Phenolic and Metabolic Profiles, Antioxidant Activities, Glycemic Control, and Anti-Inflammatory Activity of Three Thai Papaya Cultivar Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Determination of Proximate Composition of PL Powder
2.3. Preparation of Papaya Leaf Extract (PLE)
2.4. Determination of Bioactive Constitutes of PLE
2.4.1. Total Phenolic Content (TPC)
2.4.2. Determination of Total Flavonoid Content (TFC)
2.4.3. Determination of the Tannin Content (TC)
2.5. Analysis of Metabolic Profiles of PLE Using Liquid Chromatography-High-Resolution Tandem Mass Spectrometry (LC-HRMS/MS)
2.6. Determination of Antioxidant Activities of PLE
2.6.1. DPPH Radical-Scavenging Activity
2.6.2. Reducing Power
2.6.3. Chelating Activity
2.7. Determination of In Vitro Glycemic Index
2.7.1. In Vitro α-Amylase Inhibitory Activity
2.7.2. In Vitro α-Glucosidase Inhibitory Activity
2.8. MTT Assay for RAW 264.7 Cell Cytotoxicity
2.9. Determination of Nitric Oxide (NO) Inhibitory Activity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Proximate Compositions of PL
3.2. Bioactive Constituents of PLE
3.3. Phenolic and Metabolic Profiles of PLE
3.4. Antioxidant Activities of PLE
3.5. α-Amylase and α-Glucosidase Inhibitory Activity
3.6. Correlation Analysis between Phytochemical Compositions and Bioactivities
3.7. Cytotoxicity of PLE
3.8. Nitric Oxide (NO) Inhibitory Activity of PLE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, N.S.; Sreeja, P.S.D. The surprising health benefits of papaya seeds: A review. J. Pharmacogn. Phytochem. 2017, 6, 424–429. [Google Scholar]
- The Department of Agriculture. Agricultural Production Statistics Report; Ministry of Agriculture and Cooperatives: Bangkok, Thailand, 2019.
- Ongom, A.B.L.; Pranamornkith, P.; Pranamornkith, T. Intermittent warming affects postharvest quality and chilling injury of ‘Holland’ papaya fruit. J. Thai Interdiscip. Res. 2019, 14, 20–27. [Google Scholar]
- Somsri, S. Current status of papaya production in Thailand. Acta Hortic. 2014, 1022, 31–45. [Google Scholar] [CrossRef]
- Indra, M.; Mahmood, A.A.; Kuppusamy, U.R. Protective effect of Carica papaya linn leaf extract against alcohol induced acute gastric damage and blood oxidative stress in rats. West Indian Med. J. 2008, 127, 760–767. [Google Scholar]
- Sarala, N.; Paknikar, S.S. Papaya extract to treat dengue: A novel therapeutic option? Ann. Med. Health Sci. Res. 2014, 4, 320–324. [Google Scholar] [PubMed]
- Otsuki, N.; Dang, N.H.; Kumagai, E.; Kondo, A.; Iwata, S.; Morimoto, C. Aqueous extract of Carica papaya leaves exhibits anti-tumor activity and immunomodulatory effects. J. Ethnopharmacol. 2010, 127, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Roshan, A.; Verma, N.K.; Gupta, A. A brief study on Carica papaya—A review. Int. J. Curr. Trends Pharm. Res. 2014, 2, 541–550. [Google Scholar]
- Hariono, M.; Julianus, J.; Djunarko, I.; Hidayat, I.; Adelya, L.; Indayani, F.; Auw, Z.; Namba, G.; Hariyono, P. The future of Carica papaya leaf extract as an herbal medicine product. Molecules 2021, 26, 6922. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Alara, J.A. Carica papaya: Comprehensive overview of the nutritional values, phytochemicals and pharmacological activities. Adv. Tradit. Med. 2022, 22, 17–47. [Google Scholar] [CrossRef]
- Sharma, A.; Bachheti, A.; Sharma, P.; Bachheti, R.K.; Husen, A. Phytochemistry, pharmacological activities, nanoparticle fabrication, commercial products and waste utilization of Carica papaya L.: A comprehensive review. Curr. Res. Biotechnol. 2020, 2, 145–160. [Google Scholar]
