for assessing the impact of food matrix and gastrointestinal effects on the biokinetics and cellular toxicity of ingested engineered nanomaterials. Part. Fibre Toxicol. 2017, 14, 40. [Google Scholar] [CrossRef]
- Freshney, R.I. Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications, 6th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; Volume 106, p. 99. ISBN 978-0-470-52812-9. [Google Scholar]
- Grauso, M.; Lan, A.; Andriamihaja, M.; Bouillaud, F.; Blachier, F. Hyperosmolar environment and intestinal epithelial cells: Impact on mitochondrial oxygen consumption, proliferation, and barrier function in vitro. Sci. Rep. 2019, 9, 11360. [Google Scholar] [CrossRef] [PubMed]
- Arranz, E.; Segat, A.; Velayos, G.; Flynn, C.; Brodkorb, A.; Giblin, L. Dairy and plant-based protein beverages: In vitro digestion behaviour and effect on intestinal barrier biomarkers. Food Res. Int. 2023, 169, 112815. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.F.S.; Martins, J.T.; Abrunhosa, L.; Vicente, A.A.; Pinheiro, A.C. Nanoemulsions for enhancement of curcumin bioavailability and their safety evaluation: Effect of Emulsifier Type. Nanomaterials 2021, 11, 815. [Google Scholar] [CrossRef] [PubMed]
- Araiza-Calahorra, A.; Wang, Y.; Boesch, C.; Zhao, Y.; Sarkar, A. Pickering emulsions stabilized by colloidal gel particles complexed or conjugated with biopolymers to enhance bioaccessibility and cellular uptake of curcumin. Curr. Res. Food Sci. 2020, 3, 178–188. [Google Scholar] [CrossRef]
- Monte, M.J.; Marin, J.J.; Antelo, A.; Vazquez-Tato, J. Bile acids: Chemistry, physiology, and pathophysiology. World J. Gastroenterol. 2009, 15, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.J.; Macias, R.I.; Briz, O.; Banales, J.M.; Monte, M.J. Bile acids in physiology, pathology and pharmacology. Curr. Drug Metab. 2015, 17, 4–29. [Google Scholar] [CrossRef] [PubMed]
- Kalantzi, L.; Goumas, K.; Kalioras, V.; Abrahamsson, B.; Dressman, J.B.; Reppas, C. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm. Res. 2006, 23, 165–176. [Google Scholar] [CrossRef]
- Riethorst, D.; Mols, R.; Duchateau, G.; Tack, J.; Brouwers, J.; Augustijns, P. Characterization of human duodenal fluids in fasted and fed state conditions. J. Pharm. Sci. 2016, 105, 673–681. [Google Scholar] [CrossRef]
- Santos-Hernández, M.; Amigo, L.; Recio, I. Induction of CCK and GLP-1 release in enteroendocrine cells by egg white peptides generated during gastrointestinal digestion. Food Chem. 2020, 329, 127188. [Google Scholar] [CrossRef]
- Barrasa, J.I.; Olmo, N.; Lizarbe, M.A.; Turnay, J. Bile acids in the colon, from healthy to cytotoxic molecules. Toxicol. Vitr. 2013, 27, 964–977. [Google Scholar] [CrossRef]

| Oral Phase (pH7) Simulated Salivary Fluid (SSF, 1×) | Gastric Phase (pH3) Simulated Gastric Fluid (SGF, 1×) | Intestinal Phase (pH7) Simulated Intestinal Fluid (SIF, 1×) |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Digestion Product (%) | Osmolality (mOsmol/kg) |
|---|---|
| 1.6 | 305 |
| 3.1 | 306 |
| 6.3 | 309 |
| 12 | 301 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vital, N.; Gramacho, A.C.; Silva, M.; Cardoso, M.; Alvito, P.; Kranendonk, M.; Silva, M.J.; Louro, H. Challenges of the Application of In Vitro Digestion for Nanomaterials Safety Assessment. Foods 2024, 13, 1690. https://doi.org/10.3390/foods13111690
Vital N, Gramacho AC, Silva M, Cardoso M, Alvito P, Kranendonk M, Silva MJ, Louro H. Challenges of the Application of In Vitro Digestion for Nanomaterials Safety Assessment. Foods. 2024; 13(11):1690. https://doi.org/10.3390/foods13111690
Chicago/Turabian StyleVital, Nádia, Ana Catarina Gramacho, Mafalda Silva, Maria Cardoso, Paula Alvito, Michel Kranendonk, Maria João Silva, and Henriqueta Louro. 2024. "Challenges of the Application of In Vitro Digestion for Nanomaterials Safety Assessment" Foods 13, no. 11: 1690. https://doi.org/10.3390/foods13111690
APA StyleVital, N., Gramacho, A. C., Silva, M., Cardoso, M., Alvito, P., Kranendonk, M., Silva, M. J., & Louro, H. (2024). Challenges of the Application of In Vitro Digestion for Nanomaterials Safety Assessment. Foods, 13(11), 1690. https://doi.org/10.3390/foods13111690