- Nowak, J.; Kiss, A.K.; Wambebe, C.; Katuura, E.; Kuźma, Ł. Tentative qualitative and quantitative analysis of phenolic compounds in leaf extract from Carica papaya Linn. plant growing in Uganda. Herba Pol. 2021, 67, 1–9. [Google Scholar] [CrossRef]
- Soib, H.H.; Ismail, H.F.; Husin, F.; Abu Bakar, M.H.; Yaakob, H.; Sarmidi, M.R. Bioassay-guided different extraction techniques of Carica papaya (Linn.) leaves on in vitro wound-healing activities. Molecules 2020, 25, 517. [Google Scholar]
- Indran, I.R.; Tufo, G.; Pervaiz, S.; Brenner, C. Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim. Biophys. Acta 2011, 1807, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, R.; Sharma, M.; Kumar, M.; Barbhai, M.D.; Lorenzo, J.M.; Mekhemar, M. Carica papaya L. leaves: Deciphering its antioxidant bioactives, biological activities, innovative products, and safety aspects. Oxid. Med. Cell. Longev. 2022, 2022, 2451733. [Google Scholar]
- Marie-Solange, T.; Emma, A.A.; Noël, Z.G. Ethnobotanical study of plants used to treat arterial hypertension, in traditional medicine, by Abbey and Krobou populations of Agboville (Côte-d’Ivoire). Eur. J. Sci. Res. 2009, 35, 85–98. [Google Scholar]
- Nisa, F.Z.; Astuti, M.; Haryana, S.M.; Murdiati, A. Antioxidant activity and total flavonoid of Carica papaya L. leaves with different varieties, maturity and solvent. Agritech 2019, 39, 54–59. [Google Scholar] [CrossRef]
- Kumar, S.S.; Krishnakumar, K.; John, M. Flavonoids from the butanol extract of Carica papaya L. cultivar ’Red Lady’ leaf using UPLC-ESI-Q-ToF-MS/MS analysis and evaluation of the antioxidant activities of its fractions. Food Chem. Adv. 2022, 1, 100126. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 17th ed.; AOAC: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Adenowo, A.; Ilori, M.F.; Balogun, F.O.; Kazeem, M.I. Protective effect of ethanol leaf extract of Carica papaya Linn (Caricaceae) in Alloxan-induced diabetic rats. Trop. J. Pharm. Res. 2014, 13, 1877–1882. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Bravo, L.; Gómez-Guillén, L.M.C.; Alemán, A.; Montero, P. Antioxidant properties of tuna-skin and bovine-hide gelatin films induced by the addition of oregano and rosemary extracts. Food Chem. 2009, 112, 18–25. [Google Scholar] [CrossRef]
- Irondi, A.E.; Oboh, G.; Akintunde, J.K. Comparative and synergistic antioxidant properties of Carica papaya and Azadarichta indica leaf. Int. J. Pharm. Sci. Res. 2012, 3, 4773–4779. [Google Scholar]
- Pothitirat, W.; Chomnawang, M.T.; Supabphol, R.; Gritsanapan, W. Comparison of bioactive compounds content, free radical scavenging and anti-acne inducing bacteria activities of extracts from the mangosteen fruit rind at two stages of maturity. Fitoterapia 2009, 80, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S. Quantification of Tannins in Tree and Shrub Foliage: A Laboratory Manual; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Uawisetwathana, U.; Jamboonsri, W.; Bamrungthai, J.; Jitthiang, P.; Nookaew, I.; Karoonuthaisiri, N. Metabolite profiles of brown planthopper-susceptible and resistant rice (Oryza sativa) varieties associated with infestation and mechanical stimuli. Phytochemistry 2022, 194, 113044. [Google Scholar] [CrossRef]
- Fernández, N.J.; Damiani, N.; Podaza, E.A.; Martucci, J.F.; Fasce, D.; Quiroz, F.; Meretta, P.E.; Quintana, S.; Eguaras, M.J.; Gende, L.B. Laurus nobilis L. extracts against Paenibacillus larvae: Antimicrobial activity, antioxidant capacity, hygienic behavior and colony strength. Saudi J. Biol. Sci. 2019, 26, 906–912. [Google Scholar] [CrossRef]
- Canabady-Rochelle, L.L.S.; Harscoat-Schiavo, C.; Kessler, V.; Aymes, A.; Fournier, F.; Girardet, J.-M. Determination of reducing power and metal chelating ability of antioxidant peptides: Revisited methods. Food Chem. 2015, 183, 129–135. [Google Scholar] [CrossRef]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, M.L.M. Action of phenolic derivates (acetoaminophen, salycilate and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Turan, B.; Sendil, K.; Sengul, E.; Gultekin, M.S.; Taslimi, P.; Gulcin, I.; Supuran, C.T. The synthesis of some β-lactams and investigation of their metal chelating activity, carbonic anhydrase and achetylcholinesterase inhibition profiles. J. Enzyme Inhib. Med. Chem. 2016, 31, 79–88. [Google Scholar] [CrossRef]
- Kim, Y.M.; Jeong, Y.K.; Wang, M.H.; Lee, W.Y.; Rhee, H.I. Inhibitory effects of pine bark extract on alpha-glucosidase activity and postprandial hyperglycemiak. Nutrition 2005, 21, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yin, Y.; Zhao, W.; Liu, J.; Chen, F. Anti-diabetic activity peptides from albumin against α-glucosidase and α-amylase. Food Chem. 2012, 135, 2078–2085. [Google Scholar] [CrossRef] [PubMed]
- Agada, R.; Usman, W.A.; Shehu, S.; Thagariki, D. In vitro and in vivo inhibitory effects of Carica papaya seed on α-amylase and α-glucosidase enzymes. Heliyon 2020, 6, e03618. [Google Scholar] [CrossRef] [PubMed]
- Khummueng, W.; Rakhman, S.A.; Utaipan, T.; Pakhathirathien, C.; Boonyanuphong, P.; Chunglok, W. Phytochemicals, antioxidant and cytotoxicity of Stachytarpheta jamaicensis (L.) Vahl extracts. Thaksin. J. 2020, 23, 45–54. [Google Scholar]
- Chen, X.; Tang, S.-A.; Lee, E.; Qiu, Y.; Wang, R.; Duan, H.-Q.; Dan, S.; Jin, M.; Kong, D. IVSE, isolated from Inula japonica, suppresses LPS-induced NO production via NF-κB and MAPK inactivation in RAW264.7 cells. Life Sci. 2015, 124, 8–15. [Google Scholar] [CrossRef]
- Martial-Didier, A.K.; Hubert, K.K.; Parfait, K.E.J.; Kablan, T. Phytochemical properties and proximate composition of papaya (Carica papaya L. var solo 8) peels. Turk. J. Agric. Food Sci. Technol. 2017, 5, 676–680. [Google Scholar] [CrossRef]
- Anderson, J.W.; Smith, B.M.; Gustafson, N.J. Health benefits and practical aspects of high-fiber diets. Am. J. Clin. Nutr. 1994, 59, 1242S–1247S. [Google Scholar] [CrossRef]
- Nandini, G.; Gopenath, T.; Prasad, N.; Karthikeyan, M.; Gnanasekaran, A.; Ranjith, M.; Palanisamy, P.; Basalingappa, K.M. Phytochemical analysis and antioxidant properties of leaf extracts of Carica papaya. Asian J. Pharm. Clin. Res. 2020, 13, 58–62. [Google Scholar]
- Kabra, S.; Patel, S. Total phenolics & flavonoid content of the leaves of Carica papaya & Syzygium cumini. World J. Pharm. Res. 2018, 7, 734–741. [Google Scholar]
- Rahayu, S.E.; Leksono, A.S.; Gama, Z.P.; Tarno, H. The active compounds composition and antifeedant activity of leaf extract of two cultivar Carica papaya L. on Spodoptera litura F. larvae. AIP Conf. Proc. 2020, 2231, 040085. [Google Scholar]
- Chaithada, P.; Whenngean, P.; Fungfueng, R.; Maungchanburee, S. Correlation between total flavonoid content and total phenolic content on antioxidant activity of ethanol extracts from three cultivars of papaya leaves. Int. J. Res. Pharm. Sci. 2020, 11, 1883–1887. [Google Scholar] [CrossRef]
- Gaye, A.A.; Cisse, O.I.K.; Ndiaye, B.; Ayessou, N.C.; Cisse, M.; Diop, C.M. Evaluation of phenolic content and antioxidant activity of aqueous extracts of three Carica papaya varieties cultivated in Senegal. Food Nutr. Sci. 2019, 210, 276–289. [Google Scholar]
- Asghar, N.; Naqvi, S.A.R.; Hussain, Z.; Rasool, N.; Khan, Z.A.; Shahzad, S.A.; Sherazi, T.A.; Janjua, M.R.S.A.; Nagra, S.A.; Zia-Ul-Haq, M.; et al. Compositional difference in antioxidant and antibacterial activity of all parts of the Carica papaya using different solvents. Chem. Cent. J. 2016, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Ali, H.A.; Zain, N.M. Microwave-assisted extraction of phenolic compounds from Carica papaya leaves: An optimization study and LC-QTOF-MS analysis. Future Foods 2021, 3, 100035. [Google Scholar] [CrossRef]
- Fajrin, A.; Tunjung, W.A.S. The flavonoids content in leaves and fruits of papaya (Carica papaya L.) var. California and var. Gandul. KnE Life Sci. 2015, 2, 154–158. [Google Scholar] [CrossRef]
- Ugo, N.J.; Ade, A.R.; Joy, A.T. Nutrient composition of Carica papaya leaves extracts. J. Food Sci. Nutr. Res. 2019, 2, 274–282. [Google Scholar]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Liu, Y. Review in the studies on tannins activity of cancer prevention and anticancer. J. Chin. Med. Mater. 2003, 26, 444–448. [Google Scholar]
- Nugroho, A.; Heryani, H.; Choi, J.S.; Park, H.J. Identification and quantification of flavonoids in Carica papaya leaf and peroxynitrite-scavenging activity. Asian Pac. J. Trop. Biomed. 2017, 7, 208–213. [Google Scholar] [CrossRef]
- Jadaun, P.; Shah, P.; Harshithkumar, R.; Said, M.S.; Bhoite, S.P.; Bokuri, S.; Ravindran, S.; Mishra, N.; Mukherjee, A. Antiviral and ROS scavenging potential of Carica papaya Linn and Psidium guajava leaves extract against HIV-1 infection. BMC Complement. Med. Ther. 2023, 23, 82. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Saha, M.; Bandyopadhyay, P.K.; Jana, M. Extraction, isolation and characterization of bioactive compounds from chloroform extract of Carica papaya seed and it’s in vivo antibacterial potentiality in Channa punctatus against Klebsiella PKBSG14. Microb. Pathog. 2017, 111, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Gogna, N.; Hamid, N.; Dorai, K. Metabolomic profiling of the phytomedicinal constituents of Carica papaya L. leaves and seeds by 1H NMR spectroscopy and multivariate statistical analysis. J. Pharm. Biomed. Anal. 2015, 115, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.Y.; Hii, C.L.; Ong, S.P.; Lim, K.H.; Abas, F.; Pin, K.Y. Quantification of Carpaine and antioxidant properties of extracts from Carica papaya plant leaves and stalks. J. Bioresour. Bioprod. 2021, 6, 350–358. [Google Scholar] [CrossRef]
- Sangeetha, M.; Venkatalakshmi, P. In vitro antioxidant activity of the aqueous extract of Andrographis paniculata and Carica papaya leaves. World J. Pharm. Pharm. Sci. 2017, 6, 1631–1643. [Google Scholar]
- Degife, T.; Libsu, S. Studies on antioxidant activities of papaya (Carica papaya) and pineapple (Ananas cosmosus) fruits using ferric reducing antioxidant power and Rancimat methods. Chem. Mater. Res. 2018, 10, 24–29. [Google Scholar]
- Omar, S.R.; Aminuddin, F.; Karim, L.; Suhaimi, N.; Omar, S.N. Acceptability of novel antioxidant ice cream fortified with nutritious Carica papaya seed. J. Acad. 2020, 8, 7–17. [Google Scholar]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Rad. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Li, B.B.; Smith, B.; Hossain, M.M. Extraction of phenolics from citrus peels: I. Solvent extraction method. Sep. Purif. Technol. 2006, 48, 182–188. [Google Scholar] [CrossRef]
- Alqahtani, A.S.; Hidayathulla, S.; Rehman, M.T.; ElGamal, A.A.; Al-Massarani, S.; Razmovski-Naumovski, V.; Alqahtani, M.S.; Dib, R.A.E.; AlAjmi, M.F. Alpha-Amylase and alpha-glucosidase enzyme inhibition and antioxidant potential of 3-oxolupenal and katononic acid isolated from Nuxia oppositifolia. Biomolecules 2020, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Sugiwati, S.; Setiasi, S.; Afifah, E. Antihyperglycemic activity of the mahkota dewa [Phaleria macrocarpa (Scheff.) Boerl.] leaf extracts as an alpha-glucosidase inhibitor. J. Health Res. 2009, 13, 74–78. [Google Scholar]
- Marrelli, M.; Amodeo, V.; Statti, G.; Conforti, F. Biological properties and bioactive components of Allium cepa L.: Focus on potential benefits in the treatment of obesity and related comorbidities. Molecules 2019, 24, 119. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Rojop, I.E.; Tovilla-Zárate, C.; Aguilar-Domínguez, D.E.; Roa-de la Fuente, L.F.; Lobato-García, C.E.; Blé-Castillo, J.L.; López-Meraz, L.; Díaz-Zagoya, J.C.; Bermúdez-Ocaña, D.Y. Phytochemical screening and hypoglycemic activity of Carica papaya leaf in streptozotocin-induced diabetic rats. Rev. Bras. Farmacogn. 2014, 24, 341–347. [Google Scholar] [CrossRef]
- Tsimogiannis, D.I.; Oreopoulou, V. The contribution of flavonoid C-ring on the DPPH free radical scavenging efficiency. A kinetic approach for the 3′, 4′-hydroxy substituted members. Innov. Food Sci. Emerg. Technol. 2006, 7, 140–146. [Google Scholar] [CrossRef]
- Amarowicz, R.; Naczk, M.; Shahidi, F. Antioxidant activity of crude tannins of canola and rapeseed hulls. J. Am. Oil Chem. Soc. 2000, 77, 957–961. [Google Scholar] [CrossRef]
- Jo, H.J.; Chung, K.H.; Yoon, J.A.; Lee, K.J.; Song, B.C.; An, J.H. Radical scavenging activities of tannin extracted from amaranth (Amaranthus caudatus L.). J. Microbiol. Biotechnol. 2015, 25, 795–802. [Google Scholar] [CrossRef]
- Orak, H.H.; Yagar, H.; Isbilir, S.S. Comparison of antioxidant activities of juice, peel, and seed of pomegranate (Punica granatum L.) and inter-relationships with total phenolic, tannin, anthocyanin, and flavonoid contents. Food Sci. Biotechnol. 2012, 21, 373–387. [Google Scholar] [CrossRef]
- Zhang, L.L.; Lin, Y.M. Tannins from Canarium album with potent antioxidant activity. J. Zhejiang Univ. Sci. B 2008, 9, 407–415. [Google Scholar] [CrossRef]
- Mira, L.; Tereza Fernandez, M.; Santos, M.; Rocha, R.; Helena Florêncio, M.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Rad. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef]
- Li, M.; Bao, X.; Zhang, X.; Ren, H.; Cai, S.; Hu, X.; Yi, J. Exploring the phytochemicals and inhibitory effects against α-glucosidase and dipeptidyl peptidase-IV in Chinese pickled chili pepper: Insights into mechanisms by molecular docking analysis. LWT 2022, 162, 113467. [Google Scholar] [CrossRef]
- Hyun, S.B.; Ko, M.N.; Hyun, C. Carica papaya leaf water extract promotes innate immune response via MAPK signaling pathways. J. Appl. Biol. Chem. 2021, 64, 277–284. [Google Scholar] [CrossRef]
- Wang, J.; Mazza, G. Inhibitory effects of anthocyanins and other phenolic compounds on nitric oxide production in LPS/IFN-γ-activated RAW 264.7 macrophages. J. Agric. Food Chem. 2002, 50, 850–857. [Google Scholar] [CrossRef]
- Yuliani, R.; Syahdeni, F. Ethanolic extract of papaya leaves (Carica papaya) and its fractions have no potential cytotoxicity on T47D cells. Pharmacon. 2020, 17, 17–23. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Setiawan, P.Y.B.; Kertia, N.; Nurrochmad, A.; Wahyuono, S. Synergistic anti- inflammatory effects of Curcuma xanthorrhiza rhizomes and Physalis angulata herb extract on lipopolysaccharide-stimulated RAW 264.7 cells. J. Appl. Pharm. Sci. 2022, 12, 088–098. [Google Scholar] [CrossRef]
- Yarovaya, L.; Khunkitti, W. Anti-inflammatory activity of grape seed extract as a natural sun protection enhancer for broad-spectrum sunscreen. In Proceedings of the Cosmetic and Beauty International Conference 2019, Chiang Rai, Thailand, 7–9 October 2019; pp. 1–8. [Google Scholar]
- Adebayo, S.A.; Ondua, M.; Shai, L.J.; Lebelo, S.L. Inhibition of nitric oxide production and free radical scavenging activities of four South African medicinal plants. J. Inflamm. Res. 2019, 12, 195–203. [Google Scholar] [CrossRef]
- Ng, R.F.L.; Abidin, N.Z.; Shuib, A.S.; Ali, D.A.I. Inhibition of nitric oxide production by Solanum melongena and Solanum macrocarpon on RAW 264.7 cells. Front. Life Sci. 2015, 8, 241–248. [Google Scholar] [CrossRef]
- Basim, S.; Kasim, A.A. Cytotoxic activity of the ethyl acetate extract of Iraqi Carica papaya leaves in breast and lung cancer cell lines. Asian Pac. J. Cancer Prev. 2023, 24, 581–586. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumar, S.; Tomar, M.S.; Singh, R.K.; Verma, P.K.; Kumar, A.; Kumar, S.; Acharya, A. Aqueous extract of Carica papaya leaf elicits the production of TNF-α and modulates the expression of cell surface receptors in tumor-associated macrophages. Biosci. Biotechnol. Res. Commun. 2019, 4, 1115–1122. [Google Scholar] [CrossRef]
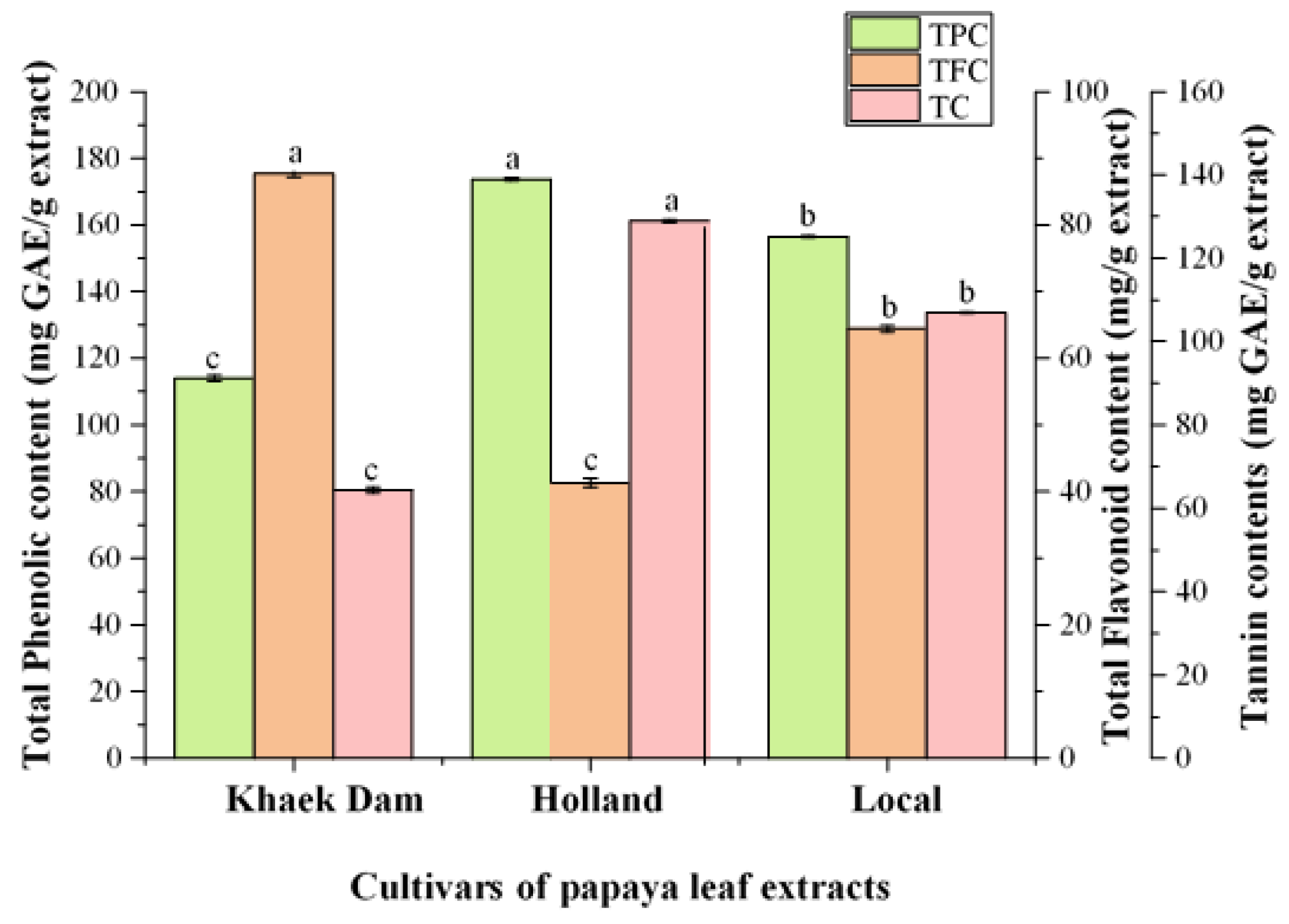
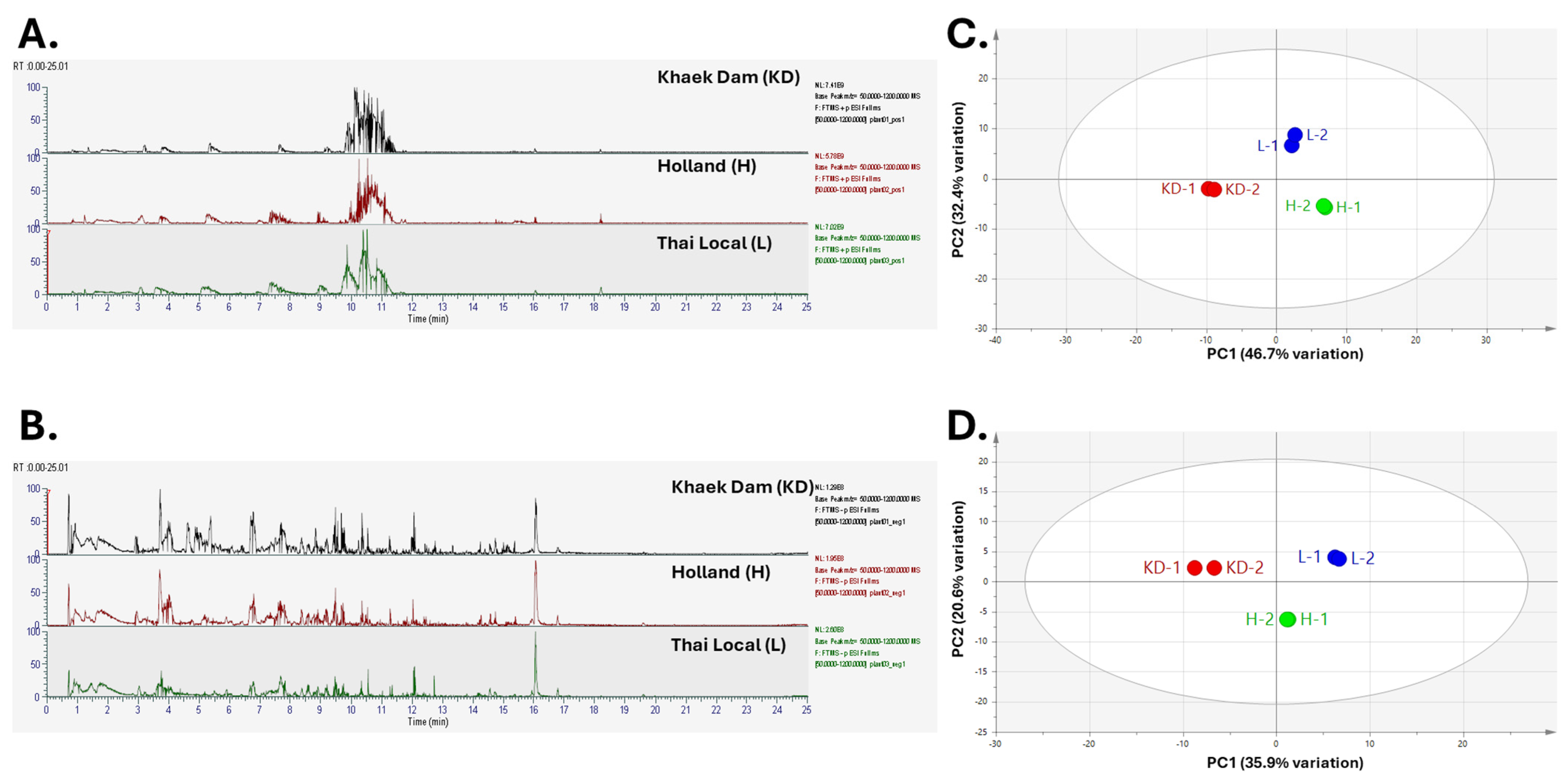

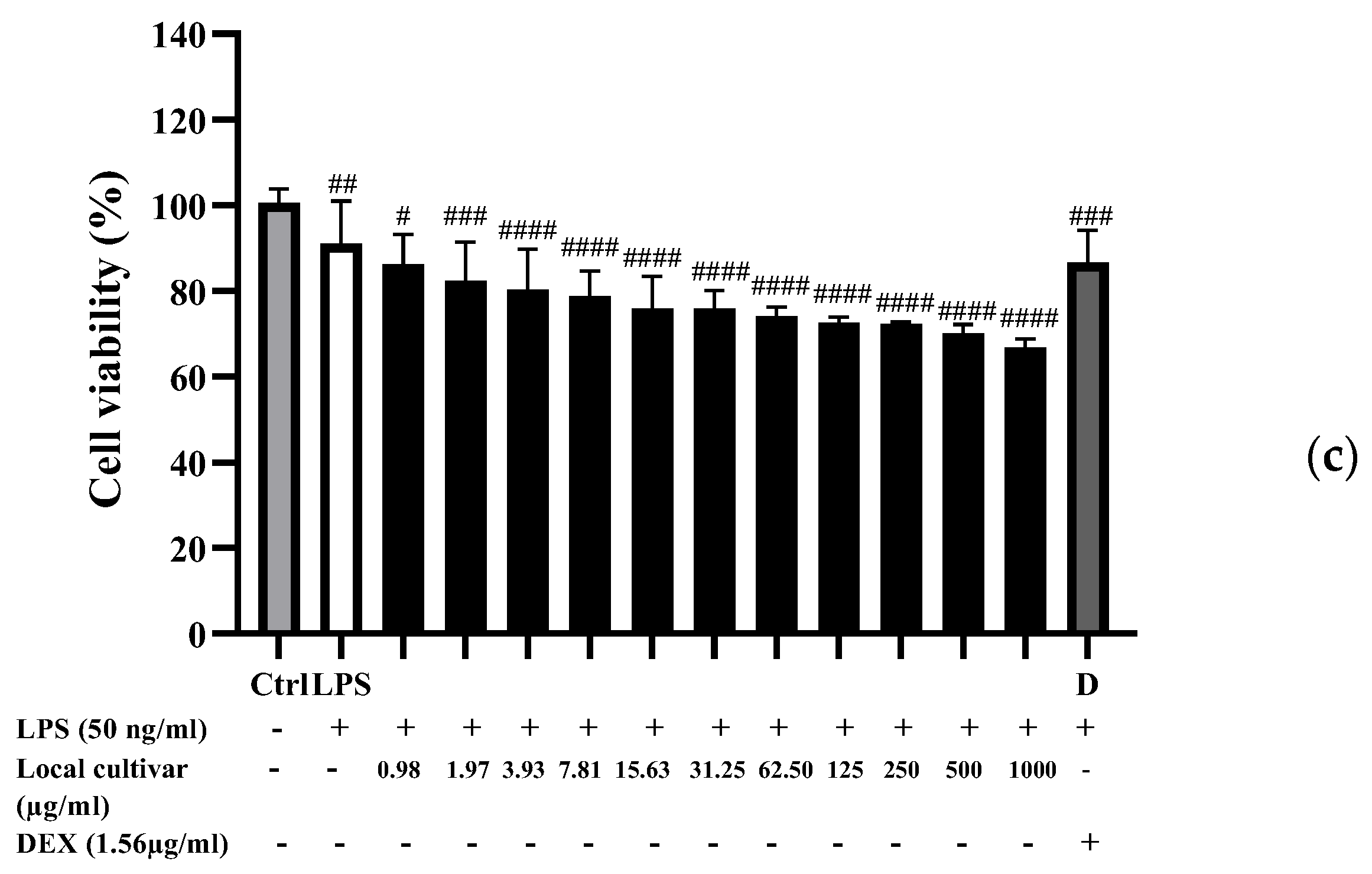
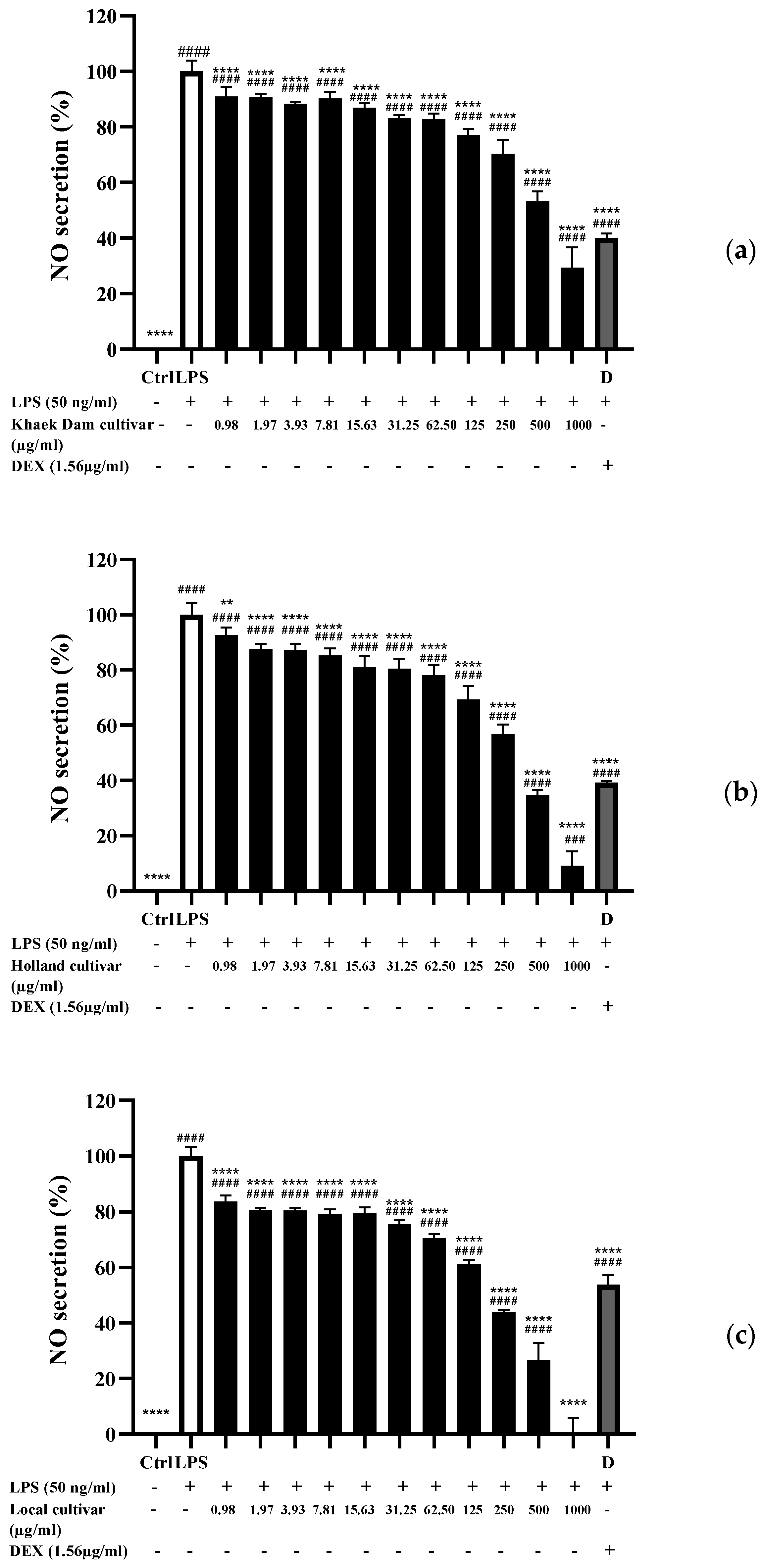
| Parameter | Khaek Dam (KD) | Holland (H) | Local (L) |
|---|---|---|---|
| Moisture (%) | 6.21 ± 0.05 b | 6.49 ± 0.04 a | 6.02 ± 0.01 c |
| Ash (%) | 11.63 ± 0.03 b | 12.40 ± 0.04 a | 11.23 ± 0.06 c |
| Protein (%) | 25.96 ± 0.20 c | 30.43 ± 0.16 b | 32.18 ± 0.34 a |
| Fat (%) | 11.66 ± 0.04 a | 7.34 ± 0.06 c | 9.42 ± 0.02 b |
| Carbohydrate (%) | 6.06 ± 0.19 b | 5.80 ± 0.11 b | 17.91 ± 0.36 a |
| Fiber (%) | 38.48 ± 0.04 a | 37.55 ± 0.30 b | 23.24 ± 0.36 c |
| Retention Time (min) | Calc. MW | m/z | Annot. DeltaMass [ppm] | Reference Ion | Putative Metabolite | Chemical Formula | Compound Class | ChemSpider (CSID) | mzCloud Match (%) | Metabolite Identification Level * | Khaek Dam (KD) | Holland (H) | Thai Local (L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3.006 | 156.06932 | 157.07659 | 3.63 | [M + H] + 1 | 3-Indoleacetonitrile | C10H8N2 | indole derivative | 312,357 | 84.5 | 2 | 3.09 × 107 | 2.48 × 107 | 3.18 × 107 |
| 3.014 | 203.05817 | 204.06543 | −0.38 | [M + H] + 1 | Indole-3-pyruvic acid | C11H9NO3 | indole | 781 | 51.5 | 2 | 5.28 × 106 | 3.01 × 106 | 5.39 × 106 |
| 4.394 | 164.04776 | 165.05504 | 2.55 | [M + H] + 1 | (E)-p-Coumaric acid | C9H8O3 | hydroxycinnamic acid | 553,148 | n/a | 2 | 2.45 × 107 | 4.13 × 107 | 3.82 × 107 |
| 4.993 | 205.07384 | 188.07056 | −0.24 | [M + H − H2O] + 1 | Indole-3-lactic acid | C11H11NO3 | indole derivative | 83,867 | 75.4 | 2 | 2.69 × 106 | 3.12 × 106 | 5.57 × 106 |
| 5.162 | 198.05277 | 199.06005 | −0.29 | [M + H] + 1 | Syringic acid | C9H10O5 | hydroxybenzoic acid | 10,289 | 85.1 | 2 | 1.26 × 107 | 7.96 × 106 | 4.59 × 106 |
| 5.19 | 198.0536 | 199.06088 | 3.93 | [M + H] + 1 | Vanillylmandelic acid | C9H10O5 | methoxyphenols | 1207 | n/a | 3 | 1.39 × 107 | 8.27 × 106 | 6.85 × 106 |
| 6.222 | 133.05266 | 132.04538 | 0.75 | [M + H] + 1 | 5-Indolol | C8H7NO | indole | 15,244 | n/a | 3 | 1.74 × 107 | 5.58 × 108 | 7.71 × 106 |
| 7.168 | 216.0898 | 217.09708 | −0.36 | [M + H] + 1 | 2,3,4,9-Tetrahydro-1H-β-carboline-3-carboxylic acid | C12H12N2O2 | indole derivative | 88,749 | 98.5 | 2 | 2.02 × 107 | 3.72 × 107 | 3.21 × 107 |
| 7.251 | 122.0373 | 123.04458 | 4.29 | [M + H] + 1 | Benzoic acid | C7H6O2 | aromatic carboxylic acid | 238 | n/a | 3 | 2.84 × 106 | 5.23 × 106 | 2.75 × 106 |
| 7.89 | 154.1363 | 155.14357 | 3.44 | [M + H] + 1 | (+/−)-Eucalyptol | C10H18O | terpenoid | 2656 | n/a | 3 | 2.99 × 106 | 2.47 × 106 | 9.75 × 106 |
| 7.9 | 108.05788 | 109.06516 | 3.37 | [M + H] + 1 | p-Cresol | C7H8O | simple phenols | 13,839,082 | n/a | 3 | 3.16 × 106 | 5.84 × 105 | 7.51 × 105 |
| 8.287 | 124.05285 | 125.06013 | 3.4 | [M + H] + 1 | Guaiacol | C7H8O2 | methoxyphenols | 447 | n/a | 3 | 6.51 × 106 | 5.36 × 106 | 2.54 × 106 |
| 8.315 | 152.04803 | 153.0553 | 4.48 | [M + H] + 1 | Vanillin | C8H8O3 | phenolic aldehyde | 13,860,434 | 80.8 | 2 | 4.02 × 107 | 4.69 × 107 | 2.66 × 107 |
| 8.376 | 302.04257 | 303.04985 | −0.28 | [M + H] + 1 | Quercetin | C15H10O7 | flavonols | 4,444,051 | 98.9 | 2 | 2.05 × 108 | 1.63 × 108 | 2.66 × 108 |
| 9.372 | 316.05952 | 317.0668 | 3.85 | [M + H] + 1 | Isorhamnetin | C16H12O7 | flavonols | 96 | 83 | 2 | 9.65 × 106 | 7.19 × 106 | 1.75 × 107 |
| 9.534 | 148.05302 | 149.0603 | 3.98 | [M + H] + 1 | Cinnamic acid | C9H8O2 | aromatic carboxylic acid | 392,447 | n/a | 3 | 2.55 × 107 | 1.69 × 107 | 1.55 × 107 |
| 10.105 | 286.04758 | 287.05485 | −0.56 | [M + H] + 1 | Kaempferol | C15H10O6 | flavonols | 4,444,395 | 97.3 | 2 | 2.60 × 108 | 1.49 × 108 | 3.81 × 108 |
| 11.294 | 194.05788 | 177.0546 | −0.13 | [M + H − H2O] + 1 | (E)-Isoferulic acid | C10H10O4 | hydroxycinnamic acid | 643,318 | n/a | 3 | 1.37 × 107 | 7.09 × 106 | 2.54 × 107 |
| 12.05 | 164.08424 | 165.09152 | 3.12 | [M + H] + 1 | Eugenol | C10H12O2 | methoxyphenols | 13,876,103 | n/a | 3 | 4.55 × 106 | 1.72 × 106 | 1.21 × 106 |
| 0.923 | 192.0633 | 191.05561 | −0.48 | [M − H] −1 | D-(−)-Quinic acid | C7H12O6 | cyclohexanecarboxylic acid | 10,246,715 | 92.5 | 2 | 1.11 × 106 | 2.49 × 106 | 2.50 × 106 |
| 3.907 | 154.02656 | 153.01929 | −0.29 | [M − H] − 1 | Protocatechuic acid | C7H6O4 | hydroxybenzoic acid | 71 | n/a | 3 | 2.94 × 105 | 3.49 × 106 | 8.47 × 105 |
| 4.097 | 332.07418 | 331.06687 | −0.51 | [M − H] − 1 | Glucogallin | C13H16O10 | tannins | 110,537 | n/a | 3 | 6.88 × 106 | 8.46 × 107 | 3.18 × 107 |
| 4.962 | 224.03211 | 223.02483 | 0.1 | [M − H] − 1 | 3-[(1-Carboxyvinyl)oxy]-4-hydroxybenzoic acid | C10H8O6 | hydroxybenzoic acid | 8,096,552 | n/a | 3 | 5.60 × 105 | 7.26 × 105 | 3.40 × 106 |
| 5.653 | 312.0485 | 293.03068 | 1.17 | [M − H − H2O] − 1 | Caftaric acid | C13H12O9 | hydroxycinnamic acid | 4,944,664 | n/a | 3 | 1.17 × 106 | 7.56 × 106 | 6.23 × 106 |
| 7.138 | 316.11503 | 315.10775 | 2.43 | [M − H] − 1 | Vanilloloside | C14H20O8 | glycosides | 24,695,215 | n/a | 3 | 7.39 × 107 | 1.08 × 108 | 3.37 × 107 |
| 8.18 | 342.0953 | 341.08803 | 0.65 | [M − H] − 1 | Caffeic acid 3-glucoside | C15H18O9 | glycosides | 4,445,073 | n/a | 3 | 1.04 × 108 | 7.46 × 107 | 6.60 × 107 |
| 8.881 | 94.04194 | 93.03467 | 0.85 | [M − H] − 1 | Phenol | C6H6O | simple phenols | 971 | 92.7 | 2 | 7.67 × 106 | 1.07 × 107 | 8.13 × 106 |
| 9.148 | 182.05809 | 181.05081 | 0.99 | [M − H] − 1 | Homovanillic acid | C9H10O4 | methoxyphenols | 1675 | n/a | 3 | 4.24 × 105 | 3.48 × 105 | 4.11 × 105 |
| 9.486 | 194.05783 | 193.05055 | −0.41 | [M − H] − 1 | Ferulic acid | C10H10 O4 | hydroxycinnamic acid | 393,368 | 97.1 | 2 | 2.32 × 108 | 2.38 × 108 | 2.49 × 108 |
| 9.657 | 224.06841 | 225.07565 | −0.27 | [M − H] − 1 | Sinapic acid | C11H12O5 | hydroxycinnamic acid | 553,361 | 90.4 | 2 | 1.29 × 107 | 4.46 × 107 | 1.22 × 108 |
| 9.668 | 340.07935 | 339.07207 | −0.24 | [M − H] − 1 | Aesculin | C15H16O9 | glycosides | 4,444,765 | n/a | 3 | 7.82 × 106 | 6.74 × 106 | 8.54 × 107 |
| 10.166 | 360.14088 | 359.13491 | 0.44 | [M − H] − 1 | 8-Epideoxyloganic acid | C16H24O9 | glycosides | 391,568 | n/a | 3 | 1.93 × 107 | 1.49 × 107 | 1.39 × 107 |
| 10.574 | 138.03179 | 137.02451 | 0.66 | [M − H] − 1 | Salicylic acid | C7H6O3 | hydroxybenzoic acid | 331 | 99 | 2 | 3.26 × 107 | 4.84 × 107 | 2.79 × 107 |
| 15.237 | 126.03179 | 125.02452 | 0.78 | [M − H] − 1 | Pyrogallol | C6H6O3 | dihydroxyphenols | 13,835,557 | n/a | 3 | 6.20 × 106 | 4.01 × 106 | 9.28 × 105 |
 the most,
the most,  the middle, and
the middle, and  the least.
the least.| Metabolite | Compounds |
|---|---|
| Amino acid and its derivative | L-Isoleucine, L-Phenylalanine, L-Valine, D-(+)-Proline, L-(−)-Methionine, D-(+)-Tryptophan, L-Histidine, Asparagine, DL-Glutamine, DL-Arginine, 2-Aminobutyric acid, 4-Acetamidobutanoic acid, Phenylacetaldehyde, 4-Guanidinobutyric acid, L-Glutathione, N-Acetylornithine, Acetylarginine, N6,N6,N6-Trimethyl-L-lysine, Glycine, L-(+)-Alanine, L-(−)-Serine, L-(−)-Threonine, Aminolevulinic acid, Asparagine, DL-Glutamic acid, L-Tyrosine |
| Lipid and fatty acids and their derivatives | (R)-3-Hydroxy myristic acid, α-Linolenic acid, 9-Oxo-ODE, (±)12(13)-DiHOME, 16-Hydroxyhexadecanoic acid, Corchorifatty acid F, Hexadecanamide, Oleoyl ethanolamide, α-Linolenoyl ethanolamide, Erucamide, 5α-Dihydrotestosterone, Butyl palmitate, Stearidonic acid, (+/-)9-HODE, 13(S)-HpOTrE, 3-oxopalmitic acid, (±)9-HpODE, 2-Hydroxy-4-(methylthio)butanoic acid |
| Saccharide and its derivatives | D-(+)-Glucose, D-Xylonic acid, D-(−)-Fructose, D-(+)-arabitol, Gluconic acid, D-(+)-Galactose, D-(−)-Mannitol, L-Iditol |
| Organic compound | Caffeic acid, Citric acid, Guvacine, 4-Methoxycinnamaldehyde, DL-Malic acid, Myristicin, Fumaric acid, Glutaric acid, 4-Acetyl-2-prenylphenol, Isoamylamine, 2-Ethyl-2-phenylmalonamide, N-Phenylacetylglutamine, L-(+)-Lactic acid, Methylmalonic acid, Caprolactam, N-Acetylputrescine, Picolinic acid, 4-Hydroxybenzaldehyde, 4-Pyridoxic acid, Acetamide, Malondialdehyde, 2-Pyrrolidone, 2-Furoic acid, Maleic acid, Malonic acid, Maleamic acid, Levulinic acid, Nicotinamide, Nicotinic acid, 2-morpholinoacetic acid, Adipic acid, Safrole, Hippuric acid, Azelaic acid, (+/−)-Camphoric acid, Porphobilinogen, Indican, 5′-S-Methyl-5′-thioadenosine |
| Parameter | Khaek Dam (KD) | Holland (H) | Local (L) |
|---|---|---|---|
| DPPH assay 1 (IC50, mg/mL) | 4.08 ± 0.004 c | 5.92 ± 0.009 a | 5.14 ± 0.017 b |
| Reducing power (mg AAE/g) | 15.28 ± 0.02 b | 19.14 ± 0.04 a | 14.66 ± 0.04 c |
| Metal chelation 2 (IC50, mg/mL) | 0.31 ± 0.001 b | 0.33 ± 0.001 a | 0.31 ± 0.001 b |
|
α-Amylase inhibitory activity 3 (IC50, mg/mL) | 2.28 ± 0.02 c | 2.36 ± 0.01 b | 2.69 ± 0.03 a |
| α-Glucosidase inhibitory activity 4 (IC50, mg/mL) | 1.73 ± 0.01 c | 2.56 ± 0.02 b | 4.20 ± 0.05 a |
| TPC | TFC | TC | Ferulic Acid | Kaempferol | Quercetin | Caffeic Acid 3-Glucoside | |
|---|---|---|---|---|---|---|---|
| DPPH assay (IC50, mg/mL) | 0.988 ** | −0.996 ** | 0.995 ** | 0.429 | −0.400 | −0.324 | −0.794 * |
| Reducing power (mg AAE/g) | 0.628 | −0.794 * | 0.672 * | −0.292 | −0.913 ** | −0.877 ** | −0.177 |
| Metal chelation (IC50, mg/mL) | 0.840 ** | −0.942 ** | 0.869 ** | 0.024 | −0.736 ** | −0.679 * | −0.4778 |
|
α-Amylase inhibitory activity (IC50, mg/mL) | 0.413 | −0.189 | 0.361 | 0.981 ** | 0.768 * | 0.817 ** | −0.798 ** |
| α-Glucosidase inhibitory activity (IC50, mg/mL) | 0.545 | −0.332 | 0.496 | 1.000 ** | 0.671 * | 0.729 * | −0.881 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaijan, S.; Chaijan, M.; Uawisetwathana, U.; Panya, A.; Phonsatta, N.; Shetty, K.; Panpipat, W. Phenolic and Metabolic Profiles, Antioxidant Activities, Glycemic Control, and Anti-Inflammatory Activity of Three Thai Papaya Cultivar Leaves. Foods 2024, 13, 1692. https://doi.org/10.3390/foods13111692
Chaijan S, Chaijan M, Uawisetwathana U, Panya A, Phonsatta N, Shetty K, Panpipat W. Phenolic and Metabolic Profiles, Antioxidant Activities, Glycemic Control, and Anti-Inflammatory Activity of Three Thai Papaya Cultivar Leaves. Foods. 2024; 13(11):1692. https://doi.org/10.3390/foods13111692
Chicago/Turabian StyleChaijan, Sirinet, Manat Chaijan, Umaporn Uawisetwathana, Atikorn Panya, Natthaporn Phonsatta, Kalidas Shetty, and Worawan Panpipat. 2024. "Phenolic and Metabolic Profiles, Antioxidant Activities, Glycemic Control, and Anti-Inflammatory Activity of Three Thai Papaya Cultivar Leaves" Foods 13, no. 11: 1692. https://doi.org/10.3390/foods13111692
APA StyleChaijan, S., Chaijan, M., Uawisetwathana, U., Panya, A., Phonsatta, N., Shetty, K., & Panpipat, W. (2024). Phenolic and Metabolic Profiles, Antioxidant Activities, Glycemic Control, and Anti-Inflammatory Activity of Three Thai Papaya Cultivar Leaves. Foods, 13(11), 1692. https://doi.org/10.3390/foods13111692







